NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Clinical Guideline Centre (UK). Blood Transfusion. London: National Institute for Health and Care Excellence (NICE); 2015 Nov. (NICE Guideline, No. 24.)
As part of the Department of Health initiative on patient blood management in addition to safety of blood transfusion, there is a clear focus to improve clinical outcomes in patients by preventing exposure to donor blood. This has resulted in the need for appropriate use of blood and use of alternatives to blood transfusion.
Cell salvage and tranexamic acid (TXA) have both been used in surgical patients to reduce the requirement for transfusion of allogeneic red cells.
Cell salvage
Cell salvage is a procedure whereby blood loss during or after surgery is collected and then transfused back to the patient.
Salvage of blood both intra-operatively and post-operatively and its re-transfusion has been used for many years with the aim of reducing the frequency and the volume of allogeneic blood transfusion for a number of surgical procedures. Intra-operative cell salvage involves collection of shed blood during surgery followed by re-transfusion. This is carried out by using a cell salvage device. Post-operative cell salvage involves collection of blood from post-operative drains and re-transfusion.
Cell salvage has been used in many hospitals in combination with other measures for minimising blood use in surgical patients. This has contributed to a marked decreased use of red cell transfusion in surgical patients in England over the last 15 years.
Tranexamic acid
Tranexamic acid (TXA) is an antifibrinolytic. It is a synthetic derivative of the amino acid lysine that inhibits fibrinolysis (clot break down) by blocking the lysine binding sites on plasminogen.
The recent CRASH-2 randomised placebo-controlled trial which assessed the effects of tranexamic acid on death, vascular occlusive events and blood transfusion in trauma patients with significant haemorrhage reported tranexamic acid safely reduced all-cause mortality and risk of death due to bleeding. The haemostatic response to vascular injury in trauma is similar to the response to major surgery. The data have been reviewed to determine the clinical and cost-effectiveness of tranexamic acid in reducing blood transfusion requirements in surgical patients in light of widespread use and safety data post the CRASH-2 trial on trauma patients.
The use of tranexamic acid and cell salvage alone or in combination with each other is examined in this review.
6.1. Review question: What is the clinical and cost-effectiveness of using alternatives to blood transfusion (cell salvage or tranexamic acid alone or in combination with one another) to reduce blood transfusion requirements?
The objective of this review question was to evaluate if the combination of cell salvage and tranexamic acid was more clinically and cost-effective than either of them alone.
For full details see review protocol in Appendix C.
6.2. Methodology of clinical evidence review
6.2.1. Background
The GDG was keen to identify the best combination of alternatives to blood transfusion in surgical patients.
Two preliminary clinical evidence reviews showed that cell salvage and tranexamic acid were both clinically and cost-effective when compared independently with standard treatment. Standard treatment was defined as either the administration of placebo or usual care. However, the GDG was keen to understand whether:
- one intervention was more effective than the other
- the combination of cell salvage and tranexamic acid was better than either intervention
- there were specific population groups in which one intervention or combination may be more effective.
To this end, the evidence was reviewed again, based on the stratification of the surgical populations into three groups (see section 6.2.2 below), as proposed by the GDG. Further details of the methodology of the review are explained in subsequent sections.
6.2.2. Stratification of risk groups and pre-defined subgroup analysis
The GDG stratified the population according to baseline risk of requiring a blood transfusion which was noted to be collectively dependent on a number of factors including:
- the type of surgery
- the use of different transfusion protocols and blood transfusion at different thresholds
- the baseline and pre-operative haemoglobin level of the patient
- any pre-operative management received by the patient to correct anaemia
- autologous donation of blood prior to surgery.
Accurate stratification of the population by baseline risk requires classification of individual patients within trials into different risk groups taking into account all of the above factors. It was acknowledged that the data for such an exercise were not available from randomised controlled trials. No individual participant data meta-analysis was available in this topic area.
The GDG agreed that stratification of patients into risk groups based on the expected volume of blood loss determined solely by the type of surgery was an acceptable approximation of the above classification. Although it was not possible to stratify the population accurately taking into account all factors influencing the risk of receiving a blood transfusion, it was agreed that these factors would be explored by way of subgroup analysis in case of heterogeneity.
The surgeries were grouped into three strata:
- High risk surgeries were defined as surgeries where blood loss is expected to be greater than 1 litre.
- Moderate risk surgeries were defined as surgeries where blood loss is expected to be between 500 ml and 1 litre.
- Low risk surgeries were defined as surgeries where blood loss is expected to be less than 500 ml.
It was noted that cell salvage is appropriate only for surgeries in the high and moderate risk groups where blood loss is expected to be greater than 1 unit (approximately 500 ml).
As blood loss is expected to be less than 500 ml, cell salvage is not a feasible option in the low risk surgery group and, therefore, effectiveness of only tranexamic acid compared with standard treatment was evaluated in this group.
It was noted that the volumes outlined in the above classification may not be applicable to surgeries in children. In children, the classification was therefore done by taking into consideration both the type of surgery and the blood volume.
- In children the GDG agreed that moderate blood loss would be defined as blood loss greater than 10% of blood volume.
- In adults, high degree of blood loss was defined as blood loss greater than 1 litre; the GDG agreed that a corresponding equivalent blood loss with respect to body weight in children would qualify as a high degree of blood loss.
This is further reflected in the outcomes analysed in the review for children where the volume of blood transfused was used an outcome rather than the units of allogeneic blood transfused.
6.2.3. Exclusion of studies published before 2003
Change in surgical practice over the last decade has resulted in less blood loss due to more attention being given to achieving haemostasis to avoid unnecessary bleeding. A change in surgical practice by some practitioners to not use post-operative drains has largely eliminated post-operative cell salvage as a blood conservation technique and the accepted indications for intra-operative cell salvage have extended.
The GDG agreed that substantial changes in transfusion practice over time with respect to the use of cell salvage meant that studies published prior to 2003 were not relevant to current clinical practice and would not inform the decision making process or the economic model. These changes were in relation to:
- selection of patients for cell salvage (intra-operative cell salvage and post-operative cell salvage)
- surgical technique.
The GDG noted that the above rationale does not impact on the effectiveness of tranexamic acid, and all RCTs evaluating the effectiveness of tranexamic acid were included in the clinical review (no date restriction was applied). The effectiveness of tranexamic acid is related to inhibition of fibrinolysis and not to changes in surgical technique and therefore it is relevant to include all studies regardless of when they were published.
A preliminary subgroup analysis of all trials for both interventions showed that there were differences between studies on cell salvage conducted before and after 2003. This finding was not observed in trials conducted to evaluate the efficacy of tranexamic acid. This reinforced the GDG's decision to exclude trials on cell salvage conducted prior to 2003, but include the data for tranexamic acid with no date restrictions.
6.2.4. Grouping of doses and routes of administration of tranexamic acid
The GDG agreed that all doses and routes of administration of tranexamic acid should be evaluated together. This was based on GDG consensus and supported by a preliminary subgroup analysis which showed no differences between different routes of administration of tranexamic acid.
6.3. Clinical evidence
We searched for randomised controlled trials comparing the effectiveness of different interventions in the protocol (see matrix of treatment comparisons).
Five Cochrane reviews were identified in this topic area which met the inclusion criteria38,130,162,163,245; These reviews independently evaluated the effect of either cell salvage or tranexamic acid in reducing allogeneic blood transfusion requirements. However, these reviews did not evaluate the effectiveness of combinations of cell salvage or tranexamic acid. As the studies included in these reviews do provide data on the effectiveness of combination of these interventions, these reviews have been included as part of this clinical evidence review and data from individual studies have been extracted again with respect to combinations of cell salvage and tranexamic acid as interventions (if present), and the analysis has been adapted accordingly.
The process of re-extracting the data included checking all studies identified in the previously published reviews and in the update searches (since the cut off dates of the Cochrane reviews) which compared cell salvage or tranexamic acid with standard treatment. All studies were checked to confirm the concomitant treatments in both arms (these may have included either cell salvage or tranexamic acid). The study interventions were reclassified based on this and the studies were grouped into a specific risk category as defined above (see section 1.3.2). The GDG discussed the patient population in each study and stratified them in one of the risk groups based on the expected blood loss.
Data relevant to the comparisons in the current review protocol on combinations of cell salvage and tranexamic acid were extracted from these reviews and reanalysed.
Pairwise meta-analysis was conducted for each risk group (high, moderate and low) based on the stratification agreed by with the GDG and for adults and children separately. Results are presented for each risk group.
For the outcome on number of units of allogeneic transfusions received by participants, the mean number of units was analysed in participants who received transfusions. In some studies where it was unclear if the mean number of units was calculated over the number of participants who received transfusions or over the total number of participants randomised to that intervention, we assumed the former and have downgraded the evidence for outcome reporting bias.
Evidence was found for the following comparisons in each risk group and the results have been presented accordingly.
A summary of the studies, classified by risk groups and included in this review is presented in the tables 22-25 below.
6.3.1. Results from pair wise meta-analysis
For summary GRADE profiles from pairwise meta-analysis, refer to sections 6.3.1.1– 6.3.1.4.
The results of the pairwise meta-analysis are presented for each comparison and outcome in each risk group. Results are presented separately for adults and children. For forest plots of pairwise comparisons, refer to Appendix K.
6.3.1.1. Evidence from pair wise comparisons: adults - high risk group
6.3.1.2. Evidence from pairwise comparisons: adults - moderate risk group
6.3.1.3. Evidence from pairwise comparisons: adults - low risk group
6.3.1.4. Evidence from pair wise comparisons: Children - high risk group
6.3.2. Network meta-analysis
A network meta-analysis (NMA) was performed for the treatments outlined in the matrix to help inform the recommendations.
Separate networks were formed for outcomes in each of the risk groups as follows.
In the high risk group, networks were developed for:
- Number of patients exposed to allogeneic transfusions
- Number of units transfused
- Length of stay in hospital
In the moderate risk group, networks were developed for:
- Number of patients exposed to allogeneic transfusions
- Number of units transfused
The baseline risk was defined as the risk of achieving the outcome of interest in the standard treatment group.
For details on the network meta-analysis, refer Appendix L
For results from network meta-analysis, refer to sections 6.3.2-6.3.7.
For rank-o-grams of the network meta-analysis, refer to section 6.3.7.
6.3.3. Adults: high risk group
Table 47 to Table 50 summarise the results of the conventional meta-analyses in terms of risk ratios generated from studies directly comparing different interventions, together with the results of the NMA in terms of risk ratios for every possible treatment comparison per outcome in the high risk group.
6.3.4. Adults: Moderate risk group
Table 51 to Table 53 summarise the results of the conventional meta-analyses in terms of risk ratios generated from studies directly comparing different interventions, together with the results of the NMA in terms of risk ratios for every possible treatment comparison per outcome in adults in the moderate risk group.
6.3.5. Adults: Low risk group
Table 54 summarises the results of the conventional meta-analyses in terms of risk ratios generated from studies directly comparing different interventions for tranexamic acid versus standard treatment per outcome in adults in the low risk group.
6.3.6. Children: High risk group
Table 55 summarises the results of the conventional meta-analyses in terms of risk ratios generated from studies directly comparing different interventions for tranexamic acid versus standard treatment per outcome in children in the high risk group.
6.3.7. Rank-o-grams from network meta-analysis
6.3.7.1. Adults: High risk
6.3.7.2. Adults: Moderate risk
6.4. Economic evidence
Published literature
Three economic evaluations were identified comparing cell salvage with no cell salvage and have been included in this review.78,169,266 Two economic evaluations were identified comparing TXA with placebo or no TXA and have been included in this review.9,251 These are summarised in the economic evidence profile below (Table 56 and Table 57) and the economic evidence tables in Appendix I.
Eight economic evaluations relating to cell salvage were identified but were excluded due to a combination of methodological limitations and the availability of more applicable evidence.26,37,70,89,200,201,204,254,270 Six economic evaluations relating to TXA were identified but were excluded due to the availability of more applicable evidence.115,116,141,186,240,316 These are summarised in Appendix Q, with reasons for exclusion given.
No economic evaluations were identified comparing cell salvage with TXA or relating to the combination of both.
See also the economic article selection flow diagram in Appendix F.
Economic evidence profile
6.4.1. New cost-effectiveness analysis
A key clinical issue identified by the GDG was which intervention to offer at the time of surgery to reduce the need for allogeneic blood transfusions: cell salvage, tranexamic acid (TXA) or both in combination. The GDG wanted to understand if one intervention was more effective than the other, if the combination of cell salvage and TXA was better than either intervention and if there were specific population groups in which one intervention or combination may be more effective.
Cell salvage is a procedure whereby blood loss during or after surgery is collected, processed and then transfused back to the patient with the aim of reducing the need of allogeneic blood transfusion. TXA is an antifibrinolytic pharmacological agent administered at the time of surgery with the aim of reducing bleeding and thus reducing the need for allogeneic blood transfusion. Reducing the use of allogeneic blood is of economic importance as it is a scarce and costly resource. In addition, transfusion of allogeneic blood is potentially associated with transfusion-related complications.
The clinical evidence suggested that cell salvage and TXA were both clinically effective compared to placebo. In addition, it suggested that in some patient groups cell salvage in combination with TXA is more effective at reducing the number of people transfused and volume transfused compared to TXA alone. As described above, economic evaluations identified in the systematic literature search indicated that cell salvage and TXA are likely to be cost-effective individually compared to standard treatment (no intervention or placebo). However, uncertainty remained regarding whether one may be more cost-effective than the other (head-to-head comparison) or whether they are more cost-effective when given in combination. As a result, this topic was identified by the GDG as the highest economic priority for original economic modelling.
Below is a summary of the analysis that was undertaken. For full details please see Appendix M Cost-effectiveness analysis: tranexamic acid and cell salvage.
6.4.1.1. Methods
A cost-utility analysis was undertaken to evaluate whether cell salvage (intra-operative and post-operative), TXA, a combination of both or standard treatment (no cell salvage or TXA) is the most cost-effective option for reducing allogeneic blood transfusion in adults undergoing surgery at moderate or high risk of bleeding. A decision tree-based model was used to estimate lifetime quality-adjusted life years (QALYs) and costs from a current UK NHS and personal social services perspective. The analysis was conducted in accordance with the NICE reference case unless otherwise stated including discounting at 3.5% for costs and QALYs.
Two population subgroups were analysed in the model: adults undergoing surgery at moderate risk of bleeding (0.5-1 litres) and high risk of bleeding (>1 litre). These subgroups were selected in line with the analysis of the clinical data (see Section 6.2 for further details). Studies that were categorised as high risk were predominantly RCTs on cardiovascular surgery and for the moderate risk they were orthopaedic surgery. Adults undergoing surgery at low risk of bleeding (<0.5 litres) were not included in the analysis as cell salvage would not be a feasible option due to insufficient blood loss. Children undergoing surgery were not included in this analysis as insufficient clinical evidence was identified for this population to allow for modelling.
The comparators for each population subgroup were selected based on the availability of evidence from the clinical review in discussion with the GDG. It was agreed that only interventions with data on both proportion transfused and volume transfused would be included in the model as the GDG felt that it was not possible to make assumptions for these critical outcomes.
Comparators for the high risk of bleeding subgroup were: standard treatment, TXA, intra-operative cell salvage (ICS), post-operative cell salvage (PCS), ICS+TXA. Comparators for the moderate risk of bleeding subgroup were: standard treatment, TXA, PCS, ICS+PCS.
Key inputs in the model were the proportion of people receiving an allogeneic transfusion and the volume of allogeneic blood transfused (in those that received a transfusion). Differences in proportions of patients transfused and volumes of blood transfused will translate to differences in costs between interventions. The clinical evidence also suggested a clinically and statistically significant decrease in 30-day mortality with TXA in the high risk group and therefore it was felt it was important to incorporate mortality into the model. The GDG also wished to incorporate differences between interventions in terms of adverse events as this may impact costs and QALYs. Adverse events could be intervention-related or transfusion-related. This impact was incorporated into the model in terms of differences in length of hospitalisation - this was then associated with a reduced quality of life and additional costs. Although the model did not explicitly model acute transfusion and treatment-related adverse events, the GDG judged length of stay to be a reasonable proxy for these acute events. This is because the ultimate impact of acute adverse event will be to prolong the patient's hospital stay while they are managed. For more information regarding the rationale behind this approach to modelling, please refer to the technical report in Appendix M.
A number of assumptions were made when developing the model. The key assumptions are outlined below but are also discussed in more detail in subsequent sections of this report and in Appendix M:
- People entering the model are eligible for each intervention listed for that subgroup.
- All allogeneic transfusions given in the model were red blood cell transfusions.
- The mortality rate after 30 days was the same for all people entering the model, irrespective of the intervention received or transfusion.
- TXA was administered intravenously.
- Cell salvage technicians were already trained and therefore the cost of training was not incorporated.
- Cell salvage equipment was available on lease via consumable charges.
- Post-operative cell salvage was unwashed.
- ICS and / or PCS were conducted for all people assigned to that intervention.
Model inputs were based on clinical evidence identified in the systematic review and network-meta analyses (NMA) undertaken for the guideline, supplemented by additional data sources as required. These are described in full in the technical report in Appendix M. All model inputs and assumptions were validated by the GDG.
The cost of each intervention took into account staff time (where additional to no intervention), drug costs, equipment and consumables. The cost of each intervention is summarised in Table 58 and is described in full in Appendix M.
The cost of allogeneic red blood cell transfusion used in the analysis was £192.17 for the first unit transfused, and £167.31 per subsequent unit transfused. A cost of £22.02 per person was applied to those who were not transfused in the model; this cost covers the cost blood grouping and antibody screening which is required for all surgical patients. Note that for those that are transfused this cost is incorporated into the cost of the first unit of red blood cells. The breakdown of resource use, costs and assumptions are described in full in Appendix M.
6.4.1.2. Results
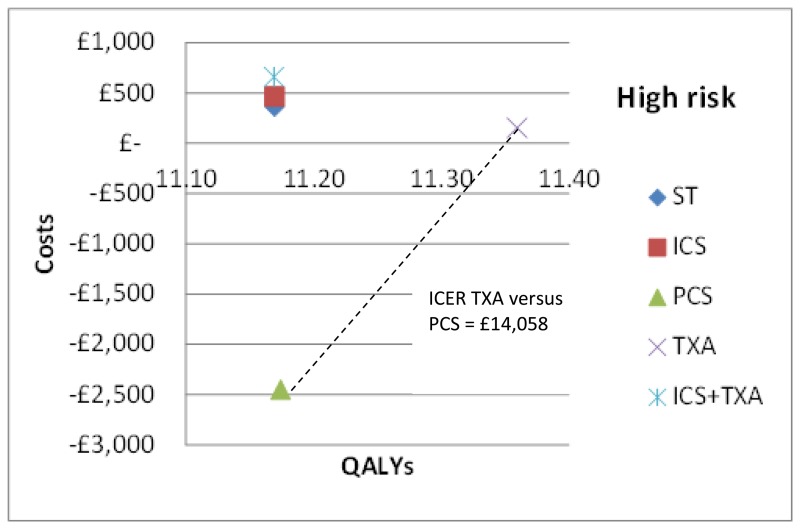
In the base case analysis for the high risk subgroup (treatment options: standard treatment, ICS, PCS, TXA and ICS+TXA), TXA was found to be the most cost-effective option. Results are summarised below in Table 59 in terms of costs, QALYs and cost-effectiveness (incremental net monetary benefit, probability costs effective and ranking). TXA was found to have the greatest benefits for patients (highest QALYs) largely due to a reduction in mortality at 30 days that was not seen with other treatment options. TXA had the second lowest cost after PCS; this was driven by a combination of the lowest intervention cost, moderate blood savings and a small saving due to a reduced length of stay. Of note, TXA was not the most blood saving intervention; it was the combination of ICS and TXA that resulted in the greatest blood savings.
The mean costs and QALYs from the probabilistic analysis have also been presented graphically on the cost-effectiveness plane in Figure 8. All interventions with the exception of PCS are dominated by TXA which has both lower costs and greater health benefits. PCS has lower costs than TXA but also lower QALYs. The incremental cost-effectiveness ratio of TXA versus PCS is £14,058 per QALY.
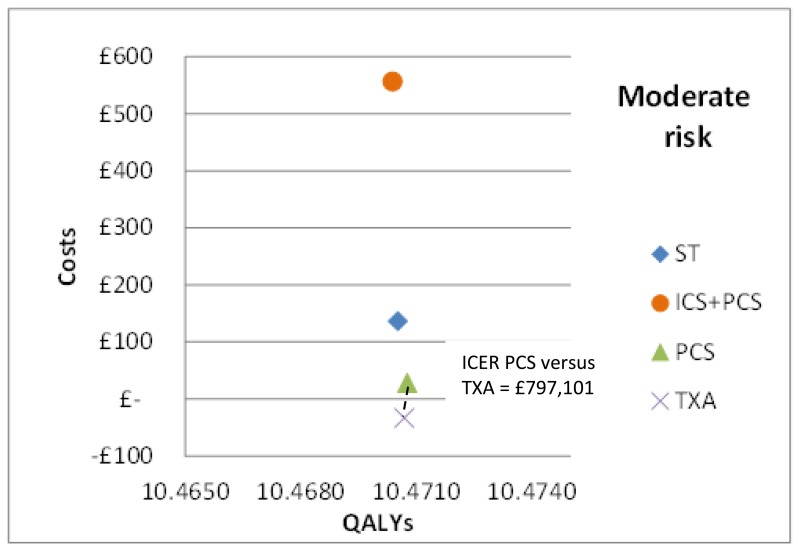
In the base case analysis for the moderate risk subgroup (treatment options: standard treatment, ICS+PCS, PCS and TXA), TXA was found to be the most cost-effective option. Results are summarised below in Table 60 in terms of costs, QALYs and cost-effectiveness (incremental net monetary benefit, probability costs effective and ranking). There was no difference in the incremental QALYs versus standard treatment between interventions to the 3rd decimal place. TXA had the lowest costs compared to all other interventions due to a combination of the lowest intervention cost, greatest savings associated with blood costs and length of stay.
The mean costs and QALYs from the probabilistic analysis have also been presented graphically on the cost-effectiveness plane in Figure 9. All interventions with the exception of PCS are dominated by TXA which has both lower costs and greater health benefits. PCS has higher costs than TXA but lower QALYs. The incremental cost-effectiveness ratio of PCS versus TXA is £797,101 per QALY.
A wide range of sensitivity analyses were undertaken, these included exploring uncertainty in the clinical effectiveness in terms of mortality, number transfused, volume transfused and length of stay; baseline transfusion and mortality rates; cost of interventions and blood transfusion and use of hospital length of stay as a proxy. For full details of the sensitivity analyses undertaken please see the technical report in Appendix M.
This conclusion was robust to all sensitivity analyses with the exception of three in the high risk group. The first was where the baseline mortality rate at 30 days was reduced to 0%. In this analysis, PCS became most cost-effective strategy. However, while this mortality rate was the lower end of the range observed in the RCTs included in the review, the GDG considered this scenario implausible for a high risk subgroup and likely due chance as a result of low event rates and so it did not impact decision making. A further two sensitivity analyses in the high risk group resulted in PCS becoming the most cost-effective option. These were analyses where the mortality after 30 days and the quality of life were adjusted to reflect MI and stroke populations. The results indicated that the QALY difference between TXA and PCS was reduced compared to difference observed in the base case. This impact on QALYs occurs because patients are less well (higher mortality rate and worse quality of life) and therefore they have less to gain from TXA's mortality benefit. When combined with the very low total costs of PCS (which are driven by the length of stay savings), PCS is the most cost-effective option. The GDG highlighted concerns with the length of stay data for PCS in the high risk group, that is that the length of stay estimate was informed by one study only and that this study had an unusually high baseline length of stay which likely accounted for the large difference in length of stay reported. To explore this further, these two sensitivity analyses were combined with a sensitivity analysis to account for the unusually large difference in length of stay for PCS. When these analyses were combined, TXA returned to being the most cost-effective option, thus indicating that the length of stay data for PCS is a key driver. The GDG considered that these sensitivity analyses highlighted some uncertainty in the base case; however, the further exploration mitigated the need for this to impact their decision making.
The GDG felt that, while TXA alone was found to be the most cost-effective option overall, for certain patients with particularly high blood loss, the addition of cell salvage to TXA may still be a cost-effective option on the basis that:
- The mechanisms of action are different for TXA and cell salvage and so it was considered that the relative benefit of cell salvage over TXA is likely to be greater with increased blood loss:
- TXA is an anti-fibrinolytic drug that is administered in advance and reduces the risk of blood loss, therefore reducing the need for allogeneic transfusions
- With cell salvage, lost blood is collected and re-transfused to the patient, thus also reducing the need for allogeneic transfusions
- The GDG considered that while TXA would help reduce allogeneic transfusion up to a point (due to reducing blood loss), the potential to collect blood lost and re-transfuse it with cell salvage is unlimited – the greater the volume of blood lost the greater the volume that can be salvaged
- Due to this, it was felt that at very high levels of blood loss the relative benefit of TXA in combination with cell salvage over TXA alone was likely to be greater.
- The mortality benefit seen with TXA alone was likely to also be achieved with ICS+TXA.
It was not possible to explore this within the context of RCT level clinical data. On this basis a series of exploratory threshold analyses were undertaken to quantitatively explore this scenario, for details of the methodology see the technical report in Appendix M. These analyses indicated that the combination of ICS and TXA could potentially become the cost-effective strategy in particular patients or patient groups where the probability of being transfused and the volume transfused is expected to be very high, if it was assumed that ICS+TXA had the same mortality benefit as TXA and that relative treatment benefits for ICS were maintained or increased. These analyses assumed that cell salvage is set up and used for all patients (as in the primary analyses).
6.4.1.3. Interpretation & limitations
This analysis suggests that TXA is the most cost-effective strategy for reducing allogeneic blood transfusion in adults undergoing surgery. Uncertainties in the analysis were explored through probabilistic sensitivity analyses of the base case for each subgroup and extensive sensitivity analyses which did not change conclusions with the exception of three sensitivity analyses in the high risk group. In the first sensitivity analysis, the baseline 30-day mortality was reduced to 0%. The GDG discussed this input and agreed that a 0% mortality rate in this risk group was not plausible and likely due to chance as a result of low event rates observed in the trials. The group therefore felt the results of this sensitivity analysis were not significant and did not change the overall conclusion. A further two sensitivity analyses, where the mortality after 30 days and the quality of life were adjusted to reflect MI and stroke populations, resulted in PCS becoming the most cost-effective option. This outcome was due to the smaller difference in QALYs between PCS and TXA and the very low total costs of PCS (as a result of length of stay savings). To explore this further, these two sensitivity analyses were combined with a sensitivity analysis to account for the unusually large difference in length of stay for PCS. This resulted in TXA returning to being the most cost effective option. The GDG considered that these sensitivity analyses highlighted some uncertainty in the base case; however, the further exploration mitigated the need to change the overall conclusion.
PCS was the most cost saving intervention in the high risk group; this was due primarily to the large reduction in hospital length of stay. As described above, when the mortality effect of TXA was removed, PCS had the highest QALYs which were attributable to the reduced length of stay. Furthermore, when the QALY difference between PCS and TXA was reduced, as seen with the MI and stroke sensitivity analyses, the length of stay savings were a key driver in establishing the most cost-effective option. The length of stay data for this comparator was based on one RCT with a high baseline length of stay. The GDG had concerns about the applicability of this evidence and therefore sensitivity analyses adjusting for this length of stay and excluding length of stay were undertaken. These resulted in TXA remaining the most cost-effective option.
The GDG highlighted that PCS may have use when blood is lost in chest drains in cardiac surgical patients, which is in a minority of cases. However, they acknowledged that in current practice it may not be considered an appropriate intervention for all high risk surgeries on its own, particularly in patients who have extensive bleeding post-operatively and therefore may require reoperation to stem the bleeding (rather than PCS). The GDG noted that this was unlike ICS, which could be used across all high risk surgeries.
Intra-operative cell salvage is used widely across the NHS in current practice, particularly in surgeries with high risk of bleeding. The GDG accepted that TXA alone was the most cost-effective option overall based on the available evidence, but considered that for certain patients with particularly high blood loss the addition of ICS to TXA may be a cost-effective option. This was on the basis that the mechanisms of action are different for TXA and cell salvage and so it was considered that the relative benefit of cell salvage over TXA in terms of avoiding allogeneic transfusions is likely to increase with greater blood loss. The evidence identified in the clinical review was not able to support or refute this because no data was available in such a population and it was not possible to explore this very high risk population within the context of RCT level clinical data. In addition, they felt that in reality the mortality benefit seen with TXA alone was likely to also be achieved with TXA+ICS and the reason that this has not been observed in the evidence could be attributed to a lack of data. A series of exploratory threshold analyses were therefore undertaken within the cost-effectiveness analysis to help the GDG explore whether conclusions might change under these assumptions. These exploratory threshold analyses indicated that under certain circumstances, like those described above, it is plausible that the combination of ICS and TXA may become a cost-effective option. However, it is highlighted that these scenarios are theoretical and not based on evidence.
As in the base case analysis, these exploratory threshold analyses assumed that patients bleeding risk is assessed in advance and if they are considered to be very high risk then ICS is set up and used for all patients, that is the cost is incurred for all patients. This implies that the patients or patient group this analysis applies to is identifiable in advance. However, the GDG acknowledged the difficulty of predicting a patient's bleeding risk. They noted that for some cases, it may be possible to predict risk prior to surgery based on type of surgery and patients' characteristics, thus allowing ICS to be set-up in advance. In other cases, troublesome bleeding may occur during surgery, for example when there is trauma to a vessel, and the equipment would need to be set up during surgery. The costs may be cheaper than those reported in this analysis if ICS is only set up for those who need it during surgery; however, some of the benefit of ICS may be lost due to delays in setting up equipment. Furthermore, in hospitals where the number of surgical patients eligible for ICS is expected to be low, hiring cell salvage equipment may not be feasible due to the requirement from manufacturers of having a minimum disposable order. For these hospitals, purchasing the equipment may be the only solution and this may make the intervention no longer a cost-effective option.
The objective of this analysis was to identify the intervention that provided the greatest health benefit (quantified in terms of QALYs) at an acceptable cost to the NHS (that is with an acceptable incremental cost-effectiveness ratio as per NICE methodological guidance). The GDG highlighted that another objective for these interventions is to conserve allogeneic blood, as it is a scarce resource. Although this was not the objective set out in our analysis, if this objective were to be considered, the combination of ICS and TXA would be the favoured intervention for the high risk group in terms of effectiveness, but cost-effectiveness would be unclear as there is no threshold for this. The group did highlight that there is currently no shortage of allogeneic blood in the UK and so were satisfied that using the cost per QALYs analysis was appropriate for decision making for the guideline. As well as conserving allogeneic blood, another objective may be to limit exposure to allogeneic blood to account for unquantifiable unknown risks.
Another benefit of avoiding allogeneic transfusion, which was not incorporated into the model, is that it eases cross-matching if these individuals need transfusions in the future, as they will not have antibodies.
This new economic analysis was assessed as directly applicable with minor limitations.
Mortality differences
The results of the high risk subgroup analysis are dependent on the mortality benefit obtained with TXA and not with other treatments. The GDG discussed why the mortality benefits might be seen with TXA and no other treatment options, especially those with similar or greater blood savings. While they felt it was not possible to establish this, they noted the different mechanisms of actions of TXA versus cell salvage options and they were satisfied that the clinical evidence for TXA was robust. They did also consider it plausible that this benefit would be seen with combination treatments of cell salvage with TXA and that it may be a lack of data that accounts for the lack of effect seen in the evidence review. This was explored in a series of sensitivity analyses and even when ICS+TXA was attributed the same mortality benefit as TXA alone, TXA remained the most cost-effective option due to the high cost of ICS relative to the additional blood savings.
The data from the clinical review for the other comparators demonstrated a great deal of uncertainty around the estimates. As a result, the GDG decided not to use the clinical review data in the base case for these comparators, and instead assumed there was no mortality difference compared to standard treatment. A sensitivity analysis was conducted where the clinical review data was used and it found that TXA remained the most cost-effective option.
Cost of cell salvage
The GDG noted that the cost of ICS disposables in the analysis was likely to be higher than prices available to hospitals through negotiations with suppliers. These lower costs could not be included as they are not publicly available. The cost of the disposables was explored in a sensitivity analysis; this demonstrated that the conclusion was not sensitive to changes in this input. The GDG considered the results of this sensitivity analysis to be important as it indicates that even if the cost of the ICS disposables was lower, TXA would remain the dominant strategy. The GDG noted that this sensitivity analysis along with the exploratory threshold analyses imply that ICS (alone or in combination with TXA) should not be used for all high risk surgeries but rather it should be reserved for those cases with high baseline risk of transfusion and high expected volume of blood loss.
Length of stay data as a proxy for the impact of acute adverse events
A limitation of this analysis is the use of length of stay as a proxy for the impact of acute transfusion-and treatment-related adverse events. Alternatives were considered during development such as explicitly modelling these events; however it was felt that this would be overly complicated and there was a lack of data to inform this approach. The GDG concluded that in principle length of stay was a reasonable proxy for the impact of these acute events. The GDG noted the general issue of length of stay data being impacted by setting (e.g. country) and in particular that there was an unusually large difference in length of stay for PCS in the high risk group that might be accounted for due to the unusually high baseline length of stay in that study. The GDG considered omitting length of stay from the base case analysis but felt that attempting to capture the impact on patients outweighed this concern. Furthermore they felt it was preferable to maintain the link with the clinical data review in the base case analysis. It was agreed that this issue required exploration in sensitivity analyses and taking into consideration when interpreting results.
A further limitation of this approach was that it used utility values from a different patient population which was not surgical patients receiving or not receiving transfusions. However, more relevant data was not identified.
To address these limitations, as part of the sensitivity analyses, length of stay was excluded, and therefore differences in quality of life and related costs. Removing length of stay did not change the conclusions.
ICS in moderate risk group
The GDG noted that ICS is still being used for orthopaedic surgeries (first time knee or hip replacements) which are considered to be at moderate risk of bleeding. There was limited evidence for the use of ICS in these types of surgery, half of which was from prior to 2003 and therefore was not incorporated in the analysis. As highlighted in Section 6.2.3, the GDG agreed that substantial changes in transfusion practice over time with respect to the use of cell salvage meant that studies published prior to 2003 were not relevant to current clinical practice. Studies published before 2003 therefore should not inform the decision making process or the economic model. Although the use of ICS in moderate risk surgery was not assessed in our economic analysis, the GDG highlighted that as blood loss has decreased now in these surgery types, ICS may not be a cost-effective strategy.
Adverse events
A further limitation is the exclusion of long term transfusion-related adverse events. Between 2010 and 2013, SHOT reported two transfusion-transmitted infections with hepatitis B, two of hepatitis E and one of Parvovirus B19 in the UK.27 The GDG acknowledged the importance of these infections in considerations of transfusion safety, but observed that they were extremely rare and were unlikely to impact on the results of the economic model. Had these infections been incorporated into the analysis, they would have favoured the interventions that reduced the exposure to allogeneic blood. For the moderate risk group, this would have further supported the use of TXA, which was the most blood saving intervention. In the high risk group, this would have increased the benefit of ICS+TXA. However, it is considered unlikely to change the conclusions.
The main adverse event for TXA was considered to be thrombotic complications. The clinical evidence review suggested there was a non-significant reduction of risk of thrombotic complications for TXA compared to placebo; therefore the GDG decided that it was unnecessary to include this outcome in the model. If it had been modelled explicitly, the results would have been even more favourable towards TXA as the thrombotic events were lower in those receiving TXA compared to placebo.
6.5. Evidence statements
Clinical
Adults - High risk group
- A network meta-analysis of 56 studies comparing seven treatments suggested that PCS is ranked as the best treatment, ICS+TXA is ranked second, TXA, ICS+PCS+TXA and ICS+PCS are jointly ranked fourth and standard treatment ranked least effective at reducing the number of adult patients receiving allogeneic transfusions in the high risk group; there was, however, considerable uncertainty. Based on the pair-wise meta-analysis, efficacy as assessed by number of patients receiving allogeneic transfusions favours tranexamic acid, post-operative cell salvage, intra-operative cell salvage and the combination of intra-operative and post-operative cell salvage over standard treatment and the combination of intra-operative cell salvage and tranexamic acid over intra-operative cell salvage.
- A network meta-analysis of 23 studies comparing five treatments suggested that ICS+TXA is ranked as the best treatment, PCS is ranked second, TXA and ICS are jointly ranked third, and standard treatment ranked least effective at reducing the number of units of allogeneic blood transfusions in adult patients in the high risk group, but there was considerable uncertainty. Based on the pair-wise meta-analysis, efficacy as assessed by reduced number of units of allogeneic transfusions received favours intra-operative cell salvage, post-operative cell salvage, tranexamic acid over standard treatment, and the combination of intra-operative cell salvage and tranexamic acid over intra-operative cell salvage.
- A network meta-analysis of 10 studies comparing six treatments suggested that PCS is ranked as the best treatment, ICs and TXA are jointly ranked third, standard treatment is ranked fourth, ICS+TXA is ranked fifth and ICS+PCS is ranked least effective at reducing length of stay in hospital in adult patients in the high risk group; there was, however, considerable uncertainty. Based on the pair-wise meta-analysis, efficacy as assessed by reduced length of stay in hospital favours post-operative cell salvage over standard treatment.
- Based on the pairwise meta-analysis, efficacy as assessed by the reduction in mortality favours tranexamic acid over standard treatment. The evidence also suggests that tranexamic acid may be better with respect to infections and thrombotic complications than standard treatment, but there is some uncertainty.
Adults - Moderate risk group
- A network meta-analysis of 73 studies comparing eight treatments suggested that PCS+TXA is ranked as the best treatment, ICS +TXA is ranked second, TXA is ranked fourth, ICS+TXA, ICS+PCS and PCS are jointly ranked fifth, ICS is ranked sixth and standard treatment is ranked least effective at reducing the number of adult patients receiving allogeneic transfusions in the moderate risk group; there was, however, considerable uncertainty. Based on the pair-wise meta-analysis, efficacy as assessed by number of patients receiving allogeneic transfusions favours the use of post-operative cell salvage or tranexamic acid over standard treatment. PCS+TXA was also found to be better than PCS alone, but there was some uncertainty.
- A network meta-analysis of 16 studies comparing four treatments suggested TXA and PCS are jointly ranked as the best treatment, standard treatment is ranked third and ICS+PCS is ranked least effective at reducing the number of units of allogeneic blood transfusions in adult patients in the moderate risk group, but there was some uncertainty. Based on the pair-wise meta-analysis, efficacy as assessed by reduced number of units of allogeneic transfusions received suggests that the ICS+TXA may be better than use of ICS alone, but there is some uncertainty.
- Based on the pairwise meta-analysis, efficacy as assessed by the reduction in mortality, infections and thrombotic complications favours tranexamic acid over standard treatment but there was considerable uncertainty.
Adults- Low risk group
- Based on the pairwise meta-analysis, efficacy as assessed by the number of patients receiving allogeneic transfusions favours tranexamic acid, but there is some uncertainty.
Children-High risk group
- Based on the pairwise meta-analysis, efficacy as assessed by the number of children receiving allogeneic transfusions and the total blood transfused favours ICS+TXA over TXA alone, but there is some uncertainty. The evidence favours tranexamic acid over standard treatment for efficacy as assessed by post-operative blood loss. Efficacy as assessed by length of stay in hospital favours standard treatment over tranexamic acid, but there is some uncertainty.
Economic
- One cost-utility analysis found that intra-operative and peri-operative cell salvage were dominant (less costly and more effective) compared with no cell salvage (allogeneic blood transfusion only) in reducing blood transfusion requirements for adults undergoing elective non-urgent major surgery. This analysis was assessed as partially applicable with minor limitations.
- One cost-consequence analysis found that cell salvage (intra-operative and post-operative) was more costly and more effective than no cell salvage (allogeneic blood transfusion only) (£477 more per patient, 0.42, 0.725 and 1.63 fewer units of allogeneic red blood cells, fresh frozen plasma and platelets transfused per patient, respectively) in reducing blood transfusion requirements for adults undergoing non-emergency cardiac surgery. This analysis was assessed as partially applicable with potentially serious limitations.
- One cost-consequence analysis found that cell salvage (intra-operative) was dominant (less costly and more effective) compared with no cell salvage in reducing blood transfusion requirements for paediatric orthopaedic or cardiac surgery patients. This analysis was assessed as partially applicable with potentially serious limitations.
- Two cost-consequence analyses found that tranexamic acid was dominant (less costly and more effective) compared with placebo or no tranexamic acid for reducing blood transfusion requirements in adult surgical patients undergoing total hip replacement. These analyses were assessed as partially applicable and with potential serious limitations.
- No relevant economic evaluations were identified that included tranexamic acid or post-operative cell salvage as a comparator in reducing blood transfusion requirements for paediatric surgical patients.
- An original cost-utility analysis found that in surgical patients at high risk of bleeding, tranexamic acid was the most cost-effective option when compared with standard treatment, intra-operative cell salvage, post-operative cell salvage and the combination of tranexamic acid and intra-operative cell salvage. It was dominant (less costly and more effective) compared to all options except post-operative cell salvage. It was cost-effective compared to post-operative cell salvage (ICER: £14,058 per QALY gained). This analysis was assessed as directly applicable with minor limitations.
- An original cost-utility analysis found that in surgical patients at moderate risk of bleeding, tranexamic acid was the most cost-effective option when compared to standard treatment, post-operative cell salvage and the combination of intra-operative cell salvage and post-operative cell salvage. It was dominant (less costly and more effective) compared to all options except post-operative cell salvage. It was cost-effective compared to post-operative cell salvage (ICER: £797,101 per QALY gained). This analysis was assessed as directly applicable with minor limitations.
6.6. Recommendations and link to evidence
| Recommendations |
|
|---|---|
| Relative values of different outcomes | The GDG agreed that the number of patients transfused, number of units transfused and mortality were critical outcomes for decision making. Other outcomes which were considered to be important in the decision making process were length of stay in hospital, quality of life and adverse events (infection, thrombotic complications). |
| Trade off between clinical benefits and harms | The evidence from studies in adults showed that in surgeries which were expected to lead to moderate or high blood loss (500 ml-1 litre and >1 litre respectively), tranexamic acid (TXA) was the most clinically and cost-effective option. This was based on clinical evidence from the network meta-analysis, results from the pair wise meta-analysis and the results of the health economic model. The inclusion criteria of studies in these analyses are explained in the methodology section of this chapter (see 6.2.3 for more information). Note that cost effectiveness is discussed in the section following this one. In adults undergoing surgery, where blood loss was expected to be high, the evidence showed that TXA demonstrated clinical benefit with respect to all critical outcomes (number of patients transfused, number of units transfused and mortality). However, the network meta-analysis showed that a combination of intra-operative cell salvage (ICS) and TXA was the most clinically effective option with respect to reducing the number of patients transfused and number of units of allogeneic blood transfused. Therefore, the GDG noted that the addition of ICS to TXA reduces overall allogeneic blood requirements where blood loss is expected to be high. Furthermore, results from the pair wise meta-analysis comparing TXA with standard treatment showed a significant decrease in mortality and no significant differences were observed for any other intervention with respect to 30 day mortality. The GDG noted that it was unclear why the mortality benefit seen with TXA alone was not seen with the combination of TXA and cell salvage but it seemed possibly that it was to be due to the lack of data rather than a difference in effect. All interventions with the exception of the combination of ICS+TXA and ICS + post-operative cell salvage (PCS) showed a decrease in length of stay compared with standard treatment. The incidence of infections and thrombotic complications were less with TXA when compared to standard treatment. The pair wise meta-analysis also suggested that there was no difference between the combination of ICS and TXA and TXA alone with respect to incidence of infections. TXA had the best evidence of mortality benefit, not seen elsewhere, and good evidence of effectiveness in terms of number transfused and volume transfused over placebo, although it was not the most blood saving compared to all other interventions. The economic model which translates different outcomes into a single health metric, QALYs, found that TXA has the greatest QALYs largely due to the mortality benefit. For adults undergoing surgery, where blood loss was expected to be moderate (500 ml-1 litre), the evidence showed that TXA was the most clinically effective option. The network meta-analysis demonstrated the benefit of using TXA in comparison with all other interventions with respect to number of patients transfused. Results from the pair-wise meta-analysis suggested that TXA was more effective than standard treatment in reducing the number of units of allogeneic blood transfused, length of stay in hospital and thrombotic complications. There was no difference in the incidence of infections between TXA and standard treatment. There was also evidence of benefit for mortality for TXA versus standard treatment; however there was high imprecision and therefore the GDG were uncertain of the effect on mortality in this risk group. Overall QALYs were very similar between interventions in this risk group as mortality was not incorporated in the economic model due to uncertainty. In children, the evidence suggested that the combination of ICS with TXA may result in fewer patients transfused and a lesser volume of total blood transfused in comparison with the use of ICS alone. This evidence, however, was of very low quality from a single study in scoliosis surgery. For the comparison of TXA with standard treatment, the evidence suggested that TXA may result in reduction of post-operative blood loss. This evidence was of moderate quality. All evidence was from studies in children who were expected to have high blood loss. No evidence was identified for children who were expected to have moderate blood loss. For this group, the GDG extrapolated the evidence from adults to inform the recommendation for children. Based on the above clinical considerations and economic considerations described below, the GDG recommended the use of TXA for both adults and children undergoing surgery where blood loss is expected to be moderate or high. However, it was acknowledged that the evidence was more limited for children, including uncertainty as to the correct dose of TXA for children, and the paediatric recommendation has been extrapolated from evidence in adults. As such, the recommendation is “consider” rather than “offer” in children to enable a more individualised approach depending on the clinical situation and patient subgroup. |
| Economic considerations | No economic evaluations were identified comparing all relevant interventions, that is TXA or cell salvage, alone or in combination. Two economic evaluations were identified comparing TXA with placebo or no TXA in total hip replacement adult surgery patients and found that TXA was dominant, that is it reduced costs and improved health outcomes.9,251 These studies were assessed as partially applicable with potentially serious limitations. No economic evaluations were identified for TXA in paediatric surgical patients. Health economic considerations for cell salvage and combinations of cell salvage with TXA are discussed in subsequent LETRs. The question of whether TXA, cell salvage or a combination of both should be used in surgical patients was prioritised for original economic modelling by the GDG. Two population subgroups were analysed in the model, adults undergoing surgery at moderate risk of bleeding (0.5-1 litres) and high risk of bleeding (>1 litre) in line with how the clinical effectiveness evidence was analysed. The comparators for each population subgroup were selected based on the availability of evidence from the clinical review in discussion with the GDG. Model inputs were based on clinical evidence identified in the systematic review and network-meta analyses undertaken for the guideline, supplemented by additional data sources as required. Total costs took into account intervention costs (staff time [where additional to no intervention], drug costs, equipment and consumables) and downstream costs including blood costs and short-term treatment- and transfusion-related adverse events (through the use of hospital length of stay as a proxy). The new economic model found that in both the moderate and high risk subgroups, TXA was the most cost-effective option for reducing allogeneic blood transfusion in adults undergoing surgery. In the high risk group, TXA was found to have the greatest benefits for patients (highest QALYs) largely due to a reduction in mortality at 30 days that was not seen with other treatment options. TXA had the second lowest cost after PCS; this was driven by a combination of the lowest intervention cost, moderate blood savings and a small saving due to a reduced length of stay. Of note, TXA was not the most blood saving intervention; it was the combination of ICS and TXA that resulted in the greatest blood savings. In the moderate risk group, there was no difference in the incremental QALYs versus standard treatment between interventions to the 3rd decimal place. TXA had the lowest costs compared to all other interventions due to a combination of the lowest intervention cost, greatest savings associated with blood costs and length of stay. The conclusion that TXA alone was the most cost-effective option was robust to a wide range of sensitivity analyses including exploring uncertainty in the clinical effectiveness in terms of mortality, number transfused, volume transfused and length of stay; baseline transfusion and mortality rates; cost of interventions and blood transfusion and use of length of stay in the model. In the high risk group, two sensitivity analyses were undertaken where the mortality after 30 days and the quality of life were adjusted to reflect MI and stroke populations, resulted in PCS becoming the most cost-effective option. This outcome was driven primarily by the very low total costs of PCS (as a result of length of stay savings). To explore this further, these two sensitivity analyses were combined with a sensitivity analysis to account for the unusually large difference in length of stay for PCS. This resulted in TXA returning to being the most cost effective option. Based on these additional analyses, the GDG did not feel the need to change the overall conclusion. This new economic analysis was assessed as directly applicable with minor limitations. Paediatric surgical patients were not included in this analysis as insufficient clinical evidence was identified for this population to allow for modelling. Based on this limited clinical evidence in children, the low intervention cost of TXA and the cost-effectiveness evidence in adults, the GDG judged it highly likely that TXA would be a cost-effective option in paediatric surgical patients. |
| Quality of evidence | The evidence from pairwise meta-analysis on TXA ranged from moderate to low quality for the critical and important outcomes. The recommendation is based on the evidence of clinical and cost-effectiveness. |
| Other considerations | The GDG acknowledged the breadth of the evidence base and the conclusive nature of the evidence in relation to the use of TXA in reducing allogeneic blood transfusion requirements in adult surgical patients. The GDG had confidence in making the recommendation based on the vast evidence base for adult surgeries and felt that it was unlikely that similar future research trials would change the direction of effect seen. The GDG was aware of reports of seizures related to high doses of TXA, but no evidence of excess reports from RCTs was found to support this. The GDG noted variation in thromboprophylaxis in the trials and made the recommendation on the assumption that hospitals will be following the recommendations on adult VTE prophylaxis in NICE guidance CG 92. The GDG noted the risk of thrombosis is lower in most paediatric groups and that there are no equivalent paediatric VTE prophylaxis guidelines so local policy should be followed. The GDG members, including the lay representatives, felt it should be standard practice to offer TXA for adults and that information about TXA should be included in patient information leaflets on blood transfusion and alternatives to blood transfusion. There was variation in the doses of TXA administered in the trials. The loading dose of TXA used in the trials involving adult patients undergoing cardiac surgery ranged from 2.5 mg/kg to 100 mg/kg. The maintenance dose of TXA for the cardiac surgery trials, ranged from 0.25 mg/kg/hour to 4.0 mg/kg/hour delivered over 1 to 12 hours. When oral TXA was used, again, there was variation in the dose and prescription regimens. IV TXA was administered peri-operatively, with the first dose being administered pre-operatively in the majority of the trials evaluating its effectiveness. The prescriber should follow dosing as recommended in the summary of product characteristics. The GDG noted that the timing of first administration of TXA was important for the TXA to be effective in reducing blood transfusion requirements in surgical patients. Of note, in the economic model, for costing purposes, the following dose listed in the BNF was used for the high risk subgroup: slow IV injection (general fibrinolysis) 1 g every 6-8 hours followed by continuous IV infusion 25-50 mg/kg over 24 hours. For the moderate risk group the following dose listed in the BNF was used: slow IV injection (general fibrinolysis) 1 g every 6-8 hours. It was noted that there is wide variation in clinical practice in the dosage of TXA used in children. This was reflected in the variation in the dose of TXA used in the limited number of trials in children and, therefore, there is uncertainty regarding the optimal dose of TXA in children. Prior to analysis of studies, the GDG considered risk and amount of bleeding and defined low, moderate and high expected blood loss in adults as <500ml, 500ml-1 litre and >1 litre, respectively. This enabled recommendations to be based upon expected blood loss. This risk stratification was applicable to adults only. The GDG noted that these expected volumes of blood loss do not apply to the risk stratification for children; the GDG judged the equivalent of moderate blood loss in children to be 10% of blood volume. The GDG specifically did not list individual types of surgery for the recommendation and instead used expected blood loss as this enables type of surgery, individual bleeding risk and local practise to be taken into consideration when implementing recommendations. Evidence with respect to TXA, in surgical patients at low risk of bleeding (< 500 ml), was inconclusive and of very low quality. The GDG therefore did not make a recommendation for this risk group. |
| Recommendations |
|
|---|---|
| Relative values of different outcomes | The GDG agreed that the number of patients transfused, number of units transfused and mortality were critical outcomes for decision making. Other outcomes which were considered to be important in the decision making process were length of stay in hospital, quality of life and adverse events (infection, thrombotic complications). |
| Trade off between clinical benefits and harms | There was evidence of effectiveness for some of the critical outcomes for the use of ICS alone in the high and moderate risk groups in adult surgical patients. Although there was some evidence of clinical effectiveness of ICS and PCS alone and in combination with one another in comparison with standard treatment from the pairwise meta-analysis with respect to number of patients transfused and number of units of allogeneic blood transfused, TXA was more effective than cell salvage in the moderate risk group and the combination of ICS and TXA was more effective than cell salvage in the high risk group when reviewed in the network meta-analysis. Although the results of the network meta-analyses showed that PCS was ranked as the best treatment for number of patients transfused and length of stay, and ranked above TXA for number of units transfused in the high risk group, the GDG highlighted that PCS may have limited use, for example, when blood is lost in chest drains in cardiac surgical patients, which is in a minority of cases. They acknowledged that in current practice it may not be considered an appropriate intervention for all high risk surgeries on its own, particularly in patients who have extensive bleeding post-operatively and therefore may require reoperation to stem the bleeding (rather than PCS). The GDG noted that this was unlike ICS, which could be used across all high risk surgeries. The GDG noted that further research was therefore required in this specific group to establish the effectiveness of PCS in high risk surgeries and made a recommendation for further research in this area (see section 6.6.1). No evidence was identified for the comparison of ICS or PCS alone with standard treatment in children. There was very low quality evidence which suggested that the combination of ICS with TXA may result in fewer patients transfused and a lesser volume of total blood transfused in comparison with the use of ICS alone. All evidence was from studies in children who were at high risk of blood loss. No evidence was identified for children who were at moderate or low risk of blood loss. Based on this, and the new economic model, the GDG decided to not recommend the routine use of cell salvage alone in both adults and children. The combination of cell salvage with TXA was more likely to be cost-effective than cell salvage alone and was recommended by the GDG (see recommendation 9 below). However, it is acknowledged there are paediatric patient groups, such as some paediatric cardiac surgery patients, in whom cell salvage may be used and TXA may be considered inappropriate (please see ‘Other considerations’). |
| Economic considerations | No economic evaluations were identified comparing all relevant interventions, that is TXA or cell salvage, alone or in combination. Two economic evaluations comparing ICS+PCS with no cell savage in cardiac and or orthopaedic surgical adult patients were identified. The first was a cost-utility analysis by Davies 200678 which found that cell salvage (ICS or PCS) was dominant compared to no cell salvage. This analysis was assessed as partially applicable with minor limitations. The second by Klein 2008169 was a cost-consequence analysis based on a single RCT which found that ICS+PCS was more costly and more effective at reducing the number of units transfused than no cell salvage. This analysis was assessed as partially applicable with potentially serious limitations. Finally a cost-utility analysis by Samnaliev 2013266 comparing ICS with no cell salvage in orthopaedic and cardiac surgical paediatric patient found that ICS was dominant compared to no cell salvage. This analysis was assessed as partially applicable with potentially serious limitations. Note, the effectiveness data used in the analysis was from a non-randomised trial and therefore not reported in the clinical evidence. No economic evaluations were identified for the use of PCS alone in paediatric surgical patients. The evidence from the new economic model conducted indicated that PCS alone in the high and moderate risk of bleeding subgroups was not a cost-effective option in adult surgical patients. PCS was the most cost saving intervention in the high risk group; this was due primarily to the large reduction in hospital length of stay. When the mortality effect of TXA was removed, PCS had the highest QALYs which were attributable to the reduced length of stay. Furthermore, when the QALY difference between PCS and TXA was reduced, as seen insensitivity analyses where mortality and quality of life were adjusted to reflect MI and stroke populations, the length of stay savings were a key driver in establishing the most cost-effective option. The length of stay data for this comparator was based on one RCT with a high baseline length of stay. The GDG had concerns about the applicability of this evidence and therefore sensitivity analyses adjusting for this length of stay and excluding length of stay were undertaken. These resulted in TXA remaining the most cost-effective option. In the high risk group, ICS alone was also not a cost-effective intervention for reducing allogeneic transfusions in adult surgical patients. In the moderate risk group, there were no data identified in the clinical review for ICS in relation to the volume of allogeneic blood transfused. As a result, we were unable to include ICS alone in the analysis without making assumptions for this outcome. We were unable to establish from our analysis whether or not using ICS alone in this group would be a cost-effective intervention. The GDG felt that the new economic analysis conducted for this guideline superseded the published studies which were based on older clinical evidence78 and in the case of two analyses169, 266 on single trials and do not include all relevant treatment options. Furthermore for the two studies that found that cell salvage was dominant to usual care, the cost of cell salvage used in their analyses was less than the cost used in our analysis78,266 and were not considered by the GDG to be reflective of the current NHS context. The GDG extrapolated the findings of the new economic model in adults to children. However, as noted above in the trade-off between clinical benefits and harms, special consideration should be given for some paediatric patient groups (please see other considerations). |
| Quality of evidence | This recommendation was based on low to very low quality evidence from the pair wise meta-analysis and the network meta-analysis and the results of our economic model. |
| Other considerations | The GDG discussed the advantages of using cell salvage. One particular advantage highlighted was that, with the use of cell salvage, allogeneic blood transfusion can be avoided, minimising all complications of transfusion including red cell alloimmunisation, that is, developing red cell antibodies causing difficulties in identifying compatible blood for future transfusions if needed. Although the GDG recommended against routinely offering cell salvage alone in adults and children, it was noted that special consideration should be given for its use in paediatric cardiac surgery patients. The GDG acknowledged that cell salvage is widely used during paediatric cardiac surgery to reduce exposure to allogeneic blood whereas TXA may not always be used in the same clinical situations due to uncertainty about the optimal dose and possible side effects. |
| Recommendations |
|
|---|---|
| Relative values of different outcomes | The GDG agreed that the number of patients transfused, number of units transfused and mortality were critical outcomes for decision making. Other outcomes which were considered to be important in the decision making process were length of stay in hospital, quality of life and adverse events (infection, thrombotic complications). |
| Trade off between clinical benefits and harms | In the high risk group, the evidence from the network meta-analysis in adults showed that the combination of ICS and TXA was the most clinically effective in reducing the number of patients transfused as well as the number of units of allogeneic blood transfused. No evidence of mortality benefit observed for the combinations of ICS and TXA in the high risk group. The GDG noted that it is unclear why the mortality benefit seen with TXA alone was not seen with the combination of ICS and TXA but it seemed possibly that it was to be due to a lack of data rather than a difference in effect. Although the evidence showed that the combination of ICS and TXA was not cost-effective in patients who were expected to have blood loss greater than 1 litre and so was not recommended, the GDG noted that there may be a sub-group of patients within this group who were at very high risk of blood loss and may require more blood transfusions (for example in cardiac and complex vascular surgery, major obstetric procedures, and pelvic reconstruction and scoliosis surgery). The GDG anticipated that due to the higher volume of blood loss in these patients, the decision to use the combination of ICS with TXA may be cost-effective, as the total costs (cost of blood saved and cost of interventions) would be less than that of TXA. The GDG discussed that when the rate of blood loss is very high, TXA may be effective only up to a point in reducing blood loss. The GDG felt that in such cases, as cell salvage utilises shed blood as opposed to TXA which is an anti-fibrinolytic, it may be more effective in reducing the need of allogeneic blood transfusion. Although there was a lack of evidence to support this, the GDG felt this was a highly plausible scenario. In addition, the GDG felt that the mortality benefit of TXA may be maintained when it is administered in combination with ICS. The combination of these two assumptions would result in similar QALYs for both interventions. In children, there was very limited and low quality evidence which suggested that the combination of ICS with TXA may result in fewer patients transfused and a lesser volume of total blood transfused in comparison with the use of ICS alone. The GDG therefore felt that the combination of ICS and TXA should be an option for situations where people are at risk of very high blood loss. |
| Economic considerations | No economic evaluations were identified including the combination of ICS+TXA. As discussed above, the GDG felt that, while TXA alone was found to be the most cost-effective option overall in the original economic evaluation, for certain patients with particularly high blood loss the addition ICS to TXA may still be a cost-effective option. This was discussed on the basis that the mechanisms of action are different for TXA and cell salvage and so it was considered that the relative benefit of ICS over TXA is likely to increase with increased blood loss and that the mortality benefit seen with TXA alone was likely to also be achieved with TXA+ICS. Of note, changing this latter assumption alone did not change the conclusions on the analysis. Although the results of the base case analysis do not provide support for this recommendation, exploratory threshold analyses indicated that the combination of ICS and TXA could potentially become the cost-effective strategy in particular patients or patient groups where the probability of being transfused and the volume transfused is expected to be very high, if it was assumed that ICS+TXA had the same mortality benefit as TXA and that relative treatment benefits for ICS were maintained or increased. These analyses assumed that patients bleeding risk is assessed in advance and if they are considered to be very high risk then ICS is set up and used for all patients, that is the cost is incurred for all patients. Based on the limited clinical evidence in children and the economic analysis conducted in adults, the GDG agreed to extrapolate their conclusions about cost-effectiveness in adults to children as well. |
| Quality of evidence | The quality of evidence for the combinations of ICS+TXAin the high risk group from the network meta-analysis was of very low quality for all outcomes in both adults and children. It was not possible to explore the effectiveness of ICS+TXA in the very high risk subgroup within the context of RCT level clinical data (for details see other considerations). This recommendation was based on results from the network meta-analysis, economic modelling and further threshold analysis as well as the consensus expert opinion of the GDG members. |
| Other considerations | The GDG discussed the challenges in identifying the specific sub-group of patients in whom the combination of ICS and TXA would be clinically and cost-effective and noted that some of these patients could be identified based on the complex nature of some surgeries (for example, scoliosis surgery or reconstructive hip and knee surgeries). The GDG noted that there was a lack of clinical evidence on these specific types of surgeries with respect to the combination of ICS and TXA. The GDG also noted that it was difficult to identify this particular group of patients from RCT data as many of the patients who were classified as high risk could well require greater amounts of blood transfusion based on their individual patient characteristics, such as baseline haemoglobin levels, pre-operative anaemia management and thresholds for blood transfusion according to local protocols. We explored these factors by sub-group analyses. The analysis was limited by the non-availability of patient level data. Consequently, it was acknowledged that these factors, alone or in combination with one another, may give rise to a group of patients whose blood transfusion needs may be significantly greater than other patients in the high risk group. The GDG anticipated that the combination of ICS and TXA may prove to be clinically as well as cost-effective in this group. The GDG discussed the advantages of using cell salvage. One particular advantage highlighted was that, with the use of cell salvage, allogeneic blood transfusion can be avoided, minimising all complications of transfusion including red cell alloimmunisation, that is, developing red cell antibodies causing difficulties in identifying compatible blood for future transfusions if needed. The GDG noted that it was important that healthcare personnel using the technique should be adequately trained in order to minimise the chance of errors occurring. |
6.6.1. Research Recommendations
- Post-operative cell salvage: For patients having cardiac surgery with a significant risk of post-operative blood loss, is post-operative cell salvage and reinfusion clinically and cost effective in reducing red blood cell use and improving clinical outcomes, compared with existing practice?
- Why this is important: There was some evidence for benefit from post-operative cell salvage, but the quality was low. Reducing blood loss during cardiac surgery may reduce the risk of complications. However, post-operative cell salvage carries additional cost. Studies are needed to determine whether post-operative cell salvage is more clinically and cost effective than existing practice for patients having cardiac surgery with a significant risk of post-operative blood loss. Important outcomes should include the use of red blood cells and other blood components, clinical outcomes and quality of life.
Figures
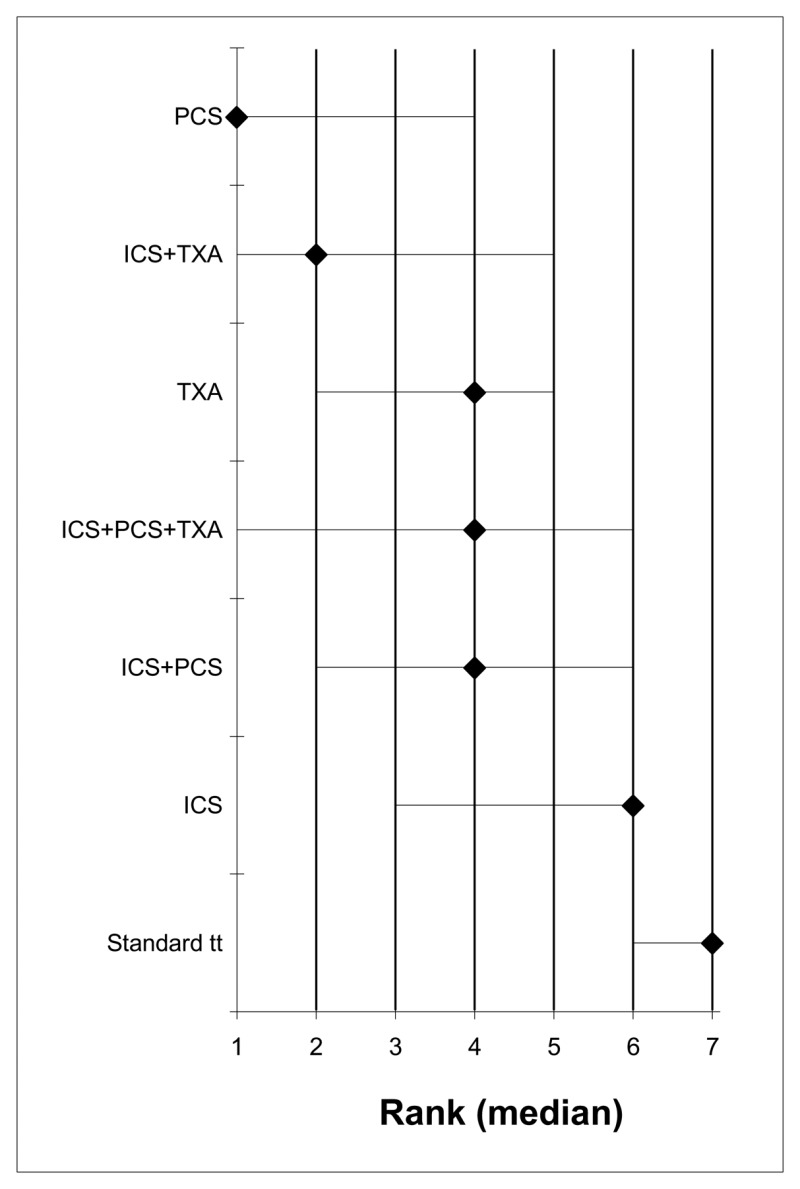
Figure 3Number of patients receiving allogeneic transfusions
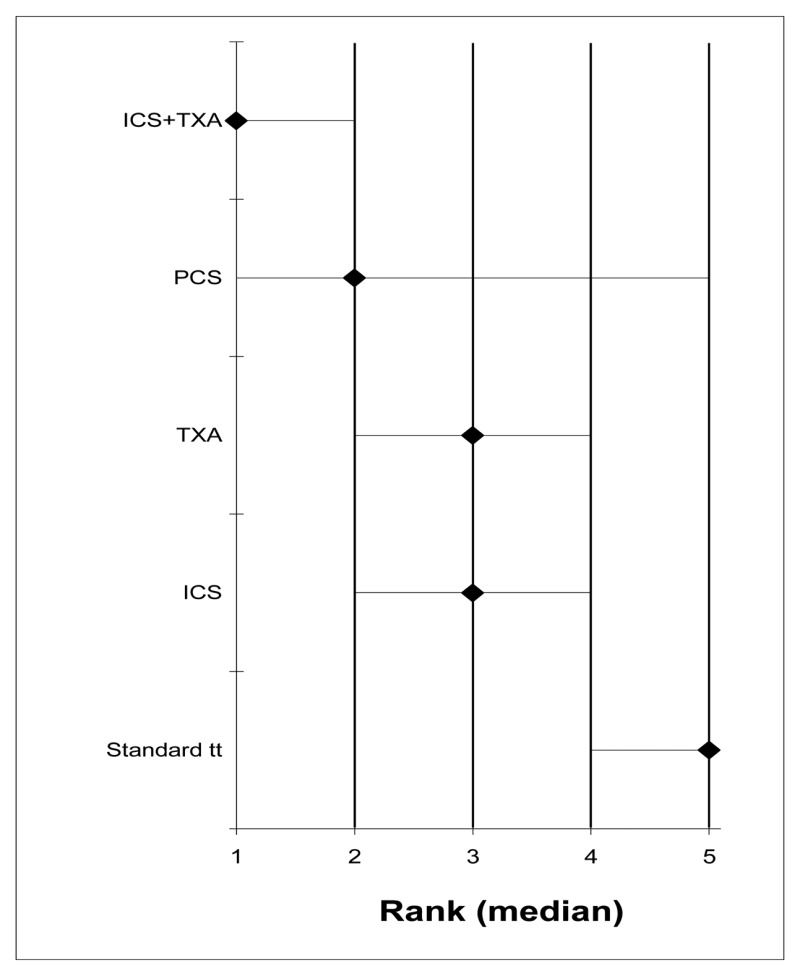
Figure 4Units of allogeneic blood transfused
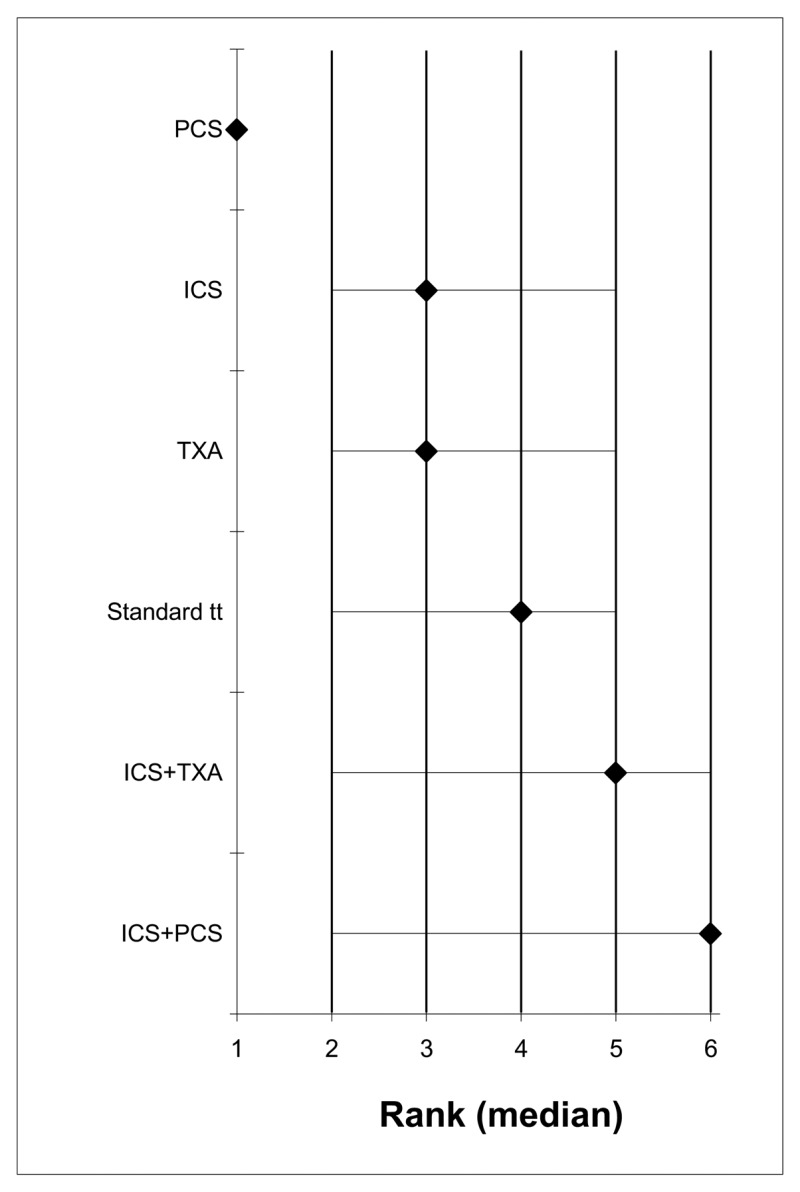
Figure 5Length of stay in hospital
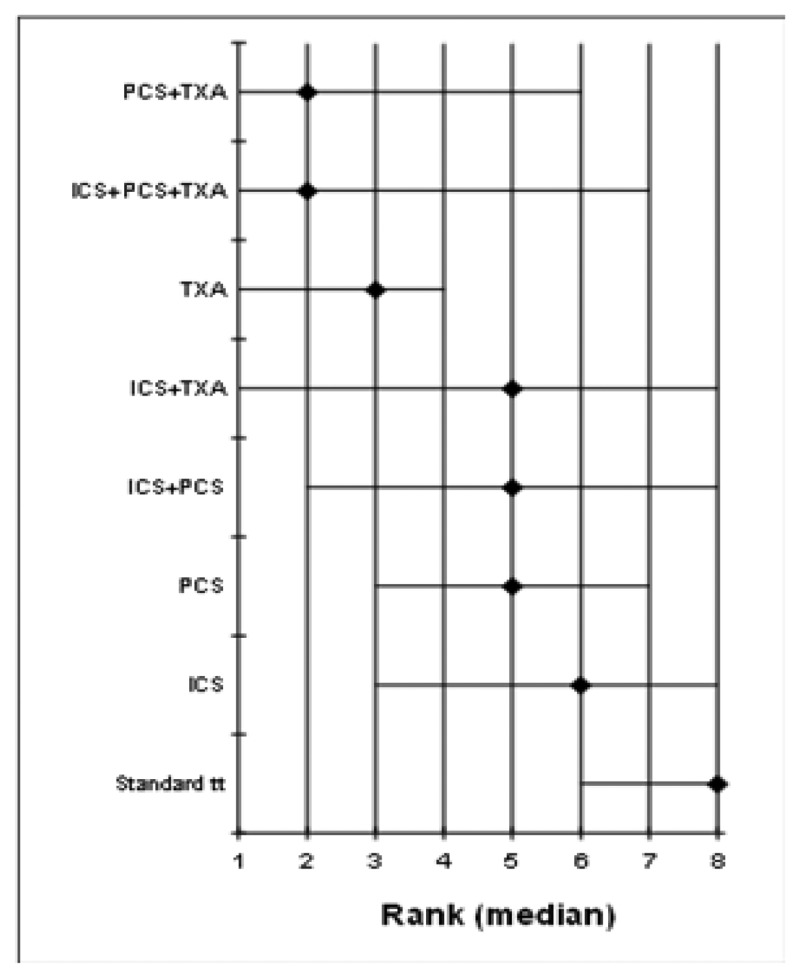
Figure 6Number of patients receiving allogeneic transfusions
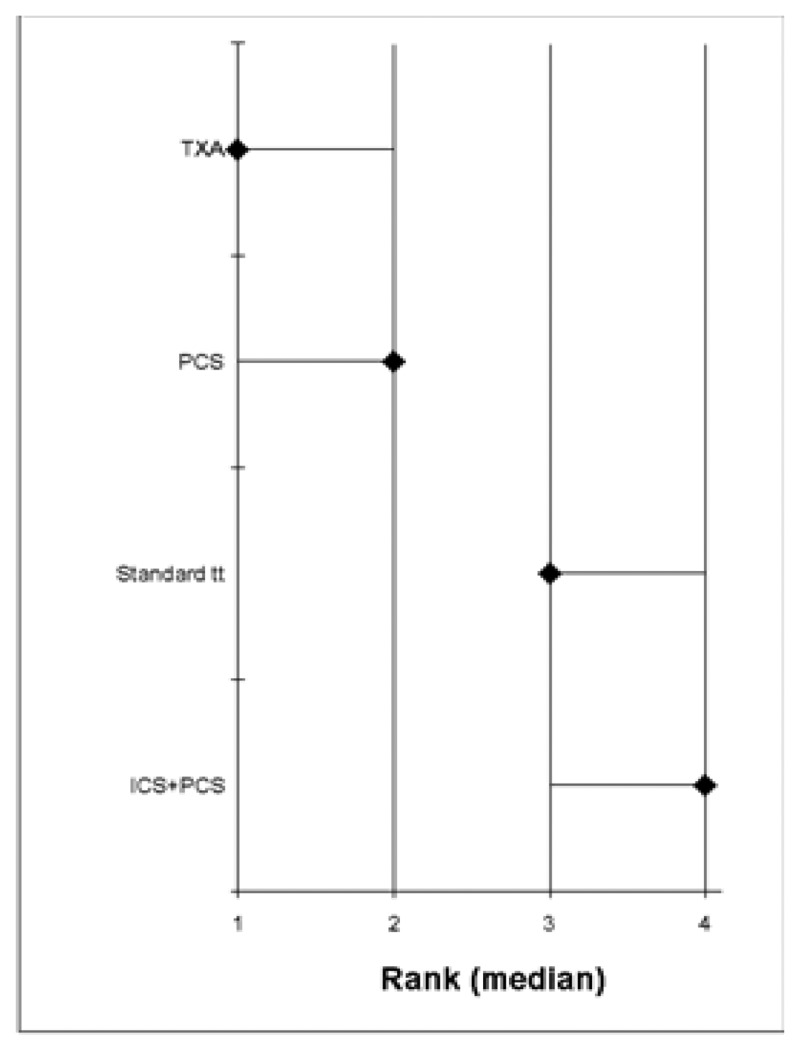
Figure 7Units of allogeneic blood transfused
Figure 8Cost-effectiveness plane, high risk
b Abbreviations: ICER = incremental cost-effectiveness ratio; ICS = intra-operative cell salvage; INMB = incremental net monetary benefit; PCS = post-operative cell salvage; QALY = quality adjusted life years; ST = standard treatment; TXA = tranexamic acid
Figure 9Cost-effectiveness plane, moderate risk
a Abbreviations: ICER = incremental cost-effectiveness ratio; ICS = intra-operative cell salvage; INMB = incremental net monetary benefit; PCS = post-operative cell salvage; QALY = quality adjusted life years; ST = standard treatment; TXA = tranexamic acid
Tables
Table 22PICO characteristics of review question
| Population | Surgical patients
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention(s) | High and moderate risk groups (see review strategy for definitions):
| ||||||||
| Comparison(s) | All of the above, alone or in combination, compared with one another within each risk group. | ||||||||
| Matrix of treatment comparisons | |||||||||
| Comparisons | ICS | P CS | ICS+PCS | TXA | ICS+TXA | PCS+TXA | ICS+PCS+ TXA | Standard treatment | |
| ICS | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| PCS | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| ICS+PCS | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| TXA | ✓ | ✓ | ✓ | ✓ | |||||
| ICS+TXA | ✓ | ✓ | ✓ | ||||||
| PCS+TXA | ✓ | ✓ | |||||||
| ICS+PCS+TXA | ✓ | ||||||||
| Standard treatment | |||||||||
| Outcomes |
| ||||||||
| Study design | Randomised controlled trials | ||||||||
Table 23Summary of studies- Adults- High risk group
| Study | Intervention | Comparator | Risk group |
|---|---|---|---|
| Adults- High risk | |||
| Aghdaii 20125 | Standard Treatment | ICS | High |
| Ahn20127 | Standard Treatment | TXA | High |
| Andreasen200413 | Standard Treatment | TXA | High |
| Armellin200114 | Standard Treatment | TXA | High |
| Baric200718 | Standard Treatment | TXA | High |
| Blauhut199425 | Standard Treatment | TXA | High |
| Casati200143 | Standard Treatment | TXA | High |
| Casati200444 | ICS | ICS+TXA | High |
| Coffey199560 | Standard Treatment | TXA | High |
| Corbeau199564 | Standard Treatment | TXA | High |
| Dalmau200075 | Standard Treatment | TXA | High |
| Damgard200676 | Standard Treatment | ICS | High |
| Debonis200081 | Standard Treatment | TXA | High |
| Dellamore201283 | Standard Treatment | TXA | High |
| Diprose200588 | ICS | ICS+TXA | High |
| Esfandiari201397 | Standard Treatment | TXA | High |
| Fawzy2009103 | Standard Treatment | TXA | High |
| Ghaffari2012114 | Standard Treatment | TXA | High |
| Ghavidel 20148 | Standard Treatment | TXA | High |
| Hardy1998124 | Standard Treatment | TXA | High |
| Horrow1991136 | Standard Treatment | TXA | High |
| Jares2003146 | Standard Treatment | TXA | High |
| Jiminez2007147 | ICS | ICS+TXA | High |
| Karski2005156 | Standard Treatment | TXA | High |
| Katoh1997158 | Standard Treatment | TXA | High |
| Katsaros1996159 | Standard Treatment | TXA | High |
| Klein2008169 | TXA | ICS+PCS+TXA | High |
| Krohn2003172 | Standard Treatment | TXA | High |
| Kuitunen2005173 | ICS | ICS+TXA | High |
| Later2009176 | ICS | ICS+TXA | High |
| Lundin2014187 | Standard Treatment | TXA | High |
| Mansour2004191 | Standard Treatment | TXA | High |
| Mehraein2007194 | Standard Treatment | TXA | High |
| Menichetti1996195 | Standard Treatment | TXA | High |
| Mercer2004196 | Standard Treatment | ICS | High |
| Murphy2004202 | Standard Treatment | ICS+PCS | High |
| Murphy2005204 | Standard Treatment | ICS | High |
| Murphy2006205 | ICS+PCS | ICS+PCS+TXA | High |
| Naumenko2003217 | Standard Treatment | PCS | High |
| Nouraei2013227 | Standard Treatment | TXA | High |
| Pleym2003248 | Standard Treatment | TXA | High |
| Pleym2005247 | Standard Treatment | PCS | High |
| Reyes2011258 | TXA | ICS+TXA | High |
| Santos2006268 | Standard Treatment | TXA | High |
| Shi2013279 | Standard Treatment | TXA | High |
| Shi2013a280 | Standard Treatment | TXA | High |
| Sirvinkas2007 281 | Standard Treatment | PCS | High |
| Speekenbrink1995289 | Standard Treatment | TXA | High |
| Taghaddomi2009295 | Standard Treatment | TXA | High |
| Vanek2005313 | Standard Treatment | TXA | High |
| Vermeijden2015315 | Standard Treatment | ICS | High |
| Wang2012323 | Standard Treatment | TXA | High |
| Wei2006327 | Standard Treatment | TXA | High |
| Wiefferink2007330 | Standard Treatment | ICS+PCS | High |
| Wu2006334 | Standard Treatment | TXA | High |
| Zhao2003342 | Standard Treatment | PCS | High |
Table 24Summary of studies-Adults- Moderate risk group
| Study | Intervention | Comparator 1 | Comparator 2 | Risk group |
|---|---|---|---|---|
| Adults – Moderate risk | ||||
| Abuzakuk20071 | Standard Treatment | PCS | - | Moderate |
| Aguilera20136 | Standard Treatment | TXA | - | Moderate |
| Alshryda201310 | Standard Treatment | TXA | - | Moderate |
| Alvarez200811 | PCS | PCS+TXA | - | Moderate |
| Amin200812 | Standard Treatment | PCS | - | Moderate |
| Atay2010i16 | Standard Treatment | PCS | - | Moderate |
| Atay2010ii16 | Standard Treatment | PCS | - | Moderate |
| Benoni199619 | Standard Treatment | TXA | - | Moderate |
| Benoni200021 | Standard Treatment | TXA | - | Moderate |
| Benoni200120 | Standard Treatment | TXA | - | Moderate |
| Bidolegui201422 | Standard Treatment | TXA | - | Moderate |
| Bradshaw201229 | Standard Treatment | TXA | - | Moderate |
| Caglar200833 | Standard Treatment | TXA | - | Moderate |
| Charoeanch201150 | Standard Treatment | TXA | - | Moderate |
| Charoeanch201249 | Standard Treatment | TXA | - | Moderate |
| Cheng200553 | Standard Treatment | PCS | - | Moderate |
| Cip201358 | Standard Treatment | ICS | - | Moderate |
| Claeys200759 | Standard Treatment | TXA | - | Moderate |
| Crescenti201168 | Standard Treatment | TXA | - | Moderate |
| Dakir201474 | Standard Treatment | TXA | - | Moderate |
| Dramis200693 | Standard Treatment | PCS | - | Moderate |
| Ellis200195 | Standard Treatment | TXA | - | Moderate |
| Engel200196 | Standard Treatment | TXA | - | Moderate |
| Farrokhi2011102 | Standard Treatment | TXA | - | Moderate |
| Garneti2004111 | Standard Treatment | TXA | - | Moderate |
| Georgiadis2013113 | Standard Treatment | TXA | - | Moderate |
| Gill2009115 | Standard Treatment | TXA | - | Moderate |
| Good2003117 | Standard Treatment | TXA | - | Moderate |
| Gungorduk2011122 | Standard Treatment | TXA | - | Moderate |
| Hiipala1995132 | Standard Treatment | TXA | - | Moderate |
| Hiipala1997133 | Standard Treatment | TXA | - | Moderate |
| Horstmann2013137 | Standard Treatment | ICS | - | Moderate |
| Horstmann2014138 | Standard Treatment | PCS | - | Moderate |
| Horstmann2014a139 | Standard Treatment | ICS+PCS | - | Moderate |
| Husted2003140 | Standard Treatment | TXA | - | Moderate |
| Ishida2011142 | Standard Treatment | TXA | - | Moderate |
| Jansen1999145 | Standard Treatment | TXA | - | Moderate |
| Johansson2005148 | Standard Treatment | TXA | - | Moderate |
| Karimi2012154 | Standard Treatment | TXA | - | Moderate |
| Kazemi2010160 | Standard Treatment | TXA | - | Moderate |
| Kim 2014ii167 | Standard Treatment | TXA | - | Moderate |
| Kim2014i167 | Standard Treatment | TXA | - | Moderate |
| Lee2013181 | Standard Treatment | TXA | - | Moderate |
| Lemay2004182 | Standard Treatment | TXA | - | Moderate |
| Macgillvray2010189 | Standard Treatment | TXA | - | Moderate |
| Moonen2007199 | Standard Treatment | PCS | - | Moderate |
| Niskanen2005225 | Standard Treatment | TXA | - | Moderate |
| Oremus2014232 | PCS | PCS+TXA | - | Moderate |
| Orpen2006235 | Standard Treatment | TXA | - | Moderate |
| Rajesparan2009251 | Standard Treatment | TXA | - | Moderate |
| Raviraj2012255 | Standard Treatment | TXA | - | Moderate |
| Roy2012262 | Standard Treatment | TXA | - | Moderate |
| Sadeghi2007265 | Standard Treatment | TXA | - | Moderate |
| Sa-ngasoongsong2011263 | Standard Treatment | TXA | - | Moderate |
| Sa-ngasoongsong2013264 | Standard Treatment | TXA | - | Moderate |
| Seo2013274 | Standard Treatment | TXA | - | Moderate |
| Shahid2013277 | Standard Treatment | TXA | - | Moderate |
| Smith2007283 | Standard Treatment | PCS | - | Moderate |
| Soosman2006285 | Standard Treatment | PCS | - | Moderate |
| Soosman2014286 | Standard Treatment | PCS | ICS+PCS | Moderate |
| Sorin1999287 | Standard Treatment | TXA | - | Moderate |
| Tanaka2001296 | Standard Treatment | TXA | - | Moderate |
| Thomassen2012299 | TXA | ICS+PCS+TXA | - | Moderate |
| Thomassen2014298 | Standard Treatment | PCS | - | Moderate |
| Tripkovic2008306 | Standard Treatment | PCS | - | Moderate |
| Vijay2013317 | Standard Treatment | TXA | - | Moderate |
| Wong2008333 | ICS | ICS+TXA | - | Moderate |
| Wong2010332 | Standard Treatment | TXA | - | Moderate |
| Yang2014336 | Standard Treatment | TXA | - | Moderate |
| Yue2015339 | Standard Treatment | TXA | - | Moderate |
| Zacharopoulos2007340 | Standard Treatment | PCS | - | Moderate |
| Zhang2008341 | Standard Treatment | ICS | - | Moderate |
| Zohar2004344 | Standard Treatment | TXA | - | Moderate |
Table 25Summary of studies-Adults- Low risk group
| Study | Intervention | Comparator | Risk group |
|---|---|---|---|
| Adults- Low risk group | |||
| Albirmawy 201310 | TXA | Standard Treatment | Low |
| Jabalameli 2006143 | TXA | Standard Treatment | Low |
| Kaewpradub 2011151 | TXA | Standard Treatment | Low |
| Rannikko 2004253 | TXA | Standard Treatment | Low |
| Sankar 2012267 | TXA | Standard Treatment | Low |
| Tsutsumimoto 2011308 | TXA | Standard Treatment | Low |
Table 27Intra-operative cell salvage versus standard treatment
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with standard treatment | Risk difference with intra-operative cell salvage (95% CI) | ||||
| No. exposed to allogeneic blood | 251 (4 studies) | VERY LOWa,b due to risk of bias, imprecision | RR 0.74 (0.58 to 0.93) | Study population | |
| 532 per 1000 | 138 fewer per 1000 (from 37 fewer to 223 fewer) | ||||
| Units of allogeneic blood transfused | 223 (4 studies) | VERY LOWa,b,c due to risk of bias, inconsistency, imprecision | The mean units of allogeneic blood transfused in the intervention groups was 0.78 lower (1.37 to 0.19 lower) | ||
| Mortality at up to 30 days | 424 (7 studies) | VERY LOWa,d due to risk of bias, imprecision | RR 0.97 (0.64 to 1.47) | Study population | |
| 89 per 1000 | 3 fewer per 1000 (from 32 fewer to 42 more) | ||||
| Any infection | 250 (4 studies) | VERY LOWa,b due to risk of bias, imprecision | RR 0.4 (0.18 to 0.87) | Study population | |
| 151 per 1000 | 90 fewer per 1000 (from 20 fewer to 124 fewer) | ||||
| Hospital length of stay | 80 (1 study) | VERY LOWa,d due to risk of bias, imprecision | The mean hospital length of stay in the intervention groups was 0.2 lower (1.26 lower to 0.86 higher) | ||
- a
The majority of the evidence was at very high risk of bias.
- b
The confidence interval crosses one MID.
- c
Downgraded by one increment due to heterogeneity, I2=65%.
- d
The confidence interval crosses both MIDs.
Table 28Post-operative cell salvage versus standard treatment
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with standard treatment | Risk difference with post-operative cell salvage (95% CI) | ||||
| No. exposed to allogeneic blood | 262 (4 studies) | VERY LOWa,b due to risk of bias, imprecision | RR 0.6 (0.45 to 0.81) | Study population | |
| 390 per 1000 | 156 fewer per 1000 (from 74 fewer to 214 fewer) | ||||
| Units of allogeneic blood transfused | 60 (1 study) | VERY LOWa,b due to risk of bias, imprecision | The mean units of allogeneic blood transfused in the intervention groups was 1.02 lower (1.19 to 0.85 lower) | ||
| Mortality at up to 30 days | 50 (1 study) | VERY LOWa,c due to risk of bias, imprecision | RR 3 (0.13 to 70.3) | Study population | |
| 0 per 1000 | - | ||||
| Any infection | 90 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 0.15 (0.02 to 1.15) | Study population | |
| 163 per 1000 | 139 fewer per 1000 (from 160 fewer to 24 more) | ||||
| Hospital length of stay | 90 (1 study) | LOWa due to risk of bias | The mean hospital length of stay in the intervention groups was 7.13 lower (9.12 to 5.14 lower) | ||
- a
The majority of the evidence was at very high risk of bias.
- b
The confidence interval crosses one MID.
- c
The confidence interval crosses both MIDs.
Table 29Intra-operative cell salvage plus post-operative cell salvage versus standard treatment
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with standard treatment | Risk difference with intra-operative cell salvage + post-operative cell salvage (95% CI) | ||||
| No. exposed to allogeneic blood | 230 (2 studies) | VERY LOWa,b due to risk of bias, imprecision | RR 0.69 (0.54 to 0.89) | Study population | |
| 632 per 1000 | 196 fewer per 1000 (from 70 fewer to 291 fewer) | ||||
| Mortality at up to 30 days | 196 (1 study) | VERY LOWa,c due to risk of bias, imprecision | RR 0.33 (0.03 to 3.09) | Study population | |
| 31 per 1000 | 21 fewer per 1000 (from 30 fewer to 65 more) | ||||
| Any infection | 196 (1 study) | VERY LOWa,c due to risk of bias, imprecision | RR 0.98 (0.14 to 6.82) | Study population | |
| 21 per 1000 | 0 fewer per 1000 (from 18 fewer to 120 more) | ||||
| Length of hospital stay | 196 (1 study) | VERY LOWa,c due to risk of bias, imprecision | The mean length of hospital stay in the intervention groups was 2.8 higher (2.11 lower to 7.71 higher) | ||
- a
The majority of the evidence is at very high risk of bias.
- b
The confidence interval crosses one MID.
- c
The confidence interval crosses both MIDs.
Table 30Intra-operative cell salvage plus tranexamic acid versus intra-operative cell salvage
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with intra-operative cell salvage | Risk difference with intra-operative cell salvage +TXA (95% CI) | ||||
| No. exposed to allogeneic blood | 514 (5 studies) | VERY LOWa,b due to risk of bias, imprecision | RR 0.71 (0.6 to 0.85) | Study population | |
| 556 per 1000 | 161 fewer per 1000 (from 83 fewer to 222 fewer) | ||||
| Units of blood transfused | 170 (2 studies) | VERY LOWa,c due to risk of bias, inconsistency | The mean units of blood transfused in the intervention groups was 1.56 lower (1.84 to 1.29 lower) | ||
| Mortality at 30 days | 352 (4 studies) | VERY LOWa,d due to risk of bias, imprecision | RR 1.04 (0.07 to 16.41) | Study population | |
| 10 per 1000 | 0 more per 1000 (from 9 fewer to 147 more) | ||||
| Length of stay in hospital | 252 (2 studies) | VERY LOWa,d due to risk of bias, imprecision | The mean length of stay in hospital in the intervention groups was 0.68 higher (0.81 lower to 2.17 higher) | ||
- a
The majority of the evidence is at very high risk of bias.
- b
The confidence interval crosses one MID.
- c
Downgraded by one increment due to heterogeneity; I2=61%.
- d
The confidence interval crosses both MIDs.
Table 31Intra-operative cell salvage and tranexamic acid versus tranexamic acid
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with TXA | Risk difference with intra-operative cell salvage +TXA (95% CI) | ||||
| No. exposed to allogeneic blood | 63 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 0.79 (0.43 to 1.45) | Study population | |
| 448 per 1000 | 94 fewer per 1000 (from 256 fewer to 202 more) | ||||
| Mortality at 30 days | 63 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 7.71 (0.43 to 137.53) | Study population | |
| 0 per 1000 | - | ||||
| Infections | 63 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 1.07 (0.32 to 3.6) | Study population | |
| 138 per 1000 | 10 more per 1000 (from 94 fewer to 359 more) | ||||
| Length of stay in hospital | 63 (1 study) | VERY LOWa,b due to risk of bias, imprecision | The mean length of stay in hospital in the intervention groups was 2.1 higher (3.36 lower to 7.56 higher) | ||
- a
The majority of the evidence is at very high risk of bias.
- b
The confidence interval crosses both MIDs.
Table 32Post-operative cell salvage plus tranexamic acid versus tranexamic acid
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with TXA | Risk difference with postoperative cell salvage +TXA (95% CI) | ||||
| No. of patients with allogeneic blood transfusion | 34 (1 study) | LOWa due to risk of bias | Not estimable | ||
- a
The majority of the evidence is at very high risk of bias.
Table 33Intra-operative cell salvage plus post-operative cell salvage plus tranexamic acid versus intra-operative cell salvage plus post-operative cell salvage
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with intra-operative cell salvage + postoperative cell salvage | Risk difference with intra-operative cell salvage + post-operative cell salvage + TXA (95% CI) | ||||
| No. exposed to allogeneic blood | 100 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 0.93 (0.49 to 1.77) | Study population | |
| 280 per 1000 | 20 fewer per 1000 (from 143 fewer to 216 more) | ||||
| Units of blood transfused | 27 (1 study) | VERY LOWa,c due to risk of bias, imprecision | The mean units of blood transfused in the intervention groups was 0.25 higher (0.32 lower to 0.82 higher) | ||
| Mortality at 30 days | 100 (1 study) | LOWa due to risk of bias | Not estimable | ||
- a
The majority of the evidence was at very high risk of bias.
- b
The confidence interval crosses both MIDs.
- c
The confidence interval crosses one MID.
Table 34Intra-operative cell salvage plus post-operative cell salvage plus tranexamic acid versus tranexamic acid
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with TXA | Risk difference with intra-operative cell salvage + post-operative cell salvage + TXA (95% CI) | ||||
| No. exposed to allogeneic blood | 213 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 1.02 (0.68 to 1.54) | Study population | |
| 297 per 1000 | 6 more per 1000 (from 95 fewer to 161 more) | ||||
| Any infection | 213 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 1.31 (0.41 to 4.15) | Study population | |
| 45 per 1000 | 14 more per 1000 (from 27 fewer to 142 more) | ||||
- a
The majority of the evidence is at very high risk of bias.
- b
The confidence interval crosses both MIDs.
Table 35Tranexamic acid versus standard treatment
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with standard treatment/placebo - high risk – adults | Risk difference with TXA (95% CI) | ||||
| No. of patients needing blood transfusions | 4105 (38 studies) | VERY LOWa,b,c due to risk of bias, inconsistency, imprecision | RR 0.71 (0.63 to 0.81) | Study population | |
| 475 per 1000 | 138 fewer per 1000 (from 90 fewer to 176 fewer) | ||||
| No. of units of blood transfused -all patients | 1918 (16 studies) | LOWa,b due to risk of bias, inconsistency | The mean no. of units of blood transfused - all patients in the intervention groups was 0.83 lower (1.17 to 0.5 lower) | ||
| Mortality | 3771 (31 studies) | VERY LOWa,c,d due to risk of bias, inconsistency, imprecision | RR 0.52 (0.31 to 0.87) | Study population | |
| 19 per 1000 | 9 fewer per 1000 (from 2 fewer to 13 fewer) | ||||
| Length of hospital stay | 182 (3 studies) | MODERATEa due to risk of bias | The mean length of hospital stay in the intervention groups was 0.08 lower (0.35 lower to 0.18 higher) | ||
| Infections | 100 (1 study) | LOWa,c due to risk of bias, imprecision | RR 0.62 (0.31 to 1.24) | Study population | |
| 320 per 1000 | 122 fewer per 1000 (from 221 fewer to 77 more) | ||||
| Thrombotic complications | 986 (10 studies) | LOWa,c due to risk of bias, imprecision | RR 0.48 (0.18 to 1.23) | Study population | |
| 25 per 1000 | 13 fewer per 1000 (from 20 fewer to 6 more) | ||||
- a
Majority of the evidence was at high risk of bias.
- b
Downgraded by one increment due to heterogeneity, I2=72%.
- c
Confidence interval crosses one MID.
- d
Downgraded by one increment as the point estimate varies widely across studies, unexplained by subgroup analysis.
Table 36Intra-operative cell salvage versus standard treatment
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with standard treatment | Risk difference with intra-operative cell salvage (95% CI) | ||||
| No. exposed to allogeneic blood | 384 (3 studies) | VERY LOWa,b due to risk of bias, imprecision | RR 0.74 (0.5 to 1.12) | Study population | |
| 250 per 1000 | 65 fewer per 1000 (from 125 fewer to 30 more) | ||||
- a
Majority of the evidence was at very high risk of bias.
- b
Confidence interval crosses one MID.
Table 37Post-operative cell salvage versus standard treatment
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with standard treatment | Risk difference with postoperative cell salvage (95% CI) | ||||
| No. exposed to allogeneic blood | 2641 (14 studies) | VERY LOWa,b,c due to risk of bias, inconsistency, imprecision | RR 0.58 (0.41 to 0.83) | Study population | |
| 163 per 1000 | 68 fewer per 1000 (from 28 fewer to 96 fewer) | ||||
| Units of allogeneic blood transfused | 1335 (8 studies) | VERY LOWa,c,d due to risk of bias, inconsistency, imprecision | The mean units of allogeneic blood transfused in the intervention groups was 0.82 lower (1.31 to 0.33 lower) | ||
| Infection | 1025 (4 studies) | VERY LOWa,e due to risk of bias, imprecision | RR 1.79 (0.53 to 6.07) | Study population | |
| 7 per 1000 | 6 more per 1000 (from 3 fewer to 37 more) | ||||
| Hospital length of stay | 205 (3 studies) | VERY LOWa,e due to risk of bias, imprecision | The mean hospital length of stay in the intervention groups was 0.37 lower (1.73 lower to 0.99 higher) | ||
- a
Majority of the evidence was at very high risk of bias.
- b
Downgraded by one increment due to heterogeneity, I2= 67%.
- c
Confidence interval crosses one MID.
- d
Downgraded by one increment due to heterogeneity, I2=88%.
- e
Confidence interval crosses both MIDs.
Table 38Intra-operative cell salvage plus post-operative cell salvage versus standard treatment
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with standard treatment | Risk difference with intra-operative cell salvage + postoperative cell salvage (95% CI) | ||||
| No. exposed to allogeneic blood | 1097 (2 studies) | VERY LOWa,b due to risk of bias, imprecision | RR 0.84 (0.54 to 1.33) | Study population | |
| 81 per 1000 | 13 fewer per 1000 (from 37 fewer to 27 more) | ||||
| Units of allogeneic blood transfused | 77 (1 study) | LOWa due to risk of bias | The mean units of allogeneic blood transfused in the intervention groups was 0.81 higher (0.49 higher to 1.13 higher) | ||
| Infection | 118 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 3.32 (0.14 to 79.77) | Study population | |
| 0 per 1000 | - | ||||
| Length of stay | 118 (1 study) | VERY LOWa,c due to risk of bias, imprecision | The mean length of stay in the intervention groups was 0.2 higher (0.2 lower to 0.6 higher) | ||
| Mortality | 118 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 3.32 (0.14 to 79.77) | Study population | |
| 0 per 1000 | - | ||||
- a
Majority of the evidence is at very high risk of bias.
- b
Confidence interval crosses both MIDs.
- c
Confidence interval crosses one MID.
Table 39Intra-operative cell salvage plus tranexamic acid versus intra-operative cell salvage
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with intra-operative cell salvage | Risk difference with intra-operative cell salvage + TXA (95% CI) | ||||
| No. exposed to allogeneic blood | 147 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 0.78 (0.5 to 1.2) | Study population | |
| 405 per 1000 | 89 fewer per 1000 (from 203 fewer to 81 more) | ||||
| Units of blood transfused | 147 (1 study) | VERY LOWa,c due to risk of bias, imprecision | The mean units of blood transfused in the intervention groups was 0.46 lower (1.1 lower to 0.18 higher) | ||
| Length of stay in hospital | 147 (1 study) | VERY LOWa,c due to risk of bias, imprecision | The mean length of stay in hospital in the intervention groups was 0.72 higher (0.85 lower to 2.29 higher) | ||
- a
Majority of the evidence was at very high risk of bias.
- b
Confidence interval crosses one MID.
- c
Confidence interval crosses both MIDs.
Table 40Post-operative cell salvage plus tranexamic acid versus post-operative cell salvage
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with postoperative cell salvage | Risk difference with postoperative cell salvage + TXA (95% CI) | ||||
| No. exposed to allogeneic blood | 193 (2 studies) | VERY LOWa,b due to risk of bias, imprecision | RR 0.37 (0.12 to 1.14) | Study population | |
| 112 per 1000 | 71 fewer per 1000 (from 99 fewer to 16 more) | ||||
| Thrombotic complications | 98 (1 study) | RR 0.2 (0.01 to 4.06) | Study population | ||
| 41 per 1000 | 33 fewer per 1000 (from 40 fewer to 125 more) | ||||
- a
Majority of the evidence is at very high risk of bias.
- b
Confidence interval crosses one MID.
Table 41Intra-operative cell salvage plus post-operative cell salvage plus tranexamic acid versus tranexamic acid
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with TXA | Risk difference with intra-operative cell salvage + post-operative cell salvage +TXA (95% CI) | ||||
| No. exposed to allogeneic blood | 197 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 0.73 (0.33 to 1.63) | Study population | |
| 129 per 1000 | 35 fewer per 1000 (from 86 fewer to 81 more) | ||||
| Units of blood transfused | 197 (1 study) | LOWa due to risk of bias | Not estimable | ||
- a
Majority of the evidence was at very high risk of bias.
- b
Confidence interval crosses both MIDs.
Table 42Tranexamic acid versus standard treatment
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with standard treatment: adults -moderate risk | Risk difference with TXA (95% CI) | ||||
| No. exposed to allogeneic transfusions | 4577 (52 studies) | LOWa,b due to risk of bias, inconsistency | RR 0.45 (0.38 to 0.52) | Study population | |
| 351 per 1000 | 193 fewer per 1000 (from 165 fewer to 218 fewer) | ||||
| No. of units of blood transfused -All Patients | 644 (9 studies) | LOWa,c due to risk of bias, inconsistency | The mean no. of units of blood transfused - all patients in the intervention groups was 0.88 lower (1.22 to 0.54 lower) | ||
| Mortality | 1071 (9 studies) | VERY LOWa,d,e due to risk of bias, inconsistency, imprecision | RR 0.73 (0.15 to 3.66) | Study population | |
| 4 per 1000 | 1 fewer per 1000 (from 3 fewer to 10 more) | ||||
| Length of hospital stay | 1332 (9 studies) | VERY LOWa,f,g due to risk of bias, inconsistency, imprecision | The mean length of hospital stay in the intervention groups was 0.25 lower (0.59 lower to 0.09 higher) | ||
| Infections | 586 (6 studies) | VERY LOWa,d,e due to risk of bias, inconsistency, imprecision | RR 0.93 (0.22 to 3.93) | Study population | |
| 10 per 1000 | 1 fewer per 1000 (from 8 fewer to 30 more) | ||||
| Thrombotic complications | 5179 (48 studies) | LOWa,g due to risk of bias, imprecision | RR 0.69 (0.44 to 1.07) | Study population | |
| 19 per 1000 | 6 fewer per 1000 (from 11 fewer to 1 more) | ||||
- a
Majority of the evidence was at high risk of bias.
- b
Downgraded by one increment due to heterogeneity, I2=55%.
- c
Downgraded by one increment due to heterogeneity, I2=89%.
- d
Downgraded by one increment due to heterogeneity; the point estimate varies widely across studies, unexplained by subgroup analysis.
- e
Confidence interval crosses both MIDs.
- f
Downgraded by one increment due to heterogeneity, I2=61%.
- g
Confidence interval crosses one MID.
Table 43Intra-operative cell salvage +Post-operative cell salvage versus Post-operative cell salvage
| Outcomes | No of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with Post op CS | Risk difference with Intraop CS+Post op CS (95% CI) | ||||
| Number of patients transfused | 642 (1 study) | LOWa,b due to risk of bias, imprecision | RR 0.70 (0.42 to 1.16) | 103 per 1000 | 31 fewer per 1000 (from 60 fewer to 16 more) |
| Units of allogeneic blood transfused | 56 (1 study) | MODERATEa due to risk of bias | The mean units of allogeneic blood transfused in the intervention groups was 2.23 higher (1.92 to 2.54 higher) | ||
- a
Majority of the evidence was at high risk of bias.
- b
Confidence interval crosses one MID.
Table 44Tranexamic acid versus standard treatment
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with placebo - low risk - adults | Risk difference with TXA (95% CI) | ||||
| No. of patients receiving allogeneic transfusions (route) | 626 (4 studies) | VERY LOWa,b due to risk of bias, imprecision | RR 0.83 (0.3 to 2.29) | Study population | |
| 23 per 1000 | 4 fewer per 1000 (from 16 fewer to 29 more) | ||||
| No. of patients receiving allogeneic transfusions (route) - Topical TXA | 400 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 0.2 (0.01 to 4.14) | Study population | |
| 10 per 1000 | 8 fewer per 1000 (from 10 fewer to 31 more) | ||||
| No. of patients receiving allogeneic transfusions (route) - Oral TXA | 136 (1 study) | VERY LOWa,b due to risk of bias, imprecision | RR 1.13 (0.36 to 3.53) | Study population | |
| 76 per 1000 | 10 more per 1000 (from 48 fewer to 192 more) | ||||
| Blood loss (type of surgery-topical TXA)) -Orthognathic surgery | 0 (1 study) | MODERATEa due to risk of bias | The mean blood loss (type of surgery-topical TXA) -orthognathic surgery in the intervention groups was 0.93 higher (0.73 to 1.2 higher) | ||
| Blood loss (type of surgery-topical TXA) - otolaryngeal surgery | 0 (2 studies) | The mean blood loss (type of surgery-topical TXA) - otolaryngeal surgery in the intervention groups was 0.74 higher (0.73 to 0.76 higher) | |||
- a
Majority of the evidence was at high risk of bias.
- b
Confidence interval crosses both MIDs.
Table 45Intra-operative cell salvage plus tranexamic acid versus intra-operative cell salvage
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with intra-operative cell salvage - type of surgery | Risk difference with intra-operative cell salvage + TXA (95% CI) | ||||
| Number of patients transfused - Post 2003 | 44 (1 study) | VERY LOWa, b due to risk of bias, imprecision | RR 0.85 (0.56 to 1.3) | Study population | |
| 714 per 1000 | 107 fewer per 1000 (from 314 fewer to 214 more) | ||||
| Total blood transfused - Post 2003 | 44 (1 study) | VERY LOWa, b due to risk of bias, imprecision | The mean total blood transfused - post 2003 in the intervention groups was 325 lower (685.06 lower to 35.06 higher) | ||
| Total blood loss -Post 2003 | 44 (1 study) | VERY LOWa, b due to risk of bias, imprecision | The mean total blood loss - post 2003 in the intervention groups was 855 lower (1408.15 to 301.85 lower) | ||
- a
Majority of the evidence was at very high risk of bias.
- b
Confidence interval crosses both MIDs.
Table 46Tranexamic acid versus standard treatment
| Outcomes | No. of Participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with Standard treatment | Risk difference with TXA (95% CI) | ||||
| Post-operative blood loss - post 2003 | 120 (1 study) | MODERATEa due to risk of bias | The mean post-operative blood loss - post 2003 in the intervention groups was 16 lower (21.13 to 10.87 lower) | ||
| Length of stay | 83 (1 study) | LOWb due to risk of bias, imprecision | The mean length of stay in the intervention groups was 0.1 higher (0.37 lower to 0.57 higher) | ||
- a
Majority of the evidence was at high risk of bias.
- b
Confidence interval crosses one MID.
Table 47Network 1: Number exposed to allogeneic transfusions
| Comparison | Risk ratio | ||
|---|---|---|---|
| Direct (mean) | NMA (median) | ||
| Versus standard treatment | TXA vs. standard treatment | 0.72 (0.64, 0.81) | 0.6189 (0.4474, 0.9006) |
| PCS vs. standard treatment | 0.60 (0.45, 0.81) | 0.3596 (0.1268, 0.8032) | |
| ICS vs. standard treatment | 0.81 (0.70, 0.93) | 0.7885 (0.517, 0.9855) | |
| ICS+PCS vs. standard treatment | 0.69 (0.54, 0.89) | 0.6482 (0.3422, 0.9548) | |
| ICS+TXA vs. standard treatment | - | 0.4924(0.2471, 0.8621) | |
| ICS+PCS+TXA vs. standard treatment | - | 0.638 (0.3177, 0.96) | |
| Versus TXA | PCS vs. TXA | - | 0.5837 (0.2341, 1.046) |
| ICS vs. TXA | - | 1.204 (0.9287, 1.766) | |
| ICS+PCS vs. TXA | - | 1.007 (0.6355, 1.552) | |
| ICS+TXA vs. TXA | 0.79 (0.43, 1.45) | 0.7957 (0.4656, 1.15) | |
| ICS+PCS+TXA vs. TXA | 1.02 (0.68, 1.54) | 0.9945 (0.5964, 1.546) | |
| Versus PCS | ICS vs. PCS | - | 2.105 (1.08, 5.768) |
| ICS+PCS vs. PCS | - | 1.717 (0.8541, 4.706) | |
| ICS+TXA vs. PCS | - | 1.338 (0.6426, 3.478) | |
| ICS+PCS+TXA vs. PCS | - | 1.685 (0.8127, 4.704) | |
| Versus ICS | ICS+PCS vs. ICS | - | 0.8406 (0.4553, 1.311) |
| ICS+TXA vs. ICS | 0.71 (0.60, 0.85) | 0.64 (0.3993, 0.9151) | |
| ICS+PCS+TXA vs. ICS | - | 0.8284 (0.4272, 1.318) | |
| Versus ICS+PCS | ICS+TXA vs. ICS+PCS | - | 0.7951 (0.3925, 1.388) |
| ICS+PCS+TXA vs. ICS+PCS | 0.93 (0.49, 1.77) | 0.9863 (0.5695, 1.649) | |
| Versus ICS+TXA | ICS+PCS+TXA vs. ICS+TXA | - | 1.235 (0.6869, 2.557) |
Table 48Network 2: Units of allogeneic blood transfused
| Comparison | Mean difference | ||
|---|---|---|---|
| Direct (mean) | NMA (median) | ||
| Versus standard treatment | ICS vs. standard treatment | -0.78 (-1.37, -0.19) | -0.818 (-1.671, -0.1148) |
| TXA vs. standard treatment | -0.83 (-1.17, -0.5) | -0.8536 (-1.343, -0.4843) | |
| PCS vs. standard treatment | -1.02 (-1.19, -0.86) | -1.021 (-2.29, 0.2511) | |
| ICS+TXA vs. standard treatment | - | -2.16 (-3.444, -0.9444) | |
| Versus ICS | TXA vs. ICS | - | -0.03479 ( -0.8862, 0.8435) |
| PCS vs. ICS | - | -0.2067 (-1.609, 1.375) | |
| ICS+TXA vs. ICS | -1.56 (-1.84, -1.29) | -1.346 (-2.291, -0.3032) | |
| Versus TXA | PCS vs. TXA | - | -0.1725 (-1.438, 1.243) |
| ICS+TXA vs. TXA | - | -1.309 (-2.589, 0.03418) | |
| Versus PCS | ICS+TXA vs. PCS | - | -1.141 (-2.965, 0.6136) |
Table 49Network 3: Length of stay in hospital
| Comparison | Mean difference | ||
|---|---|---|---|
| Direct (mean) | NMA (median) | ||
| Versus standard treatment | TXA vs. standard treatment | -0.08 (0.35, 0.18) | -0.1266 (-0.9664, 0.4938) |
| ICS vs. standard treatment | -0.22 (-1.16, 0.72) | -0.1668 (-1.346 , 1.041) | |
| PCS vs. standard treatment | -7.13 (-9.12, -5.14) | -7.123 (-9.394, -4.869) | |
| ICS+PCS vs. standard treatment | 2.80 (-2.11, 7.71) | 2.83 (-2.182, 7.842) | |
| ICS+TXA vs. standard treatment | - | 0.6375 (-1.306, 2.607) | |
| Versus TXA | ICS vs. TXA | - | -0.03038 (-1.315, 1.428) |
| PCS vs. TXA | - | -6.987 (-9.315, -4.577) | |
| ICS+PCS vs. TXA | - | 2.977 (-2.077, 8.056) | |
| ICS+TXA vs. TXA | 2.10 (-3.36, 7.56) | 0.7759 (-1.204, 2.864) | |
| Versus ICS | PCS vs. ICS | - | -6.962 (-9.537, -4.427) |
| ICS+PCS vs. ICS | - | 2.994 (-2.137, 8.15) | |
| ICS+TXA vs. ICS | 0.68 (-0.81, 2.17) | 0.8029 (-0.8243, 2.432) | |
| Versus PCS | ICS+PCS vs. PCS | - | 9.961 (4.498, 15.46) |
| ICS+TXA vs. PCS | - | 7.748 (4.834, 10.78) | |
| Versus ICS+PCS | ICS+TXA vs. ICS+PCS | - | -2.196 (-7.537, 3.248) |
Table 50Results of pairwise meta-analysis
| Comparison | No. of studies | Effect size (Relative risk/Mean difference) |
|---|---|---|
| Outcome: Infections | ||
| ICS vs. standard treatment | 3 | 0.40 [0.18, 0.87] |
| PCS vs. standard treatment | 1 | 0.15 [0.02, 1.15] |
| ICS + PCS vs. standard treatment | 1 | 0.98[0.14, 6.82] |
| TXA vs. standard treatment | 2 | 0.61 (0.32, 1.18) |
| ICS +TXA vs. TXA | 1 | 1.07 (0.32, 3.60) |
| ICS+PCS+TXA vs. TXA | 1 | 1.31 (0.41, 4.15) |
| Outcome: Thrombotic complications | ||
| TXA vs. standard treatment | 4 | 0.48 (0.18, 1.23) |
| Outcome: Mortality | ||
| ICS vs. standard treatment | 5 | 0.65 (0.27, 1.59) |
| PCS vs. standard treatment | 1 | 3 (0.13, 70.30) |
| ICS + PCS vs. standard treatment | 1 | 0.33 (0.03, 3.09) |
| TXA vs. standard treatment | 17 | 0.52 (0.31, 0.87) |
| ICS + TXA vs. ICS | 1 | 1.04 (0.07, 16.41) |
| ICS + TXA vs. TXA | 1 | 7.71 (0.43, 137.53) |
Table 51Network 4: Number exposed to allogeneic transfusions
| Comparison | Risk ratio | ||
|---|---|---|---|
| Direct (mean) | NMA (median) | ||
| Versus standard treatment | TXA vs. standard treatment | 0.45 (0.38, 0.52) | 0.2637 (0.1555, 0.7161) |
| PCS vs. standard treatment | 0.58 (0.41, 0.83) | 0.5546 (0.3012, 0.9111) | |
| ICS vs. standard treatment | 0.74 (0.50, 1.12) | 0.7996 (0.2924, 1.466) | |
| ICS+PCS vs. standard treatment | 0.84 (0.54, 1.33) | 0.6637 (0.1941, 1.345) | |
| ICS+PCS+TXA vs. standard treatment | - | 0.2118 (0.02899, 0.9152) | |
| PCS+TXA vs. standard treatment | - | 0.208 (0.02931, 0.8688) | |
| ICS+TXA vs. standard treatment | - | 0.6146 (0.08566, 1.899) | |
| Versus TXA | PCS vs. TXA | - | 1.906 (1.133, 3.492) |
| ICS vs. TXA | - | 2.552 (1.102, 7.319) | |
| ICS+PCS vs. TXA | - | 2.091 (0.7929, 6.512) | |
| ICS+PCS+TXA vs. TXA | 0.73 (0.33, 1.63) | 0.7393 (0.1268 , 3.145) | |
| PCS+TXA vs. TXA | - | 0.7304 (0.1222, 2.971) | |
| ICS+TXA vs. TXA | - | 1.866 (0.3508, 9.221) | |
| Versus PCS | ICS vs. PCS | - | 1.315 (0.5614, 3.565) |
| ICS+PCS vs. PCS | 0.70 (0.42, 1.16) | 1.114 (0.3976, 2.982) | |
| ICS+PCS+TXA vs. PCS | - | 0.3891 (0.05739, 1.652) | |
| PCS+TXA vs. PCS | 0.37 (0.12, 1.14) | 0.383 (0.06477, 1.323) | |
| ICS+TXA vs. PCS | - | 1.036 (0.1687, 4.425) | |
| Versus ICS | ICS+PCS vs. ICS | - | 0.8597 (0.2174, 2.754) |
| ICS+PCS+TXA vs. ICS | - | 0.2875 (0.03492, 1.381) | |
| PCS+TXA vs. ICS | - | 0.2833 (0.03496, 1.263) | |
| ICS+TXA vs. ICS | 0.78 (0.50, 1.20) | 0.7911 (0.1602, 2.309) | |
| Versus ICS+PCS | ICS+PCS+TXA vs. ICS+PCS | - | 0.3499 (0.04127, 1.872) |
| PCS+TXA vs. ICS+PCS | - | 0.3449 (0.0427, 1.657) | |
| ICS+TXA vs. ICS+PCS | - | 0.937 (0.1237, 5.031) | |
| Versus ICS+PCS+TXA | PCS+TXA vs. ICS+PCS+TXA | - | 0.9854 (0.09827, 9.512) |
| ICS+TXA vs. ICS+PCS +TXA | - | 2.528 (0.2924, 28.61) | |
| Versus PCS+TXA | ICS+TXA vs. PCS +TXA | - | 2.583 (0.3143, 28.64) |
Table 52Network 5: Units of allogeneic blood transfused
| Comparison | Mean difference | ||
|---|---|---|---|
| Direct (mean) | NMA (median) | ||
| Versus standard treatment | TXA vs. standard treatment | -0.88 (-1.22, -0.54) | -0.9028 (-1.397, -0.4369) |
| PCS vs. standard treatment | -0.82 (-1.31, -0.33) | -0.8217 (-1.364, -0.2834) | |
| ICS+PCS vs. standard treatment | 0.81 (0.49, 1.13) | 1.11(-0.1026, 2.313) | |
| Versus TXA | PCS vs. TXA | - | 0.0816(-0.6285, 0.8177) |
| ICS+PCS vs. TXA | - | 2.013(0.7254, 3.317) | |
| Versus PCS | ICS+PCS vs. PCS | 2.23 (1.92, 2.54) | 1.932(0.7209, 3.136) |
Table 53Results of pairwise meta-analysis
| Comparison | No. of studies | Effect size (Relative risk/Mean difference) |
|---|---|---|
| Outcome: Units of allogeneic blood | ||
| ICS +TXA vs. ICS | 1 | -0.46 (-1.10, 0.18) |
| Outcome: Infections | ||
| PCS vs. standard treatment | 2 | 1.79 (0.53, 6.07) |
| ICS+PCS vs. standard treatment | 1 | 3.32 (0.14, 79.77) |
| TXA vs. standard treatment | 3 | 0.93 (0.22, 3.93) |
| Outcome: Length of stay | ||
| PCS vs. standard treatment | 3 | -0.37( -1.73, 0.99) |
| ICS+PCS vs. standard treatment | 1 | 0.20 (-0.20, 0.60) |
| ICS +TXA vs. ICS | 1 | 0.72 (-0.85, 2.29) |
| TXA vs. standard treatment | 10 | -0.25 (-0.59, 0.09) |
| Outcome: Mortality | ||
| TXA vs. standard treatment | 3 | 0.73 (0.15, 3.66) |
| ICS+PCS vs. standard treatment | 1 | 3.32 (0.14, 79.77) |
| Outcome: Thrombotic complications | ||
| PCS+TXA vs. PCS | 1 | 0.2 (0.01, 4.06) |
| TXA vs. standard treatment | 22 | 0.69(0.44, 1.07) |
Table 54Results of pair wise meta-analysis
| Comparison | Outcome | No. of studies | Effect size (Relative risks/Mean difference) |
|---|---|---|---|
| TXA vs. standard treatment | No. exposed to allogeneic blood | 2 | 0.83 (0.30, 2.29) |
| Blood loss (log odds ratio) | 3 | 0.74 (0.73, 0.76) |
Table 55Results of pair wise meta-analysis
| Comparison | Outcome | No. of studies | Effect size (Relative risks/Mean difference) |
|---|---|---|---|
| ICS +TXA vs. ICS | No. exposed to allogeneic blood | 1 | 0.85 (0.56, 1.30 |
| Total blood transfused | 1 | -325.00(-685.06, 35.06) | |
| Total blood loss | 1 | -855.00 (-1408.15, -301.85) | |
| Length of stay | 1 | 0.10 (-0.37, 0.57) | |
| TXA vs. standard treatment | Post-operative blood loss | 2 | -12.62 (-16.79, -8.45) |
| Length of stay | 1 | 0.1 (-0.37, 0.57) |
Table 56Economic evidence profile: cell salvage versus no cell salvage
| Study | Applicability | Limitations | Other comments | Incremental cost | Incremental effects (mean per patient) | Cost-effectiveness | Uncertainty |
|---|---|---|---|---|---|---|---|
| Davies 200678 (UK) | Partially applicable(a) | Minor limitations(b) | Decision tree depicting adult elective non-urgent major surgery patients (orthopaedic, cardiac and vascular) receiving either cell salvage (intra-operative or post-operative) or no cell salvage. All patients who receive a transfusion have a risk of transfusion or surgical complications and transfusion complications. For allogeneic blood transfusion there is a risk of transfusion transmitted infections. For cell salvage transfusion there is a risk of cell salvage transfusion complications. | Saves £76(c) | 0.00477 QALYs(d) | Cell salvage is dominant | A probabilistic sensitivity analysis was conducted for the base case. Probability cell salvage cost-effective (30K threshold): 91% Additional analyses were conducted to explore the impact on results of using different structural variables or data sets. Results indicate that in cardiac surgery, washed intra-operative cell salvage was more likely to be cost-effective than unwashed post-operative cell salvage. In orthopaedic surgery, unwashed post-operative cell salvage was more likely to be cost-effective than washed intra-operative cell salvage. |
| Klein 2008169 (UK) | Partially applicable(e) | Potentially serious limitations(f) | Within trial analysis (RCT) of adults undergoing non-emergency first time coronary artery bypass grafting and / or cardiac valve surgery receiving either cell salvage (intra-operative and post-operative) or no cell salvage. Analysis of individual level resource use, with unit costs applied. | £477(g) | Saves 0.42, 0.725 and 1.63 units of allogeneic red blood cells, fresh frozen plasma and platelets transfused, respectively. No difference in percentage receiving allogeneic blood product transfusions(h) | Cell salvage is more costly and more effective at reducing units of allogeneic blood components transfused | No analysis reported |
| Samnaliev 2013266 (USA) | Partially applicable (i) | Potentially serious limitations (j) | Decision tree depicting paediatric orthopaedic and cardiac surgical patients receiving either intra-operative cell salvage or no cell salvage. All patients who receive an allogeneic transfusion have a risk of transfusion-related adverse events. | Saves £387 (k) | 51% fewer patients transfused. Saves 1.096 units of allogeneic red blood cells (per patient transfused) (l) | Intra-operative cell salvage is dominant | Probabilistic sensitivity analysis conducted around costs (CI: £15 to £1,262). Subgroup analyses by surgery type (cardiac and orthopaedic). Intervention 2 remains dominant. A series of threshold analysis were conducted to estimate the maximum cost of cell salvage per patient that would still result in cell salvage being cost-saving:
|
- a
Study does not include all interventions in protocol.
- b
Model used data for resource use as well as effectiveness from clinical trials that were mostly outside the UK. Effectiveness of transfusion strategies included older technologies (systematic review included studies from 1979) that may be less effective than the newer technologies used to estimate costs (2003-2004). No discounting was reported in the sensitivity analyses where a ten and 30 year time horizon was applied.
- c
2003-2004 UK pounds. Costs incorporated are cost per case of washed cell salvage equipment, maintenance, consumables and staff (base case); transfusion and transfusion-related services; operation and index hospital admission; and adverse events (surgical and transfusion-related).
- d
Probability of transfusion, risks of adverse events related to transfusion or surgery from published systematic reviews and the authors own systematic review. Utility values for health states with long-standing illness, limiting illness and no long-standing illness from the 1996 Health Survey for England as well as a utility value for stroke was taken from published literature. Tariffs not specified.
- e
Health effects not expressed as QALYs. Study does not include all interventions in protocol.
- f
Follow up for health outcomes and cost is not the same and no analysis of uncertainty conducted. Follow up for health outcomes and cost is not the same and no analysis of uncertainty conducted
- g
2006-2007 US dollars converted into UK pounds using the purchasing power parities 234. Costs incorporated are: cost of operation room, intensive care unit stay, ward stay, adverse events, red blood cells, other blood components (fresh frozen plasma and platelets), cell salvage equipment, primary care visits and medication.
- h
Mean volume of allogeneic blood components transfused and proportion of patients transfused from within trial.
- i
Health effects not expressed as QALYs. US healthcare payer perspective. Study does not include all interventions in protocol.
- j
Effectiveness data from a non-randomised study which was not included in clinical review and therefore does not reflect full body of evidence. Baseline transfusion rates based on assumptions. Rate of discounting not reported.
- k
2010 US dollars converted into UK pounds using the purchasing power parities.234 Costs incorporated are: Cost of cell salvage (including disposables and supplies, cell salvage technician, paediatric anaesthetists), allogeneic transfusion (unit of red blood cells and blood processing including staff time), and costs of transfusion related adverse events.
- l
Mean volume of allogeneic blood components transfused and proportion of patients transfused based on data for all paediatric surgical patients who were eligible for cell salvage at the Boston Children's Hospital in 2010. Baseline mean volume of allogeneic blood components transfused and proportion of patients transfused based on assumption that number of salvaged units of red blood cells replaces an equivalent amount of units that would have been transfused in absence of cell salvage. Probabilities of transfusion related events from various sources (published literature, national data).
Table 57Economic evidence profile: Tranexamic acid versus no tranexamic acid or placebo
| Study | Applicability | Limitations | Other comments | Incremental cost | Incremental effects (mean per patient) | Cost-effectiveness | Uncertainty |
|---|---|---|---|---|---|---|---|
| Rajesparan 2009251 (UK) | Partially applicable(a) | Potentially serious limitations(b) | Within trial analysis (RCT) of adults undergoing total hip replacement. Analysis of individual level resource use with unit costs applied. TXA administered intravenously. | Saves £54(c) | Saves 0.1 units of allogeneic blood transfused (per patient transfused)(d) | Tranexamic acid is dominant | No analysis reported |
| Alshryda 2013A9 (UK) | Partially applicable(e) | Potentially serious limitations(f) | Within trial analysis (RCT) of adults undergoing total hip replacement. Analysis of individual level resource use with unit costs applied. TXA administered topically (intra-articular) | Saves £305(g) | Saves 0.46 units of allogeneic blood transfused (per patient transfused)(h) | Tranexamic acid is dominant | Bootstrapping of costs. Tranexamic acid saves £304 (CI -613 to -15, p=0.046) |
- a
Health effects not expressed in terms of QALYs. Study does not include all interventions in protocol.
- b
Short follow up which does not account for impact of potential risks and costs associated with transfusion related adverse events and illness, costs do not account for resource use such as staff time or for treatment of thrombotic complications and no analysis of uncertainty conducted.
- c
UK pounds, year NR, assumed to be 2009 UK pounds based on the date publication submission. Resource use from within study. Unit cost of allogeneic blood and cost of tranexamic acid from institution.
- d
Other health outcomes presented and available in full evidence table. Percentage requiring allogeneic blood transfusion, volume transfused, blood loss and rate of clinical post-operative deep vein thrombosis confirmed by venography from within study.
- e
Health effects not expressed in terms of QALYs. Study does not include all interventions in protocol.
- f
Short follow up which does not account for impact of potential risks and costs associated with transfusion related adverse events and illness. Costs do not account for resource use such as staff time and the cost of complications.
- g
2010 UK pounds. Resource use from within study. Resource use from within study. Source of unit cost of allogeneic blood, hospital stay per diem and cost of tranexamic acid not reported but likely to be from institution. Cost of complications and staff time not estimated.
- h
Other health outcomes presented and available in full evidence table. Percentage requiring allogeneic blood transfusion and volume transfused, blood loss, length of stay, complications including deep vein thrombosis, EQ-5D from within study.
Table 58Intervention costs
| Intervention | Cost | Source |
|---|---|---|
| ICS | £295 | PSSRU 2013,72 NHS Supply Chain Catalogue April 2014,223 BNF 67,150 NICE Clinical Guideline CG174,210 Crotty 200670 |
| PCS | £88 | PSSRU 2013,72 NHS Supply Chain Catalogue April 2014223 |
| ICS+PCS | £350 | PSSRU 2013,72 NHS Supply Chain Catalogue April 2014,223 BNF 67,150 NICE Clinical Guideline CG174,210 Crotty 200670 |
| TXA (high risk subgroup) | £19 | Total dose 6000 mg, slow IV injection followed by continuous IV infusion. BNF 67,150 eMIT July 201,462 NHS Supply Chain Catalogue April 2014,223 NICE Clinical Guideline CG174210 |
| TXA (moderate risk subgroup) | £9 | Total dose 3000 mg slow IV injection. BNF 67,150 eMIT July 201462 |
| ICS +TXA | £314 | Sum of ICS and TXA (high risk subgroup) |
Abbreviations: BNF = British National Formulary; eMIT = Electronic Market Information Tool; ICS = intra-operative cell salvage; IV = intravenous; PSSRU = Personal Social Services Research Unit; PCS = post-operative cell salvage; TXA = tranexamic acid
Table 59Base case analysis results (probabilistic analysis), cost-effectiveness, high risk
| Analysis | Incremental QALYs vs ST | Incremental costs vs ST | INMB at £20K(a) | Probability most CE option | Rank (95% CI) |
|---|---|---|---|---|---|
| ST | £0 | 0% | 3 (3, 5) | ||
| ICS | 0.000 | £104 | -£102 | 0% | 4 (3, 5) |
| PCS | 0.005 | -£2,815 | £2,908 | 28% | 2 (1, 2) |
| TXA | 0.190 | -£212 | £4,009 | 72% | 1 (1, 2) |
| ICS+TXA | 0.000 | £295 | -£303 | 0% | 5 (3, 5) |
Abbreviations: CE = cost-effective; CI = confidence intervals; ICS = intra-operative cell salvage; INMB = incremental net monetary benefit; PCS = post-operative cell salvage; QALYs = quality adjusted life years; ST = standard treatment; TXA = tranexamic acid.
- a
INMB = NMB intervention A – NMB ST; Highest INMB = most cost-effective option at a £20,000 per QALY threshold; a negative INMB means that ST is more cost-effective than this option.
Table 60Base case analysis results (probabilistic analysis), cost-effectiveness, moderate risk
| Analysis | Incremental QALYs vs ST | Incremental costs vs ST | INMB at £20K(a) | Probability most CE option | Rank (95% CI) |
|---|---|---|---|---|---|
| ST | £0 | 0% | 3 (2, 3) | ||
| ICS+PCS | 0.000 | £420 | -£423 | 0% | 4 (4, 4) |
| PCS | 0.000 | -£108 | £113 | 40% | 2 (1, 3) |
| TXA | 0.000 | -£169 | £173 | 60% | 1 (1, 2) |
Abbreviations: CI = confidence intervals; ICS = intra-operative cell salvage; INMB = incremental net monetary benefit; PCS = post-operative cell salvage; QALYs = quality adjusted life years; ST = standard treatment; TXA = tranexamic acid
- a
INMB = NMB intervention A – NMB ST; Highest INMB = most cost-effective option at a £20,000 per QALY threshold; a negative INMB means that ST is more cost-effective than this option.