Parasitic protozoans and helminths (worms) synthesize glycans with structures often different from those typically found in vertebrates and are typically antigenic. Parasites also express glycan-binding proteins (GBPs) involved in host invasion and parasitism. As part of the disease process, parasite glycans can trigger the host's innate immune system, which can lead to the induction of adaptive immune responses. This chapter discusses the major roles of glycoconjugates in parasitic infections.
BACKGROUND ON PARASITIC INFECTIONS
Parasitism is a condition in which one organism (the parasite) in some way lives at the expense of the host. Parasites infect millions of people worldwide and cause much suffering and death, especially in underdeveloped countries (Table 43.1). Like other types of infections (e.g., bacterial or viral; Chapter 42), parasite GBPs interact with host glycomes and parasite glycans interact with host GBPs and antibodies. Thus, research on parasite glycobiology and biochemistry could provide therapeutics for reducing disease morbidity and mortality. Moreover, studies on the molecular pathology of organisms, such as parasites that have evolved to deceive and compromise the host immune system, provide novel insights into the regulation of human innate and adaptive immune responses.
The majority of parasitic diseases are divided into two categories: those caused by protozoans (single-celled organisms; Table 43.2) and those caused by helminths (worms/metazoans; Table 43.3). The major classes of protozoan parasites include Plasmodium (causing malaria), Entamoeba histolytica (causing amebiasis), Leishmania (causing leishmaniasis), and Trypanosoma (causing sleeping sickness and Chagas’ disease). Parasitic worms include trematodes (ex. Schistosoma mansoni, causing schistosomiasis), nematodes (ex. Ascaris lumbricoides), and cestodes or tapeworms (ex. Taenia solium, causing taeniiasis). Worms are very large relative to host cells. Thus, most worms live in the extracellular spaces of their hosts and have evolved a variety of infective and protective strategies. Glycoconjugates are important in both the life cycles and pathology of most major parasites. For example, many parasitic protozoans and helminths have elaborated intriguing mechanisms to target GBPs (Chapter 29) or glycans in the host to promote parasitism, and to evade host immune responses.
PLASMODIA
Malaria is caused by Plasmodium species, prominently Plasmodium falciparum in humans. Malarial parasites lead a complicated life cycle, alternating between a sexual reproduction stage in the female Anopheles mosquito vector and an asexual reproduction stage in mammalian tissues (hepatocytes and erythrocytes) and the bloodstream (Figure 43.1). Cell–cell interactions between the parasite and host are critical for the successful completion of each stage.
Following inoculation into the bloodstream, the sporozoite's major circumsporozoite protein interacts with heparan sulfate (HS) on the surface of hepatocytes, which allows invasion and the first replication in the mammalian host. Liver HS possesses an unusually high degree of sulfation compared with similar glycosaminoglycans from other organs, suggesting the basis for the selective targeting of Plasmodium to hepatocytes.
Upon exit from the liver, Plasmodium merozoites use multiple ligand–receptor interactions to invade host erythrocytes. The merozoite proteins, such as the erythrocyte-binding-like (EBL) proteins (Table 43.4), vary in their dependency on sialic acid residues on the erythrocyte surface and play an important role in erythrocyte invasion.
The glycophorins are the major sialic acid–containing glycoproteins on erythrocytes. The EBL protein EBA-175 (erythrocyte-binding antigen-175) recognizes clusters of sialylated O-glycans attached to glycophorin A, particularly within a 30-amino-acid region that carries 11 O-glycans. Desialylation of erythrocytes precludes interactions of some strains of P. falciparum, and individuals lacking glycophorins A or B are refractory to invasion. Some strains of P. falciparum can reversibly switch from sialic acid–dependent to sialic acid–independent invasion, and this has important implications for vaccine design against malaria parasites.
Pregnancy-associated malaria is a major cause of suffering and arises when P. falciparum–infected erythrocytes are sequestered in the placenta of pregnant women. The parasite expresses the VAR2CSA protein, which mediates adherence of the infected erythrocytes to placental chondroitin sulfate A (CSA) with very high specificity and affinity (Kd ∼ 15 nm), promoting parasite infection and invasion.
Eruption of the merozoites from infected erythrocytes releases free glycosylphosphatidylinositols (GPIs), which are prominent virulence factors contributing to malaria pathogenesis (Chapter 12). P. falciparum GPIs mimic host GPIs and activate host GPI-associated signaling pathways. The GPIs can activate host's macrophages, mainly through Toll-like receptor-2 signaling, leading to the production of inflammatory cytokines and up-regulation of cell adhesion molecules, such as E-selectin in endothelial cells. Antibodies to these GPIs can neutralize their effects and mitigate the pathology of the disease independently of direct infection.
In addition, the P. falciparum genome encodes a number of enzymes involved in N-glycosylation, O-glycosylation (O-fucosylation), and C-mannosylation. Although N-glycans are truncated and typically contain only a chitobiosyl core, GlcNAcβ1-4GlcNAcβ-Asn, mannose is abundantly present in GPI anchors. There is also evidence suggesting that Plasmodia may express a number of unusual glycoconjugates, such as galactose- and glucose-containing glycoproteins and glucose-containing glycolipids.
TRYPANOSOMES
African trypanosomes (Trypanosoma brucei) transmitted by blood-sucking tsetse flies are the etiologic agents of nagana disease in cattle and sleeping sickness in humans. A remarkable feature of these organisms is their ability to survive extracellularly in the host bloodstream, where they are constantly exposed to the immune system. Evasion of the host immune response depends on “antigenic variation,” a highly evolved survival strategy that relies on structural variance of GPI-anchored glycoproteins (variant surface glycoproteins [VSGs]) on the trypanosome surface (Figure 43.2). VSGs are dimeric proteins, consisting of two 55-kDa monomers that each carry N-linked oligomannose-type glycans, which make up a large component of the dense glycocalyx. As parasites multiply in the host bloodstream, the host mounts an immune response that is effective against only the population of trypanosomes expressing a particular VSG. Trypanosomes that have switched to an alternative VSG coat (encoded among 1000 distinct VSG genes) escape immunological destruction.
Within the gut of the tsetse fly, the trypanosome replaces its entire VSG coat with acidic glycoproteins called procyclins (Figure 43.2). These GPI-anchored proteins form a dense glycocalyx and are composed of polyanionic polypeptide repeat domains projecting from the membrane. Unusual features are the presence of a single type of N-glycan (Man5GlcNAc2) and GPI anchors that are modified with branched poly-N-acetyllactosamine [Galβ1-4GlcNAc]n glycans. The terminal β-galactose can be substituted with α2-3-linked sialic acid by a parasitic trans-sialidase that transfers sialic acid from host glycoconjugates to the parasite surface. This sialylation protects the parasite in the insect intestine and can compromise the human immune system. Among parasites, genes encoding trans-sialidases have only been found in the protozoan genus Trypanosoma and occur in both T. brucei and Trypanosoma cruzi. Interestingly, there is also evidence for trans-sialidase activities in some bacteria and in human serum, but these are far less well studied.
T. cruzi, transmitted by reduviid bugs, is the etiologic agent of Chagas’ disease, or South American trypanosomiasis. T. cruzi has a dense coat of glycosylinositol phospholipids (GIPLs) (Chapter 12) and mucins (Chapter 10) that project above the GIPL layer (Figure 43.3). The GIPLs contain the same basic structure as other GPI anchors, except that they are heavily substituted with Gal, GlcNAc, and host-derived sialic acid. The mucins contain large amounts of O-glycans composed of a serine- or threonine-linked GlcNAc extended with one to five Gal residues that can be substituted with sialic acid by a trans-sialidase. For T. cruzi sialylation is believed to reduce the susceptibility of the parasite to anti-α-Gal antibodies that are normally present in the mammalian bloodstream.
T. cruzi also express lipopeptidophosphoglycan (LPPG), the major surface glycan of the insect stage of the parasite (Figure 43.3). Depending on the life-cycle stage, LPPG is composed of an inositolphosphoceramide-anchored glycan or an alkylacylphosphatidylinositol-anchored glycan that includes nonacetylated glucosamine, mannose, galactofuranose, and 2-aminoethylphosphonate (2-AEP). The lack of ceramide anchors and galactofuranose in mammalian cells suggests potential targets for the development of chemotherapeutic agents.
LEISHMANIA
Leishmania causes different forms of leishmaniasis, which manifest clinically in three forms: cutaneous, mucocutaneous, and visceral—the latter being fatal if untreated. Leishmania parasites have a remarkable capacity to avoid destruction in the hostile environments they encounter during their life cycle, alternating between intracellular macrophage parasitism and extracellular life in the gut of their sandfly vector (Figure 43.4).
Stage-specific adhesion is mediated by structural variation of the abundant cell-surface glycoconjugate lipophosphoglycan (LPG), which contributes to parasite survival in the hydrolytic midgut (Figure 43.5). The basic LPG structure in all Leishmania species consists of a 1-O-alkyl-2-lyso-phosphatidyl(myo)inositol lipid anchor, a heptasaccharide core, a long phosphoglycan (PG) polymer composed of (-6Galβ1-4Manα 1-PO4-) repeat units and a small oligosaccharide cap. In many species, the PG repeats contain additional substitutions that mediate key roles in stage-specific adhesion. For example, in Leishmania major, the PG repeats bear β1-3-galactosyl side-chain modifications, which provide a binding site for the sandfly midgut galectin, PpGalec. As parasites differentiate in the sandfly from amastigotes into procyclic promastigotes, and finally to infective metacyclic stages, the β1-3 Gal-modified PGs are capped by α1-2 arabinosyl residues, giving rise to a structure that does not bind to PpGalec and thus facilitating detachment of the parasite from the sandfly midgut. LPG is highly abundant on the parasite surface, suggesting a central role for the glycoconjugate in the parasite's infectious cycle, as well as allowing the parasite to establish successful infections in the mammalian host. In addition, galectin-3 in the vertebrate host may recognize some of the L. major glycans as pathogen-associated molecular patterns, facilitating leukocyte responses to infection.
Leishmania also express an abundance of other important glycoconjugates, such as GIPLs, GPI-anchored proteins, and secreted proteophosphoglycans (PPGs) (Figure 43.5) (also see Chapter 12). Most of the serine residues within PPGs are phosphoglycosylated with Gal-Man-PO4- repeats via unique phosphodiester linkages. In Leishmania mexicana, these highly anionic polysaccharides form a gel-like matrix composed of interlocking filaments. These matrices enhance parasite development in the sandfly, and contribute to the formation of a parasitophorous vacuole in macrophages of the mammalian host, where the parasite replicates.
Virtually every known glycoconjugate of Leishmania shows some intersection with the LPG biosynthetic pathway. The presence of molecules bearing similar modifications raises the possibility that they share biosynthetic steps. The early steps in LPG and GPI biosynthesis (up to Man-Man-GlcN-PI) occur in the endoplasmic reticulum, and galactosylation of the LPG glycan core and assembly of the PG repeats occurs in the Golgi apparatus (Chapter 12). The phosphoglycan portions of LPG and PPG are assembled by the sequential and alternating addition of mannose-PO4- and galactose, forming the characteristic -Galβ1-4Manα1-PO4- repeats. Depending on the species of Leishmania, additional branching sugars can then be added, creating a remarkable array of side chains that drive Leishmania–sandfly vectorial competence.
ENTAMOEBA
The life cycle of Entamoeba histolytica, which causes amoebic dysentery and hepatic abscesses, includes the disease-inducing amebic or trophozoite stage and the infectious cyst stage. Mature cysts are ingested by an animal, and once inside the host gut, excystation produces the trophozoite that causes pathology. The trophozoites can replicate or form cysts, which are passed via feces to complete the cycle. E. histolytica expresses several lectins, among them a Gal/GalNAc lectin, a GPI-anchored heterodimeric glycoprotein. This lectin mediates binding of the parasite to colonic mucins and plays a critical role in parasite viability, and is a promising vaccine candidate against the disease. The Entamoeba cyst wall is composed mostly of chitin, and chitin-binding lectins (including the Jacob and Jessie lectins) accompanied by remodeling via chitinases, help to create the cyst wall.
Entamoeba trophozoites synthesize cell-surface LPG and LPPG (Figure 43.6). The LPG consists of a lipid anchor and a phosphoglycan component that resembles the phosphoglycans of Leishmania LPG. The LPPG GPI anchors are unique in that they have a glycan backbone that contains the sequence Gal1Man2GlcN-(myo)inositol, in which α-Gal replaces the terminal α1-2 mannose residue found in typical protein anchors. Leishmania LPG and LPPG are important as virulence factors, because antibodies raised against these molecules inhibit the ability of the parasites to kill target cells. Thus, such glycans may be the target for vaccine development.
SCHISTOSOMA
Schistosomiasis, a major parasitic disease associated with a high morbidity worldwide is caused by parasitic trematodes. Three major species infect humans: Schistosoma japonicum, S. mansoni, and Schistosoma haematobium (Figure 43.7). Schistosoma is unique among helminths in that the male and female worms pair to live together in the blood vessels of their host. The female lays eggs about 4–6 weeks after infection, which adhere to the endothelium and migrate through vessels into tissues. The eggs may become lodged in the host tissues, where they sequester and induce granulomatous inflammatory responses. Eggs trapped in the peripheral circulation can cause portal hypertension and fibrosis, which are characteristic of chronic schistosomiasis and lead to morbidity and often death. However, many eggs eventually pass into the stool and continue the cycle through intermediate snail hosts, which are unique for each Schistosoma species. Thus, the geographical limitation to the spread of this disease is the range of the intermediate host snails.
Schistosomes, especially the cercarial glycocalyx and eggs, generate huge quantities of membrane-bound glycoproteins and glycosphingolipids. Many glycoproteins derived from the tegument, gut, and eggs of the parasite are highly antigenic and occur in the circulation of the infected animal. These proteins are generally rich in complex glycan structures, and contain an impressive array of O- and N-glycans. Fucosylated antigens are a common feature of most schistosome glycoconjugates, including Lewis x (Lex), LacdiNAc (LDN), and fucosylated LacdiNAc (LDNF) structures, and polyfucose branches (Figure 43.8). Glycoconjugates from helminths lack sialic acids.
The glycoconjugate structures expressed among schistosome species show some variation, but much less than between schistosomes and other helminths. For example, S. mansoni glycosphingolipids have extended difucosylated oligosaccharides, but these structures are absent in S. japonicum glycosphingolipids. In addition to schistosomes, Dictyocaulus viviparous, a parasitic nematode in cattle, is one of the few helminths to synthesize the Lex antigen. In addition to schistosomes, Echinococcus granulosus, Dirofilaria immitis, and Haemonchus contortus synthesize glycoproteins containing LDN and LDNF, in addition to other fucosylated and xylosylated glycans (Figure 43.8). The expression of many of these glycan structures is developmentally regulated and stage-specific, but their fundamental roles in parasite development and host pathogenesis are mostly unclear.
There is mounting evidence that the glycan antigens expressed by schistosomes can modulate innate and adaptive immune responses. Individuals infected with Schistosoma species develop a wide variety of antibodies to glycan antigens, which may provide partial protection against subsequent infections. A general feature of helminth infections, including chronic schistosome infections, is that T helper 2 (Th2) immune responses (promoting humoral immunity) predominate over Th1 responses (promoting cellular immunity). Such Th2-driven responses also contribute to the generation of alternatively activated macrophages with wound-healing and anti-inflammatory properties. The induction of an anti-inflammatory immune response facilitates survival of the parasites in their hosts, but also benefits the host by reducing unrelated inflammatory responses (e.g., in autoimmune diseases). Worm-derived molecules, possibly species-dependent and including glycoconjugates, play a role in such modulation of the host immune response. Helminths, and their secreted products, are currently being evaluated as novel therapy for chronic inflammatory disorders such as Crohn's disease and multiple sclerosis.
Schistosome glycans are recognized by antigen-presenting cells such as dendritic cells and macrophages via GBPs, in particular C-type lectins (Chapter 34), including pattern-recognition receptors such as DC-SIGN (dendritic cell–specific intercellular adhesion molecule-3-grabbing nonintegrin) and the mannose receptor. For immune responses to many helminths, collectins and surfactant protein D help to limit the pathology of infection. Internalization and processing of parasite glycoconjugates by dendritic cells can lead to polarization of adaptive immune responses (Chapter 34). The identification of many antigenic glycoconjugate structures from schistosomes is helping in the design of new diagnostic approaches for schistosomiasis. Because antibody responses to schistosomes are mainly directed against antigenic glycan motifs, and antibodies to such glycans can kill and inhibit parasite growth and egg deposition, such glycans may be targets of vaccine development. The characterization of schistosome glycosyltransferases responsible for antigenic glycan biosynthesis, together with enzymes from the intermediate snail hosts, may also help in the identification of new drug targets and the development of glycan-based vaccines.
GLYCOBIOLOGY OF OTHER PARASITES
Glycoconjugates and GBPs, as discussed above, play an impressive role in host infection by many different parasites. Several additional examples are shown in Table 43.4. Many protozoan parasites appear to use GBPs as a major mechanism for host-cell attachment and invasion. For example, Acanthamoeba keratitis, which causes severe eye infections involving the corneal epithelium, adheres to host cells via a lectin-glycan interaction and precludes amoeba-induced cytolysis of target cells. Adhesion is mediated by a mannose-binding protein, which is strongly inhibited by Manα1-3Man disaccharides. Furthermore, mannose and mannose-6-PO4- can inhibit adhesion of Giardia lamblia trophozoites, and these glycans may be recognized by the parasite's antigenic protein taglin. Other antigenic proteins, including several galectins, have been cloned from parasitic nematodes including Teladorsagia circumcincta. Sporozoites from Cryptosporidium parvum, an opportunistic protozoan that infects individuals with compromised immunity, have hemagglutinating activity, and a lectin on the parasite surface may play a crucial role in host-cell attachment.
In addition, the glycan antigens of many parasites are being characterized in an effort to develop vaccines and new diagnostics for the diseases they cause. For example, the major antigenic glycoconjugates synthesized by larvae of the parasitic nematodes Toxocara canis and Toxocara cati are O-methylated trisaccharides that contain 2-O-methyl fucosyl and galactosyl residues (Chapter 25). The intestinal nematode Trichinella spiralis synthesizes several highly immunogenic glycoproteins carrying complex N-glycans containing the unusual sugar tyvelose (3,6-dideoxy-D-arabino-hexose). This structure is critical to induce a strong antibody response to these glycans, which causes expulsion of the invading larvae from the intestine and results in protective immunity.
ACKNOWLEDGMENTS
The authors acknowledge contributions to previous versions of this chapter by Salvatore Turco and helpful input from Jennifer Groves.
FURTHER READING
- Camus D, Hadley TJ. 1985. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230: 553–556. [PubMed: 3901257]
- Farthing MJ, Pereira ME, Keusch GT. 1986. Description and characterization of a surface lectin from Giardia lamblia. Infect Immun 51: 661–667. [PMC free article: PMC262400] [PubMed: 3943906]
- Khoo KH, Maizels RM, Page AP, Taylor GW, Rendell NB, Dell A. 1991. Characterization of nematode glycoproteins: The major O-glycans of Toxocara excretory–secretory antigens are O-methylated trisaccharides. Glycobiology 1: 163–171. [PubMed: 1823159]
- Parodi AJ. 1993. N-glycosylation in trypanosomatid protozoa. Glycobiology 3: 193–199. [PubMed: 8358146]
- Reason AJ, Ellis LA, Appleton JA, Wisnewski N, Grieve RB, McNeil M, Wassom DL, Morris HR, Dell A. 1994. Novel tyvelose-containing tri- and tetra-antennary N-glycans in the immunodominant antigens of the intracellular parasite Trichinella spiralis. Glycobiology 4: 593–603. [PubMed: 7881173]
- Schenkman S, Eichinger D, Pereira ME, Nussenzweig V. 1994. Structural and functional properties of Trypanosoma trans-sialidase. Annu Rev Microbiol 48: 499–523. [PubMed: 7826016]
- Hoessli DC, Davidson EA, Schwarz RT, Nasir-ud-Din. 1996. Glycobiology of Plasmodium falciparum: An emerging area of research. Glycoconj J 13: 1–3. [PubMed: 8785480]
- Mengeling BJ, Beverley SM, Turco SJ. 1997. Designing glycoconjugate biosynthesis for an insidious intent: Phosphoglycan assembly in Leishmania parasites. Glycobiology 7: 873–880. [PubMed: 9363429]
- Cummings RD, Nyame AK. 1999. Schistosome glycoconjugates. Biochim Biophys Acta 1455: 363–374. [PubMed: 10571025]
- Dell A, Haslam SM, Morris HR, Khoo KH. 1999. Immunogenic glycoconjugates implicated in parasitic nematode diseases. Biochim Biophys Acta 1455: 353–362. [PubMed: 10571024]
- Ferguson MA. 1999. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci 112: 2799–2809. [PubMed: 10444375]
- Loukas A, Maizels RM. 2000. Helminth C-type lectins and host–parasite interactions. Parasitol Today 16: 333–339. [PubMed: 10900481]
- McConville MJ, Menon AK. 2000. Recent developments in the cell biology and biochemistry of glycosylphosphatidylinositol lipids. Mol Membr Biol 17: 1–16. [PubMed: 10824734]
- Guha-Niyogi A, Sullivan DR, Turco SJ. 2001. Glycoconjugate structures of parasitic protozoa. Glycobiology 11: 45R–59R. [PubMed: 11358874]
- Petri WA Jr, Haque R, Mann BJ. 2002. The bittersweet interface of parasite and host: Lectin–carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu Rev Microbiol 56: 39–64. [PubMed: 12142490]
- Nyame AK, Lewis FA, Doughty BL, Correa-Oliveira R, Cummings RD. 2003. Immunity to schistosomiasis: Glycans are potential antigenic targets for immune intervention. Exp Parasitol 104: 1–13. [PubMed: 12932753]
- Naderer T, Vince JE, McConville MJ. 2004. Surface determinants of Leishmania parasites and their role in infectivity in the mammalian host. Curr Mol Med 4: 649–665. [PubMed: 15357214]
- Nyame AK, Kawar ZS, Cummings RD. 2004. Antigenic glycans in parasitic infections: Implications for vaccines and diagnostics. Arch Biochem Biophys 426: 182–200. [PubMed: 15158669]
- Previato JO, Wait R, Jones C, DosReis GA, Todeschini AR, Heise N, Previato LM. 2004. Glycoinositolphospholipid from Trypanosoma cruzi: Structure, biosynthesis and immunobiology. Adv Parasitol 56: 1–41. [PubMed: 14710995]
- Tolia NH, Enemark EJ, Sim BK, Joshua-Tor L. 2005. Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell 122: 183–193. [PubMed: 16051144]
- Petri WA Jr, Chaudhry O, Haque R, Houpt E. 2006. Adherence-blocking vaccine for amebiasis. Arch Med Res 37: 288–291. [PubMed: 16380334]
- Jang-Lee J, Curwen RS, Ashton PD, Tissot B, Mathieson W, Panico M, Dell A, Wilson RA, Haslam SM. 2007. Glycomics analysis of Schistosoma mansoni egg and cercarial secretions. Mol Cell Proteomics 6: 1485–1499. [PubMed: 17550893]
- Debierre-Grockiego F, Schwarz RT. 2010. Immunological reactions in response to apicomplexan glycosylphosphatidylinositols. Glycobiology 20: 801–811. [PubMed: 20378610]
- van Die I, Cummings RD. 2010. Glycan gimmickry by parasitic helminths: A strategy for modulating the host immune response? Glycobiology 20: 2–12. [PubMed: 19748975]
- Schauer R, Kamerling JP. 2011. The chemistry and biology of trypanosomal trans-sialidases: Virulence factors in Chagas disease and sleeping sickness. Chembiochem 12: 2246–2264. [PubMed: 21956798]
- Frank S, van Die I, Geyer R. 2012. Structural characterization of Schistosoma mansoni adult worm glycosphingolipids reveals pronounced differences with those of cercariae. Glycobiology 22: 676–695. [PubMed: 22241826]
- Tundup S, Srivastava L, Harn DA Jr. 2012. Polarization of host immune responses by helminth-expressed glycans. Ann NY Acad Sci 1253: E1–E13. [PubMed: 22974465]
- Van Diepen A, Van der Velden NS, Smit CH, Meevissen MH, Hokke CH. 2012. Parasite glycans and antibody-mediated immune responses in Schistosoma infection. Parasitol 139: 1219–1230. [PubMed: 22423613]
- Prasanphanich NS, Mickum ML, Heimburg-Molinaro J, Cummings RD. 2013. Glycoconjugates in host–helminth interactions. Front Immunol 4: 24061. [PMC free article: PMC3755266] [PubMed: 24009607]
- Vazquez-Mendoza A, Carrero JC, Rodriguez-Sosa M. 2013. Parasitic infections: A role for C-type lectin receptors. Biomed Res Intl 2013: 456352. [PMC free article: PMC3581113] [PubMed: 23509724]
- Mickum ML, Prasanphanich NS, Heimburg-Molinaro J, Leon KE, Cummings RD. 2014. Deciphering the glycogenome of schistosomes. Front Genet 5: 262. [PMC free article: PMC4122909] [PubMed: 25147556]
- Sato S, Bhaumik P, St-Pierre G, Pelletier I. 2014. Role of galectin-3 in the initial control of Leishmania infection. Crit Rev Immunol 34: 147–175. [PubMed: 24940913]
- Cabezas Y, Legentil L, Robert-Gangneux F, Daligault F, Belaz S, Nugier-Chauvin C, Tranchimand S, Tellier C, Gangneux J-P, Ferrieres V. 2015. Leishmania cell wall as a potent target for antiparasitic drugs. A focus on the glycoconjugates. Org Biomol Chem 13: 8393–8404. [PubMed: 26130402]
- Cova M, Rodrigues JA, Smith TK, Izquierdo L. 2015. Sugar activation and glycosylation in Plasmodium. Malaria J 14: 427. [PMC free article: PMC4628283] [PubMed: 26520586]
- Fleming JO, Weinstock JV. 2015. Clinical trials of helminth therapy in autoimmune diseases: Rationale and findings. Parasite Immunol 37: 277–292. [PubMed: 25600983]
- Fried M, Duffy PE. 2015. Designing a VAR2CSA-based vaccine to prevent placental malaria. Vaccine 33: 7483–7388. [PMC free article: PMC5077158] [PubMed: 26469717]
- Singh RS, Walia AK, Kanwar JR. 2017. Protozoa lectins and their role in host–pathogen interactions. Biotech Adv 34: 1018–1029. [PubMed: 27268207]
Figures
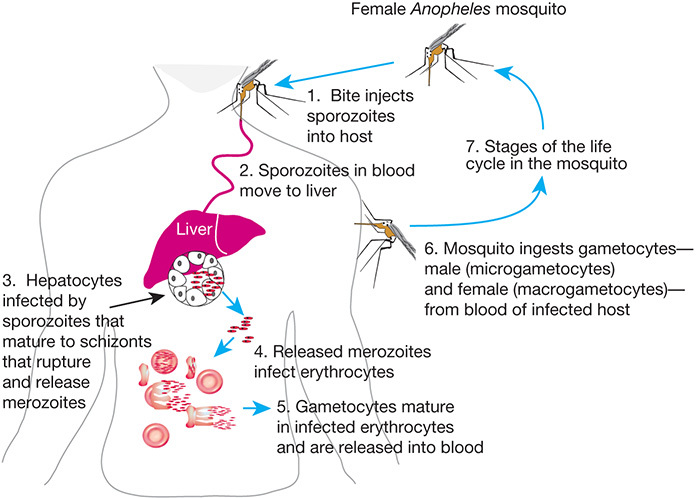
FIGURE 43.1.
Life cycle of Plasmodium falciparum, a parasitic protozoan that causes the most severe form of malaria in humans. The bite of the female Anopheles mosquito introduces sporozoites into the human host, which mature as they travel to the liver and ultimately the bloodstream. After a blood meal from an infected person, the malarial gametocytes enter the midgut of the mosquito where they transform into male microgametes and female macrogametes. Their union leads to a zygote, which transforms into an ookinete, penetrates the intestinal wall of the mosquito, and is transformed into a circular oocyst. Inside the oocyst, the sporozoites develop from germinal cells known as sporoblasts. The sporozoites emerge from the oocysts and migrate to the salivary gland where they enter the human hosts during the blood meal of the mosquito.
Download Teaching Slide (PPTX, 1.8M)
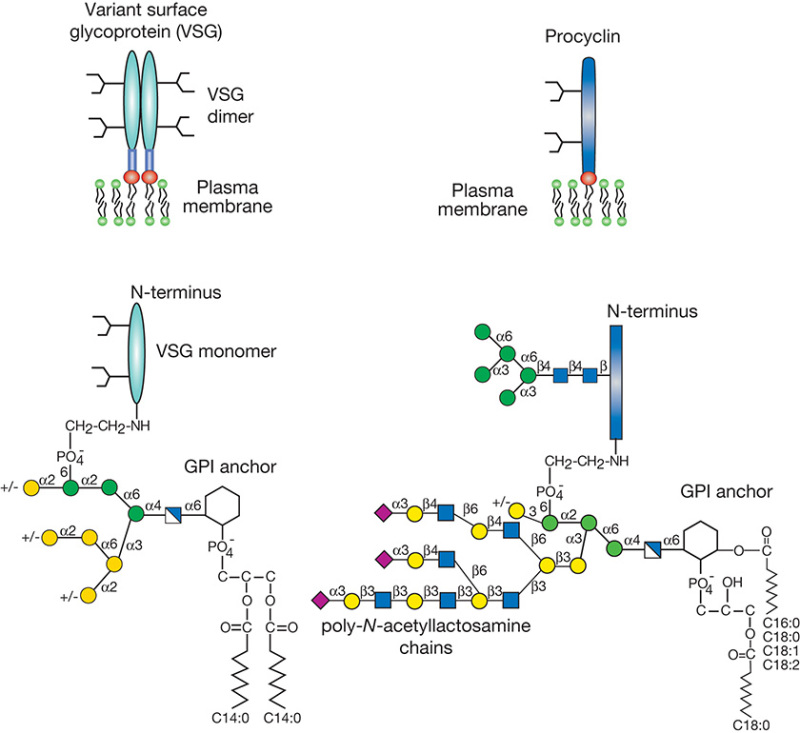
FIGURE 43.2.
Schematic representation of the major surface glycoconjugates of procyclic and metacyclic Trypanosoma. brucei. Variant surface glycoprotein (VSG) is the major component of the metacyclic form, and each molecule consists of two GPI-anchored N-glycosylated monomers (shaded ovals are the protein component). The surface of the procyclic form is densely covered with procyclins, which are GPI-anchored polypeptides with polyanionic repeat domains (shaded rectangle is the protein component). The anchor structures are detailed below the schematic.
Download Teaching Slide (PPTX, 1.9M)
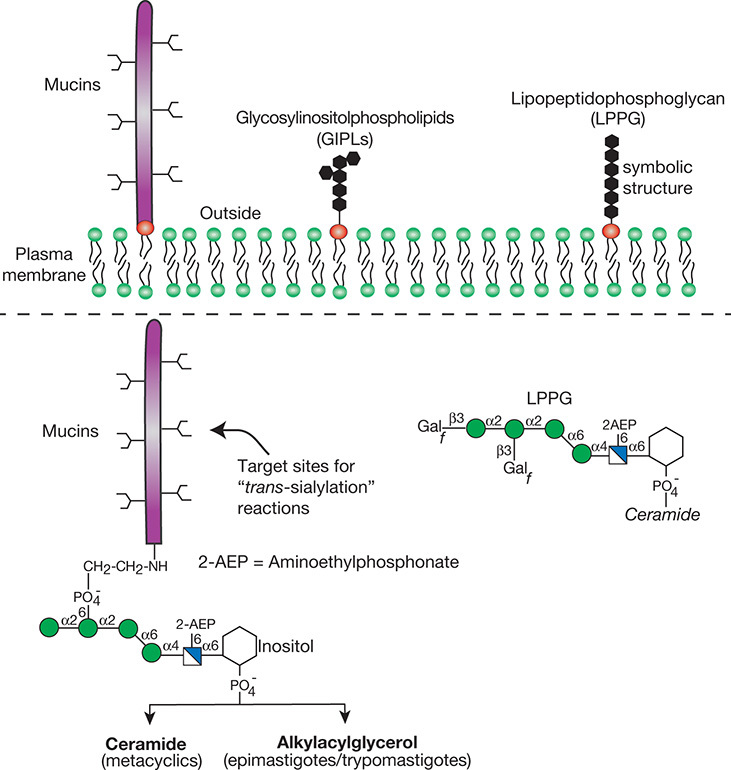
FIGURE 43.3.
Schematic representation of the major surface glycoconjugates of Trypanosoma cruzi. The cell surface of T. cruzi is covered with a dense layer of mucins, glycosylinositolphospholipids (GIPLs), and lipopeptidophosphoglycan (LPPG). The structures of the mucin anchors and the predominant LPPG species are outlined. Aminoethylphosphonate residues are indicated by 2-AEP. For the monosaccharide symbol code, see Figure 1.5, which is also reproduced on the inside front cover.
Download Teaching Slide (PPTX, 1.9M)
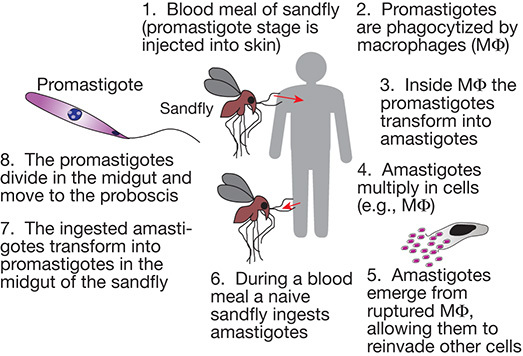
FIGURE 43.4.
Life cycle of Leishmania species, the parasitic protozoan that causes leishmaniasis in humans.
Download Teaching Slide (PPTX, 1.8M)
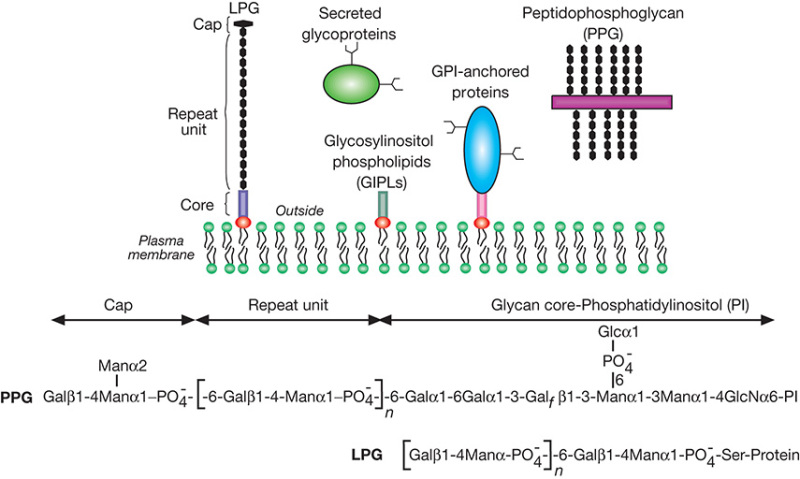
FIGURE 43.5.
Schematic representation of the major cell-surface glycoconjugates of Leishmania. Lipophosphoglycan (LPG) consists of a Gal-Man-PO4- repeat unit backbone attached to a lipid anchor via a glycan core. Secreted glycoproteins and mucin-like molecules called proteophosphoglycans (PPGs) are composed of serine/threonine-rich peptides that are heavily glycosylated on serine with phosphoglycan chains, similar to those found on LPG. The structures of L. donovani LPG and PPG are shown.
Download Teaching Slide (PPTX, 1.8M)
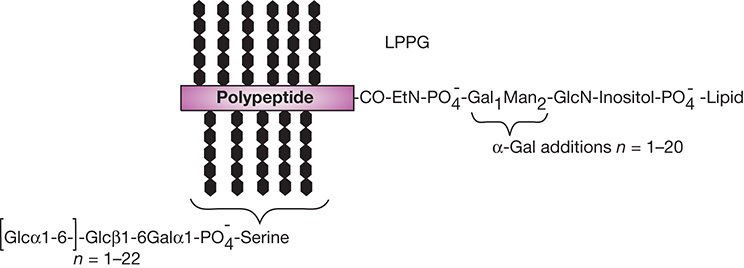
FIGURE 43.6.
Structure of Entamoeba histolytica lipopeptidophosphoglycan (LPPG).
Download Teaching Slide (PPTX, 1.7M)
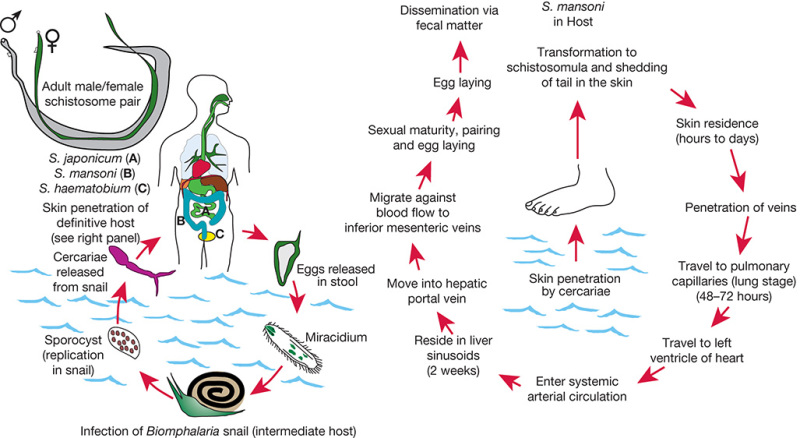
FIGURE 43.7.
Life cycle of Schistosoma species, the parasitic helminth that causes schistosomiasis in humans. (Left) Alternating cycle between the invertebrate snail host and the human definitive host; (right) passage of the worms and their maturation to adult sexually active male/female pairs within the human host.
Download Teaching Slide (PPTX, 2.0M)
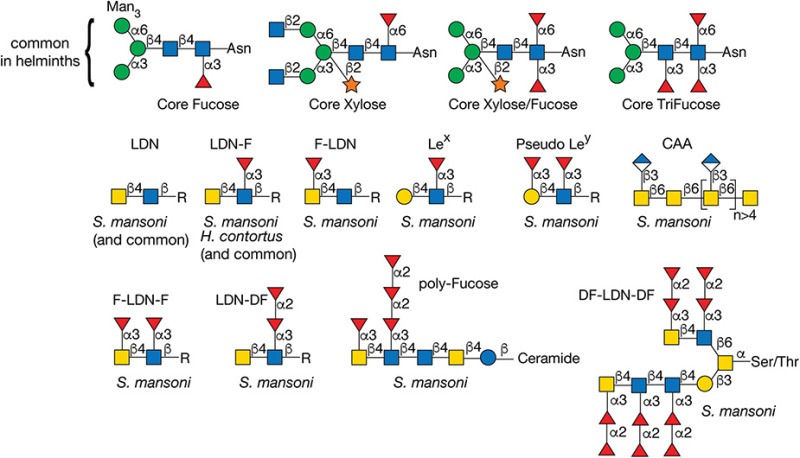
FIGURE 43.8.
Structures of glycans found in parasitic helminths, including Schistosoma mansoni and Haemonchus contortus. The antigens are indicated by the structures within the brackets. (LDN) LacdiNAc; (Lex) Lewis x; (CAA) circulating anodic antigen; (F-) fucosylated; (DF-) difucosylated.
Download Teaching Slide (PPTX, 1.9M)
Tables
TABLE 43.1.
Worldwide distribution of some major parasitic human diseases
| Type of disease | Estimated human infections (need for treatment) | Deaths per year |
|---|---|---|
| Parasitic helminths (worms) | ||
| Soil-transmitted helminths | 880 million | ∼150,000 |
| Roundworm (Ascaris lumbricoides) | ||
| Whipworm (Trichuris trichiura) | ||
| Hookworms (Necator americanus, Ancylostoma duodenale) | ||
| Strongyloidiasis (Strongyloides sterecoralis) | ||
| Schistosomiasis (Schistosoma sp.) | 258 million | uncertain but ∼20,000–200,000 |
| Lymphatic filariasis (Wuchereria bancrofti, Brugia malayi, and Brugia timori) | 120 million | ∼20,000–50,000 (plus millions disfigured or incapacitated) |
| Enterobiasis (Pinworms or threadworms) (Enterobius vermicularis) | 200 million | rare |
| Parasitic protozoans | ||
| Malaria (Plasmodium vivax and Plasmodium ovale) | 214 million | 438,000 |
| Leishmaniasis (Leishmania sp.) | 0.9 million to 1.4 million new cases/year | 20,000–30,000 |
| Onchoceriasis (river blindness) (Onchocerca volvulus) | 25 million | few deaths but 300,000 blinded |
| African trypanosomiasis (sleeping sickness) (Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense) | 60 million at risk and ∼300,000 new cases/year | 50,000 |
| American trypanosomiasis (Chagas’ disease) (Trypanosoma cruzi) | 8 million | 12,000 |
Information was condensed from the World Health Organization (www
.who.int/en/) and the Centers for Disease Control and Prevention (www .cdc.gov). This table shows the total number of individuals with infections or in need of treatment worldwide, and the approximate number of infection-related deaths per year. Note that some individuals may have more than one infection.
TABLE 43.2.
Some of the major parasitic protozoans of humans
| Parasite | |
|---|---|
| Amoeba infecting humans | |
| Entamoeba histolytica | causes amebic dysentery; can cause liver abscesses |
| Intestinal and genital flagellates | |
| Giardia lamblia | causes diarrhea; one of the most common parasites in North America |
| Trichomonas vaginalis | causes inflammation of reproductive organs; very common |
| Hemoflagellates | |
| Leishmania donovani | causes visceral leishmaniasis (kala-azar); hepatosplenomegaly |
| Leishmania mexicana | causes fulminating, cutaneous ulcers |
| Leishmania major | causes cutaneous ulcers |
| Trypanosoma brucei sp. | causes sleeping sickness in humans and nagana in cattle (African trypanosomiasis) |
| Trypanosoma cruzi | causes Chagas’ disease (South American trypanosomiasis) |
| Gregarines, coccidia, and related organisms | |
| Plasmodium falciparum | major cause of human malaria |
| Plasmodium vivax, Plasmodium ovale, Plasmodium malariae | also cause human malaria |
| Causes of opportunistic infections in immunodeficiency conditions | |
| Toxoplasma gondii | causes muscle pain |
| Pneumocystis carinii | causes interstitial cell pneumonia |
| Cryptosporidium parvum | intracellular parasite of intestinal cells that causes diarrhea |
TABLE 43.3.
Some of the major parasitic helminths of mammals
| Parasite | |
|---|---|
| Trematodes | |
| Blood flukes | |
| Schistosoma mansoni | causes human schistosomiasis (affects mesenteric veins draining large intestine) |
| Schistosoma haematobium | causes human schistosomiasis (affects urinary bladder plexus) |
| Schistosoma japonicum | causes human schistosomiasis (affects mesentery veins in the small intestine) |
| Liver flukes | |
| Fasciola hepatica | primarily infects ruminants and occasionally humans (worms live in biliary tract) |
| Clonorchis sinensis | most prevalent liver fluke in humans (can be acquired by eating raw fish) |
| Cestodes | |
| Taenia solium | long human tapeworm acquired by eating undercooked pork |
| Echinococcus granulosus | shorter human tapeworm acquired by eating undercooked lamb (parasitic cysts [hydatids] occur in liver and elsewhere) |
| Taeniarhynchus saginatus | long human tapeworm acquired by eating undercooked beef |
| Nematodes | |
| Ascaris lumbricoides | most common intestinal roundworm in humans |
| Trichuris trichiura | intestinal whipworm in humans |
| Enterobius vermicularis | tiny intestinal roundworm (causes perianal night itch in children) |
| Necator americanus | intestinal hookworm of humans (causes anemia) |
| Ancylostoma duodenale | intestinal hookworm of humans (causes anemia) |
| Strongyloides stercoralis | intestinal parasite (causes autoinfection) |
| Haemonchus contortus | intestinal parasite of sheep and goats |
| Trichinella spiralis | smallest nematode parasite of humans (trichinosis) residing in muscle fibers (acquired from eating undercooked pork) |
| Onchocerca volvulus | filarial parasite (causes river blindness) |
| Wuchereria bancrofti | filaria live in lymph nodes causing elephantiasis |
| Brugia malayi | filaria live in lymph nodes causing elephantiasis |
| Dirofilaria immitis | dog heartworm |
TABLE 43.4.
Some major parasites and their glycan-binding proteins
| Parasite | Stage | Protein | Specificity |
|---|---|---|---|
| Plasmodium falciparum | merozoite | EBA-175 | Neu5Acα2-3Gal/glycophorin A |
| merozoite | EBA-140 | sialic acid/glycophorin B? | |
| merozoite | EBA-180 | sialic acid | |
| sporozoite | circumsporozoite protein | HS | |
| parasitized erythrocytes | the anchor protein VAR2CSA | chondroitin sulfate A | |
| Trypanosoma cruzi | trypomastigote | trans-sialidase | Neu5Acα2-3Gal |
| trypomastigote | penetrin | HS | |
| Entamoeba histolytica | trophozoite | Gal/GalNAc lectin | Gal/GalNAc |
| Entamoeba invadens (a reptilian pathogen) | cyst | cyst wall protein (Jacob lectin) | chitin |
| Giardia lamblia | trophozoite | taglin (α-1 giardin) | Man-6-PO4-, HS |
| Cryptosporidium parvum | sporozoite | Gal/GalNAc lectin | Gal/GalNAc |
| sporozoite | Cpa135 protein | ? | |
| Acanthamoeba keratitis | trophozoite | 136 kDa mannose-binding protein | mannose |
| Toxocara canis | larval | TES-32 | ? |
| Haemonchus contortus | gut-localized | galectin (Hco-gal) | β-galactosides |
HS, heparan sulfate.
