NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.034

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsC-type lectins (CTLs) are Ca++-dependent glycan-binding proteins (GBPs) that share primary and secondary structural homology in their carbohydrate-recognition domains (CRDs). The CRD of CTLs is more generally defined as the CTL domain (CTLD), because not all proteins with this domain bind either glycans or Ca++. CTLs include collectins, selectins, endocytic receptors, and proteoglycans, some of which are secreted and others are transmembrane proteins. They often oligomerize, which increases their avidity for multivalent ligands. CTLs differ significantly in the types of glycans that they recognize with high affinity. These proteins function as adhesion and signaling receptors in many pathways, including homeostasis and innate immunity, and are crucial in inflammatory responses and leukocyte and platelet trafficking.
DISCOVERY OF C-TYPE LECTINS AND COMMON STRUCTURAL MOTIFS
The CTL family is remarkably diverse and is the largest family of known glycan-binding proteins (GBPs). The first CTL identified in animals was the hepatic asialoglycoprotein receptor (ASGPR), also termed the hepatic Gal/GalNAc receptor or Ashwell–Morell receptor (AMR). The sequences of the AMR and other CTLs revealed a CRD unique to this family of proteins. Glycan binding by CTLs is typically Ca++-dependent because of specific amino acid residues that coordinate Ca++ and bind the hydroxyl groups of sugars, but some CTLDs bind glycans without coordinating Ca++. The CTLD is defined by the sequence of amino acids and Cys positions, as well as the folded structure. Interestingly, comparisons of the folded structure of the CTLD to that in other proteins revealed a common folded structure, termed the CTL fold (CTLF), which is a structurally rigid scaffold that comprises a remarkable number of sequence variations, yet may have no sequence in common with CTLs, such as the major tropism determinant (Mtd), which is a receptor-binding protein of Bordetella bacteriophage. The evolutionarily ancient CTLF may occur in at least 1013 different sequences, a diversity that rivals the immunoglobulin fold in its conservation of structure using millions of different primary amino acid sequences.
The CRD of CTLs is a compact region of 110–130 amino acid residues with a double-looped, two-stranded antiparallel β-sheet formed by the amino- and carboxy-terminal residues connected by two α-helices and a three-stranded antiparallel β-sheet (Figure 34.1). The CRD has two conserved disulfide bonds and up to four sites for binding Ca++, with site occupancy depending on the lectin. Amino acid residues with carbonyl side chains are often coordinated to Ca++ in the CRD, and these residues directly bind to sugars when Ca++ is bound in site 2. A ternary complex may form between a sugar, the Ca++ ion in site 2, and amino acids within the CRD, whereas the specific residues within the CRD determine sugar specificity. Key conserved residues that bind sugars include the “EPN” motif (which promotes binding to Man, GlcNAc, Fuc, and Glc) and “WND” motif (which promotes binding to Gal and GalNAc), as seen in mouse L-selectin and rat mannose-binding protein C (Figure 34.1). However, because the CTLD is relatively shallow with few contacts to sugars, it is not possible to predict the glycan structures that bind to a particular CTL. In several CTLs, such as P-selectin and the AMR, Ca++ binding induces structural changes in the CRD that stabilize the double loop region. Loss of Ca++ can lead to destabilization of these loops and loss of ligand binding, even when Ca++ is not directly involved in complexing the ligand, as seen in the macrophage mannose receptor. This destabilization is also important in pH-induced changes that lead to loss of ligand-binding affinity, because of the pH-induced loss of Ca++. In CTLD-containing proteins such as human tetranectin, which is not known to bind glycans, the CTLD can bind Ca++, but in the absence of Ca++ the CTLD is important for interactions with kringle-domain-containing proteins, such as plasminogen.
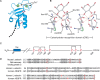
FIGURE 34.1.
Structure of C-type lectins (CTLs). (A) Ribbon diagram of the carbohydrate-recognition domain (CRD) of rat mannose-binding protein A (MBP-A). (Light green spheres) Ca++-binding sites, where 1 is the auxiliary binding site and 2 is the principal binding (more...)
CTLs occur as both monomers and oligomers, such as the trimeric rat mannose-binding protein (MBP)-A (Figure 34.2). The rat MBP was the first CTL structurally characterized with ligand by crystallography. The CRD of trimeric lectins is angled to the side of the stalk domain through which the protein associates to form the trimer. The CRDs are at the top of the trimer and enhance multivalent interactions with glycan ligands.

FIGURE 34.2.
Crystal structure of trimeric rat mannose-binding protein-A complexed with α-methylmannoside. (Created from PDB deposited structure 1kwu and with permission of the American Society for Biochemistry and Biology from Ng KK, Kolatkar AR, Park-Snyder (more...)
DIFFERENT SUBFAMILIES OF C-TYPE LECTINS
CTLDs have been categorized into 16 groups that are distinguished by their domain architecture. Many more than 100 different proteins encoded in the human genome contain a CTLD (Figure 34.3). Most of these groups have a single CTLD, but the macrophage mannose receptor (group 6) is an example of a multi-CTLD protein and has eight of these domains. Several groups have CTLDs that lack critical Ca++ residues, but can bind glycans (e.g., dectin-1 and layilin in group 5); the REG group 7 lacks Ca++ binding but it is unclear whether they bind glycans, whereas tetranectin in group 9 binds Ca++ and again it is unclear if it binds glycans. From a functional perspective, we know most about collectins, endocytic receptors, myeloid lectins, and selectins, as discussed below.
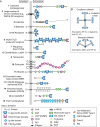
FIGURE 34.3.
Different groups of C-type lectins (CTLs) and their domain structures. (A) Sixteen groups are shown, defined by their phylogenetic relationships and domain structures. Some of the groups are soluble proteins and others are transmembrane proteins. (B) (more...)
CTLF-containing proteins are found in all metazoans and many nonmetazoans. This latter group includes bacterial toxins (e.g., pertussis toxin), outer-membrane adhesion proteins (e.g., invasin from Yersinia pseudotuberculosis), and viral proteins (e.g., envelope protein in Epstein–Barr virus). Interestingly, the viral proteins have more similarity to mammalian CTLD-containing proteins than the bacterial proteins. There are at least 278 genes encoding CTLDs within the genome of Caenorhabditis elegans, but only a small number have the key amino acid residues required to form the primary Ca++ binding site.
THE ASHWELL–MORELL RECEPTOR
The AMR is expressed primarily on the sinusoidal surface of hepatocytes and was discovered serendipitously during studies on clearance of the serum glycoprotein ceruloplasmin, a major copper carrier in mammalian plasma. Glycosylation of ceruloplasmin was found to regulate its lifetime in the rabbit circulation; enzymatic desialylation of radiolabeled ceruloplasmin exposed penultimate β-linked Gal residues and caused rapid accumulation of asialoceruloplasmin into hepatocytes. Removal or modification of the exposed Gal residues lessened the rate of clearance. In 1974, the Ca++-dependent AMR was purified from rabbit liver by affinity chromatography on asialoorosomucoid-Sepharose. The rabbit AMR is a heterooligomer containing two subunits of 48 kDa and 40 kDa, whereas the human AMR contains two subunits of 50 kDa (H1) and 46 kDa (H2) that occur as a heterooligomeric type I transmembrane protein (trimers and high-order oligomers) comprised of small and large subunits.
The purified rabbit AMR agglutinated desialylated human and rabbit erythrocytes and induced mitogenesis in desialylated peripheral lymphocytes, which represented the first demonstration that an animal GBP could profoundly affect cellular metabolism. Interestingly, hepatic AMR can also recognize some sialylated ligands (Siaα2-6Galβ1-4GlcNAc-R). A homologous GBP identified in chicken hepatocytes recognizes glycoproteins containing terminal GlcNAc, rather than Gal, residues. Interestingly, circulating glycoproteins in birds constitutively lack sialic acid (Sia) compared with their mammalian counterparts.
The AMR was one of the first proteins shown to participate in receptor-mediated endocytosis (RME) and, along with the low-density lipoprotein (LDL) receptor, transferrin receptor, and mannose-6-phosphate receptor (M6PR), represent some of the best characterized receptors. The AMR internalizes its ligands captured at the cell surface at physiological pH; via coated pits, the AMR in complex with its ligand is internalized into coated vesicles (Figure 34.4), and changes in pH in late endosomes lead to dissociation of Ca++ from the AMR and release of ligand. The uncomplexed AMR then recycles to the plasma membrane, whereas the ligand is delivered to lysosomes where it is degraded. For some nonrecycling receptors (e.g., dendritic cell [DC]-specific intercellular adhesion molecule-3-grabbing nonintegrin [DC-SIGN]), both the ligand and receptor are targeted to lysosomes and degraded. Internalization of ligands by the AMR in hepatocytes is rapid, occurring in 2–3 minutes, and the receptor recycles to the surface within 4–5 minutes. The efficiency of the AMR in endocytosis has led it to be a target for gene therapy and delivery of molecules to hepatocytes.
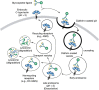
FIGURE 34.4.
Some C-type lectins (CTLs) are endocytic receptors. Ligands are internalized by clathrin-dependent pathways and delivered to early and then late endosomes. Receptors may be recycled or degraded, depending on the receptor and the type of ligand it endocytoses. (more...)
The precise orientation of the trimers within the AMR can dictate its affinity to specific glycans that have multivalent presentations. The AMR binds glycans with terminal β-linked Gal or GalNAc residues; however, tri- and tetra-antennary N-glycans, with appropriate branching and presentation of nonreducing terminal Gal/GalNAc, bind to the rat AMR with >100,000 × higher affinity (∼nM range) than ligands with a single terminal Gal/GalNAc residue.
Surprisingly, mice null for the AMR were found to be phenotypically normal without challenge. However, it is now known that the AMR is required to clear platelets (thrombocytes) during Streptococcus pneumonia–related sepsis, which is accompanied by microbial-dependent desialylation of platelets and consequent thrombocytopenia. Clearance helps to limit disseminated intravascular coagulation (DIC) caused by the infection. Such clearance of platelets during sepsis or as a result of platelet aging is also linked to production of thrombopoietin that regulates platelet production.
OTHER ENDOCYTIC C-TYPE LECTINS
Many other CTLs, both type I and type II transmembrane proteins, also deliver bound ligands to lysosomes via RME through their cytoplasmic motifs (Figure 34.4). Endocytosis of ligands by CTLs in DCs and macrophages can lead to receptor accumulation and degradation in phagolysosomes or to recycling of the receptor to the cell surface. The pathway taken is dependent on the bound ligand. Dectin-1 is degraded when it internalizes zymosan, but it is recycled when it endocytoses soluble ligands. Stimulation of CTLs such as dectin-1 in myeloid cells activates mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB), and enhances transcription of genes important in innate immune responses. Internalization of antigens via the CTLs in DCs induces production of reactive oxygen species (ROS) and other responses.
Clustering and density of the CTLDs may determine both their specificity and affinity for ligands because each individual CRD can act independently to bind sugar. Although the hepatic lectins subunits have a single CTLD, the macrophage mannose receptor has eight CTLDs in a single polypeptide. The adjacent CTLDs may promote binding to specific multivalent, mannose-containing glycans. The macrophage mannose receptor internalizes lysosomal enzymes containing oligomannose-type N-glycans and facilitates phagocytosis of several pathogens such as yeast, Pneumocystis carinii, and Leishmania. Interestingly, domains in some CTLs outside of the CTLD may also have receptor activity. For example, the cysteine-rich domain of the mannose receptor is an R-type domain (Chapter 31) that binds to glycans containing R-GalNAc-4-SO4 on pituitary glycoprotein hormones and thus acts to clear these hormones from the circulation.
THE COLLECTINS
The collectins are a family of soluble and membrane-bound CTLs that contain a collagen-like domain amino-terminal to the CTLD and usually assemble in large oligomeric complexes containing 9–27 subunits (Figure 34.3). Bovine conglutinin was identified in the early 1900s by Bordet and Streng as a plasma protein that agglutinated erythrocytes following reactions with antibody and complement, now known to be through Ca++-dependent interactions with the exposed glycans on iC3b after proteolytic conversion from C3b. Bovine conglutinin and human conglutinin bind yeast glycans, and GlcNAc can inhibit interactions. The term conglutinin was coined in 1993 based on evidence that conglutinin had a collagen-like sequence and exhibited lectin activity. To date, nine different collectins have been identified: conglutinin, mannose-binding lectin (MBL), surfactant proteins SP-A and SP-D (which were originally found in the lung but also expressed in the intestine), and collectins CL-43, CL-46, CL-P1, CL-L1, and CL-K1. MBL, conglutinin, CL-43, CL-46, CL-K1, SP-A, and SP-D are soluble, whereas CL-L1 and CL-P1 are membrane proteins.
Collectins are pattern-recognition receptors (PRRs) functioning in innate immunity that bind to surfaces of microbes or fungi expressing glycan ligands termed pathogen-associated molecular patterns (PAMPs). Thus, collectins and many other CTLs in myeloid cells, as discussed above, function as PRRs and cooperate with Toll-like receptors (TLRs) in recognition and responses of cells to pathogens. Examples of PAMPS include lipopolysaccharides (LPSs), β-glucans, lipoteichoic acids (LTAs), and glycoproteins of parasites. Collectins stimulate in vitro phagocytosis by recognizing PAMPS, and binding promotes leukocyte chemotaxis and stimulates the production of cytokines and ROS by immune cells. Lung surfactant lipids have the ability to suppress a number of immune cell functions such as proliferation, and this suppression of the immune response is further augmented by SP-A.
Binding of MBL and other collectins initiates the lectin pathway of complement activation. Such collectins are associated with proenzyme forms of MBL-associated serine proteases 1 and 2 (MASP-1 and -2), in which binding to ligand by the CRD activates MASP-1 to cleave MASP-2, which then activates complement via the classical pathway and generates opsonic C3b fragments that coat pathogens and lead to their destruction and phagocytosis. Some individuals with MBL deficiency syndrome have mutations in the Gly-X-Y repeat encoded within exon-1 of the MBL gene (MBL2). Mutations within exon-1, which are highly variable among human populations, inhibit assembly of the MBL subunit, leading to increased risk of microbial infections. Furthermore, several polymorphisms within the promoter region of MBL2 are associated with MBL deficiency and enhanced susceptibility to infections. Genetic variants of MBL2 are also associated with Crohn's disease. In addition, polymorphisms in MASP1 and MASP2 are associated with altered serum levels and compromised activity of collectins. Activation of these proteases can also lead to prothrombin cleavage and promote clot formation.
THE MYELOID C-TYPE LECTINS
Myeloid cells, which include monocytes, macrophages, neutrophils, and DCs, have a large number of proteins with CTLDs, which belong mainly to groups 2, 5, and 6 (see Figure 34.3). Myeloid cells also express many members of the galectin and Siglec families of lectins. All leukocytes express L-selectin (group 4), which is discussed below in a separate section on selectins. Group 2 CTLs are DC-SIGN (in humans, but there is no murine homolog), CD209a (also termed SIGN-R1 in mice, but there is no human homolog), macrophage CTL (MCL), dectin-2 (DC-associated C-type lectin-2), langerin, the macrophage galactose-binding lectin (MGL), and the macrophage inducible Ca++-dependent lectin (MINCLE) (CLEC4E); in group 5, dectin-1, myeloid-DAP12-associating lectin (MDL-1), CLEC-1, CLEC-2, and dendritic cell–associated lectin-1 (DCAL-1); and in group 6, macrophage mannose receptor and DEC-205.
There are approximately 20 members of the natural killer (NK) cell receptor group 5 of CTLD-containing proteins in humans, and additional ones in mice. Many of the genes encoding the NK cell receptor group 5 are present on human chromosome 12 and mouse chromosome 6. The dectin-1 cluster that is part of the NK gene complex of CTLDs (mouse chromosome 6 and human chromosome 12) includes dectin-1, CLEC-1, CLEC-2, LOX-1, CLEC12b, and CLEC9a. These CTLDs bind various ligands. The dectin-2 cluster of CTLDs (whose genes are clustered in the centromeric region of the NK gene cluster) includes dectin-2, blood DC antigen 2 (BDCA-2), DC immunoactivating receptor (DCAR), DC immunoreceptor (DCIR), CTL superfamily 8 (CLECSF8), and MINCLE (CLEC4E). Mice have the Ly49 family of receptors that contain CTLDs and are on murine chromosome 6. But the Ly49 members, also termed Ly49 NK cell receptors, may not bind sugar but primarily bind to major histocompatibility complex (MHC) class I ligands and MHC class I-like molecules expressed by viruses. These are most functionally related to the killer-cell immunoglobulin-like receptors (KIRs) in human NK cells and some T cells.
The myeloid CTLDs act as PRRs and collectively can recognize a wide variety of glycans acting as PAMPs, as well as nonglycan determinants. The glycan ligands for the myeloid CTLs are diverse. MINCLE, which was identified originally as a protein that recognizes dying cells, binds α-mannose-containing glycans and especially interacts with mannans and trehalose dimycolate, a key glycolipid virulence factor from Mycobacterium tuberculosis and Mycobacterium bovis. hBDCA-1 binds HIV-1 gp120 and galactose-containing glycans. Dectin-1, which binds ligands in the absence of Ca++, and dectin-2, which binds Ca++, are important in fungal defense, and mice deficient in either one are more susceptible to specific yeast and fungal infections. Dectin-1 is the functional receptor for β-glucans, which are polymers of a backbone of β1-3-linked glucose and side chains of β1-6-linked glucose. Dectin-2 binds α-mannans, which are polymers of α1-6-linked mannose with α1-2-linked mannose side chains. However, the ligands of many myeloid proteins with CTLDs are not well understood, including myeloid inhibitory CTL-like receptor (MICL) and DNGR-1. There is some evidence that DNGR-1 binds damage-associated molecular patterns (DAMPs) exposed in dying cells (DNGR-1 binds F-actin). Thus, the CTLDs in such proteins may not be involved in glycan binding.
The various signaling pathways by which many of these myeloid CTLDs function is illustrated in Figure 34.5. Most of these proteins express immunoreceptor tyrosine-based activation motif (ITAM) motifs in their cytoplasmic domain. An example is dectin-1, which contains an activating ITAM-like motif, and like other activatory ITAM CTLs, also contains a tri-acidic motif that promotes downstream signaling. Some CTLs in myeloid cells, such as MICL and macrophage antigen h (MAH), contain an immunoreceptor tyrosine-based inhibitory (ITIM) motif. Such motifs on phosphorylation recruit tyrosine phosphatases to negatively regulate signaling pathways.
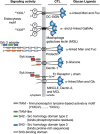
FIGURE 34.5.
Signaling activity of C-type lectins (CTLs) in innate immune responses. CTLs expressed on dendritic cells (DCs) and macrophages interact with different glycans, often expressed on pathogen-derived glycans. Activation of CTLs occurs through glycan binding, (more...)
Among the myeloid CTLDs, CLEC-2 is unusual in that it may not be involved directly in innate immunity; it is expressed by NK cells, DCs, megakaryocytes, and platelets, but it is also expressed on some tumor cells. CLEC-2 is a type II transmembrane glycoprotein whose signaling is initiated by tyrosine phosphorylation of a single tyrosine in the YXXL motif of its cytoplasmic domain. Signaling involves Src, Syk kinases, and phospholipase Cγ2. CLEC-2 is also a receptor for the platelet activating snake toxin rhodocytin, from the Malayan viper, Calloselasma rhodostoma.
The major endogenous glycoprotein ligand for CLEC-2 is podoplanin (also called Aggrus), expressed by lymphatic endothelial cells and lymph node fibroblastic reticular cells (FRCs). CLEC-2 binds to the sialylated O-glycans and peptide portions of podoplanin. This is physiologically important and serves both as an alternative pathway for in vivo platelet activation, as well as fundamentally serving to drive lymphangiogenesis through the signaling functions of podoplanin. In the absence of extended O-glycans on podoplanin, as engineered in mice lacking C1GalT1 (T-synthase), due to loss of T-synthase or its chaperone Cosmc (C1GalT1C1), podoplanin is dysfunctional. This leads to defects in lymphangiogenesis, due to lack of platelet involvement, a phenotype that closely phenocopies that seen in podoplanin-deficient mice.
Another potential adhesion molecule is prolectin (CLEC17A), which is expressed in dividing B cells in germinal centers, and interacts with glycans containing terminal α-linked mannose or fucose residues. It may function within lymph nodes as an adhesion or colonization factor for invading tumor cells and shows preference for binding to epithelial rather than mesenchymal cells, which is relevant for tumor cells undergoing epithelial–mesenchymal transition (EMT).
Both macrophages and DCs are antigen-presenting cells (APCs) that internalize antigens via specific endocytic receptors or by fluid-phase pinocytosis; they process antigens for presentation to CD8+ cytotoxic T cells. DCs also contribute to the balance between tolerance and the induction of immunity and help to distinguish harmless “self-antigens” from pathogens. When DCs are activated, they migrate to lymph nodes where they interact with T cells. The types of antigens that DCs encounter lead to their differentiation into different subtypes, which function to instruct the differentiation of T cells. TLRs and CTLs (for myeloid cells, these have also been termed CTL receptors or CLRs) act to help DCs discriminate between pathogens and self-antigens. TLRs are PRRs that interact with PAMPs, but, unlike CLRs, TLRs cannot directly promote phagocytosis of bound ligands. Ligation of TLRs can lead to DC maturation in a pathogen-specific manner. In contrast, DC interaction with pathogens through CLRs, in the absence of TLR activation, leads to internalization and processing of antigens and can result in DCs remaining immature. Interactions of T cells with antigens presented by immature DCs may lead to tolerogenic, rather than inflammatory, responses. However, if both TLRs and CLRs are activated and “cross-talk” ensues, DCs mature in different ways. For example, mycobacteria interact with DCs via TLR-2 and TLR-4, resulting in strong T-helper 1 (Th1) responses by the activated DCs. However, some virulent strains of mycobacteria secrete glycosylated factors (e.g., ManLAM) that are bound by CLRs, but apparently not by TLRs, which lead to down-regulation of TLR activation and limitation of DC maturation.
THE SELECTINS
A seminal experiment in the 1960s by Gesner and Ginburg showed that 32P-labeled rat lymphocytes injected back into rats homed to the spleen and lymph nodes, but when they were first treated with crude glycosidases homing was diminished. They hypothesized that “… sugars serve a physiological function by acting as sites of cellular interactions … .” This exceptional insight eventually led to the discovery of L-selectin and its role in lymphocyte homing.
There are three members of the selectin family, P-, E-, and L-selectin, and they are among the best-characterized family of CTLs because of their extensively documented roles as cell adhesion molecules. They mediate (1) the earliest stages of leukocyte trafficking; (2) constitutive migration of lymphocytes to peripheral lymph nodes and to skin; and (3) hematopoietic stem cell trafficking to marrow. The three mammalian selectins are type-1 transmembrane glycoproteins that are expressed on platelets, endothelial cells, and leukocytes, hence the origin of their names (Figure 34.6A). Interactions between the selectins and cell-surface glycoconjugate ligands promote tethering and rolling of leukocytes and platelets in postcapillary venules, and are important for leukocyte recruitment to sites of inflammation and injury. Rolling is a form of adhesion that requires rapid formation and dissociation of bonds between selectins and their ligands. Rolling adhesion enables leukocytes to encounter endothelium-bound chemokines. Signaling through chemokine receptors cooperates with signaling through selectin ligands to activate leukocyte integrins, which bind to immunoglobulin superfamily ligands on endothelial cells to slow rolling velocities and arrest leukocytes on vascular surfaces. The arrested leukocytes then crawl across between endothelial cells into the underlying tissues (diapedesis or extravasation) (Figure 34.6D).
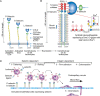
FIGURE 34.6.
Structures and functions of selectins. (A) Overall domain structures of P-selectin, E-selectin, and L-selectin and their expression patterns are indicated. P-selectin also forms homodimers in the membrane. (The key for the domain structures is shown in (more...)
Each selectin has a C-type CRD at the amino terminus followed by a consensus epidermal growth factor (EGF)-like domain and a number of short consensus repeats composed of sushi domains (also called complement control protein [CCP] modules) (Figure 34.6A). The proteins have a single transmembrane domain (TM) and a cytoplasmic domain and are relatively rigid, extended molecules. The CRD of each selectin has modest affinity for the sialylated, fucosylated structure known as the sialyl Lewis x antigen (SLex) and its isomer sialyl Lewis a (SLea) (Chapter 14) and increased binding to some sulfated versions of these motifs. In addition, P- and L-selectin, but not E-selectin, also bind to some forms of heparin/heparan sulfate (HS) in a divalent cation-dependent manner. However, each of the selectins binds with higher affinity to specific macromolecular ligands, and most contain sialylated, fucosylated O-glycans. The major ligand for P-selectin, termed P-selectin glycoprotein ligand-1 (PSGL-1), has sulfated tyrosine residues adjacent to SLex expressed on a core-2-based O-glycan. This glycoform of PSGL-1 can also bind to L- and E-selectin. Another major ligand for E-selectin is a glycoform of CD44 called “hematopoietic cell E/L-selectin ligand” (HCELL) constitutively expressed on human hematopoietic stem cells that carries SLex on its N-glycans. Murine leukocytes require core 1-derived O-glycans on CD44 and other glycoproteins to bind E-selectin. Ligands for L-selectin on endothelial cells of lymph node high endothelial venule (HEV) contain 6-sulfo-SLex determinants containing MECA-79 antigen on mucin-type O-glycans and on N-glycans.
P-Selectin
P-selectin (CD62P) was discovered as an antigen expressed on the surface of activated platelets. It is constitutively expressed in megakaryocytes, where it is packaged into the membranes of α-granules of circulating platelets. It is also expressed in the Weibel–Palade bodies of vascular endothelial cells. Within minutes following activation of either platelets or endothelial cells by proinflammatory secretagogues such as histamine, thrombin, or complement components, P-selectin is expressed on the cell surface because of fusion of the intracellular storage membranes with the plasma membrane. Sequences within the cytoplasmic domain of P-selectin mediate its sorting to secretory granules as well as its rapid endocytosis from the plasma membrane and movement from endosomes to lysosomes, where it is degraded. Splice variants of human P-selectin transcripts yield forms of P-selectin that lack a TM, thus contributing to low-level soluble forms of P-selectin in the circulation. Leukocyte adhesion also stimulates proteolytic cleavage of the ectodomain of P-selectin from the plasma membrane, releasing it into the circulation. The inflammatory mediators tumor necrosis factor (TNF)-α, interleukin 1-β (IL1-β), and LPS augment transcription of mRNA for P-selectin in endothelial cells in mice and non-primate mammals but not in humans.
P-selectin contributes to leukocyte recruitment in both acute and chronic inflammation. Mice that lack P-selectin exhibit defective rolling of their leukocytes on endothelial cells of postcapillary venules, diminished recruitment of neutrophils or monocytes into tissues following injection of inflammatory mediators, and impaired recruitment of T cells into skin or other tissues following challenge with specific antigens. Mobilization of P-selectin to the surfaces of activated endothelial cells is important for all these responses. In addition, P-selectin expressed on the surfaces of activated platelets contributes to inflammation as well as to hemostasis and thrombosis. Activated platelets adhere through P-selectin to neutrophils, monocytes, NK cells, and some subsets of T lymphocytes. This adhesion augments the recruitment of leukocytes and platelets to sites of vascular injury. Expression of P-selectin on both platelets and endothelial cells contributes to experimental atherosclerosis in mice. Platelet-expressed P-selectin also stimulates monocytes to synthesize tissue factor, a key cofactor of blood coagulation that facilitates fibrin deposition during clot formation.
As discussed below, although the major leukocyte counter receptor for P-selectin is PSGL-1, P-selectin (and L-selectin) also binds less avidly to some forms of heparin/HS and to some other glycoproteins that express SLex. Athough the physiological significance of this non-PSGL-1-dependent binding by P-selectin is unclear, clinically prescribed levels of heparin can block P-selectin functions. In addition, P-selectin can interact with mucins containing highly clustered O-glycans bearing SLex antigens and sulfate esters, which appear to be important in the metastasis of tumors bearing such ligands (Chapter 47). The therapeutic effect of heparin in blocking cancer metastases may thus be mediated at least partly through blocking P- and/or L-selectin-dependent adhesion of leukocytes and platelets to tumor cells expressing SLex/a rich sulfated mucins. Results from a phase 2 trial suggest that a chemical selectin inhibitor (GMI-1070) may reduce the severity of vasoocclusive crisis in patients with sickle cell disease (SCD). Complementary phase 2 trials results using blockade of P-selectin in humans with an inhibitory humanized antibody, has also shown efficacy in prophylactic treatment of SCD.
PSGL-1
PSGL-1 (CD162) is a homodimeric, disulfide-bonded mucin with subunits of ∼120 kDa. It is the major physiological ligand on leukocytes for P- and L-selectin and is also an important ligand for E-selectin (Figure 34.6B). PSGL-1 contains 16 decapeptide repeating units (mucin repeats) with the consensus sequence spanning residues 118–277 in the ectodomain of the long form of the protein, which is the major form expressed in humans. Murine PSGL-1 has some sequence similarity to the human sequence, but the mouse protein has only 10 decameric repeats with the consensus sequence -E-T-S-Q/K-P-A-P-T/M-E-A-, which is different from human PSGL-1. The highest homology between human and murine PSGL-1 occurs in the transmembrane and cytoplasmic domains, and unexpectedly not in the P-selectin-binding domain.
The PSGL-1 polypeptide is expressed in most leukocytes (including neutrophils, monocytes, eosinophils, basophils, and subsets of T cells), and also on hematopoietic stem cells of mice and humans. Additionally, PSGL-1 is expressed in some activated endothelial cells, most notably the inflamed microvessels of the ileum in a spontaneous model of chronic ileitis in mice. In leukocytes, PSGL-1 is enriched in microvilli and is associated with lipid rafts.
In neutrophils, monocytes, and activated T cells, PSGL-1 undergoes the appropriate posttranslational modifications to express the SLex structure on core 2 O-glycans (Figure 34.6C). This glycoform of PSGL-1 interacts with each of the three selectins to support leukocyte rolling under flow. Engagement of PSGL-1 during rolling also transduces signals into leukocytes that activate leukocyte integrins to slow rolling velocities. Signaling through PSGL-1 cooperates with signaling through chemokine receptors to elicit other effector responses in leukocytes.
PSGL-1 has multiple functions in addition to being a selectin ligand. PSGL-1 is a check-point regulator in T cells, promotes T-cell exhaustion, and partly regulates the programmed cell death protein 1 (PD-1) expression. PSGL-1 via its sulfated tyrosine residues can interact with certain chemokines, including CCL21 and CCL19, which may promote T-cell entry into secondary lymphoid organs. PSGL-1 is an adhesion molecule for pathogens, including enterovirus 71, Anaplasma phagocytophilum (the tick-transmitted obligate intracellular bacterium that causes human granulocytic anaplasmosis), and S. pneumonia. In some cases, as for A. phagocytophilum, the SLex-containing O-glycan of PSGL-1 is a recognition factor, whereas for the other pathogens, sulfated tyrosines are recognized.
The key determinants for binding P-selectin are in the extreme amino terminus of PSGL-1. Antibodies to this region block binding of PSGL-1 to P- and L-selectin (but not to E-selectin, which may interact with other fucosylated sites on PSGL-1 and does not interact with sulfated tyrosines). Treatment of neutrophils with selective proteases that remove the first ten amino acids from the amino terminus of PSGL-1 abrogates its binding to P- and L-selectin. Site-directed mutagenesis of a specific O-glycosylated amino-terminal threonine and/or the three tyrosine residues in the amino terminus of PSGL-1 prevents binding to P- and L-selectin. Finally, a synthetic glycosulfopeptide (GSP) with the structure shown in Figure 34.6C binds to P-selectin with similar high affinity as that of native PSGL-1.
The data support the model in Figure 34.6B in which the combination of tyrosine sulfate residues and a glycan on PSGL-1 are required for high-affinity binding to P-selectin. The cocrystal structure of a PSGL-1-derived GSP with P-selectin confirms this model. The interactions between the GSP and P-selectin result from a combination of hydrophobic and electrostatic contacts. These include contacts of at least two of the three tyrosine sulfate residues as well as other PSGL-1 amino acids with multiple residues within the P-selectin CRD, and hydroxyl groups in the fucose residue of SLex that ligate the lectin domain-bound Ca++, and there are additional binding interactions with the hydroxyl groups of galactose and the –COOH group of Neu5Ac.
E-Selectin
E-selectin (CD62E) was discovered as a leukocyte adhesion molecule expressed by activated vascular endothelial cells. In most tissues (the bone marrow and skin are exceptions), endothelial cells do not constitutively express E-selectin. Cytokine-dependent transcriptional processes lead to an inducible expression of E-selectin on the surface of the endothelium. Inducible transcription of the E-selectin locus by TNF-α, IL1-β, and LPS is mediated at least in part through NF-κB-dependent events. In vitro, cytokine treatment increases E-selectin expression after 2 hours, with maximal expression at 4 hours. E-selectin expression then declines to basal levels within 12–24 hours in vitro, but may be expressed chronically at sites of inflammation in vivo. Decline of E-selectin expression is associated with decreased transcription of the E-selectin locus, degradation of E-selectin transcripts, and internalization and turnover of E-selectin protein. Acute and chronic inflammatory conditions associated with E-selectin expression include trauma, sepsis, rheumatoid arthritis (RA), and organ transplantation.
E-selectin cooperates with P- and L-selectin to recruit leukocytes to sites of inflammation. Physiological ligands for E-selectin contain the SLex antigen and occur on leukocytes (neutrophils, monocytes, eosinophils, memory/effector T cells, and NK cells) and on human hematopoietic stem cells. Each of the leukocyte subsets is found in acute and chronic inflammatory sites in association with the expression of E-selectin. PSGL-1 is one of the physiological ligands for E-selectin, but E-selectin can also interact with several other glycoproteins that express the SLex antigen on either N- or O-glycans, including HCELL (a human CD44 glycoform), E-selectin ligand-1 (in mice), and L-selectin (in humans), and possibly long-chain glycosphingolipids expressing the SLex antigen. In human hematopoietic stem cells (HSCs), via adhesive interactions on bone medullary microvessels that constitutively express E-selectin, HCELL directs trafficking of HSCs into marrow. In addition to effects on leukocyte and hematopoietic stem cell migration, E-selectin receptor/ligand interactions may have a potential role in cancer metastasis (see Chapter 47).
L-Selectin
L-selectin (CD62L) is expressed on the microvilli of most leukocytes, including all myeloid cells, naïve T and B cells, and some memory/effector T cells. L-selectin was the first selectin discovered through efforts to define molecules that facilitate recirculation of lymphoid cells from the intravascular compartment to secondary lymphoid organs, including lymph nodes and Peyer's patches, from which the lymphoid cells then return to the circulation through the lymphatic system, as discussed above in work by Gesner and Ginsburg. This recirculation process provides lymphocytes with the opportunity to encounter foreign antigens displayed by APCs within secondary lymphoid organs. Early studies indicated that blood lymphocytes enter lymph nodes in specialized postcapillary HEVs. Endothelial cells in the HEV are cuboid in shape. Their surfaces are decorated with mucins that mediate L-selectin-dependent adhesion of lymphocytes. These mucins are called peripheral node addressins. They include CD34, Sgp200, GlyCAM-1, MAdCAM-1, endoglycan, endomucin, and podocalyxin-like protein (PCLP). L-selectin is proteolytically shed from the surface of activated leukocytes by a metalloproteinase TNF-α-converting enzyme (TACE).
Rolling mediated by L-selectin exhibits a counterintuitive “shear threshold” requirement (e.g., a minimum flow rate is required for leukocytes to roll). As the flow rate drops below this threshold, leukocytes roll faster and more unstably and then detach. Flow-enhanced rolling operates through a force-dependent mechanism. As the flow rate increases, the force applied to adhesive bonds between L-selectin and its ligands increases. At threshold levels, the force actually strengthens the bonds, which prolongs their lifetimes. These are called “catch bonds.” As the flow rate increases further, the applied force begins to weaken the bonds, which shortens their lifetimes. These are called “slip bonds.” Catch bonds are regulated by force-dependent straightening of the angle between the lectin and EGF domains of L-selectin, which affects how ligand dissociates from the binding interface on the lectin domain. Transitions between catch and slip bonds are also seen for interactions of P-selectin and E-selectin with their ligands. However, these transitions occur at lower forces with less dramatic effects in the circulation.
A unique feature of the L-selectin ligands on HEV is the requirement for sulfated glycans, such as 6-sulfo-SLex on both core-2 O-glycans and on extended core-1 O-glycans. The 6-sulfo-SLex determinant is associated with the MECA-79 epitope on O-glycans (Figure 34.6), an antibody that binds to 6-sulfo-N-acetyllactosamine on extended core-1 O-glycans. The biosynthesis of the 6-sulfo-SLex determinant depends on two key α1-3 fucosyltransferases, FuT-VII and FuT-IV, along with at least four different sulfotransferases that may form the 6-sulfo-SLex determinant. Two of these sulfotransferases, GlcNAc6ST-1 and GlcNAc6ST-2, are expressed in HEV and appear to be most important. Mice lacking FuT-VII or both FuT-VII and FuT-IV have dramatically reduced homing of lymphocytes to lymph nodes. Mice lacking both GlcNAc6ST-1 and GlcNAc6ST-2 do not express 6-sulfo-SLex or the MECA-79 epitope and exhibit markedly diminished lymphocyte homing to lymph nodes. A unique β1-3GlcNAcT generates the extended core-1 O-glycan; mice lacking both this β1-3GlcNAcT and the β1-6GlcNAcT branching enzyme for core-2 O-glycan biosynthesis do not express the MECA-79 antigen, but they have residual lymphocyte rolling on HEV and only a minimal decrease in lymphocyte numbers in peripheral and mesenteric lymph nodes. The residual L-selectin-dependent lymphocyte homing appears to result from 6-sulfo-SLex on N-glycans, suggesting that both N- and O-glycans on HEV glycoproteins contribute to L-selectin-dependent lymphocyte recirculation through lymph nodes.
L-selectin also plays a role in adhesion of neutrophils, eosinophils, and monocytes to nonlymphoid vascular endothelium (see Figure 34.6). The major ligand for L-selectin in these inflammatory settings is PSGL-1, which is expressed on adherent leukocytes and may also be deposited on inflamed endothelial cells as fragments left behind by previously rolling leukocytes. An alternate ligand on endothelial cells is HS. As with P-selectin, clinically relevant doses of the drug heparin can block L-selectin, and likely contributes to the inhibition of tumor metastasis and of mucinous-tumor associated hypercoagulability (Trousseau's syndrome) (Chapter 47). On human hematopoietic stem cells, HCELL is also a potent L-selectin ligand, yet unlike all other naturally-expressed L-selectin ligands, its binding to L-selectin is not sulfate-dependent. Mice lacking L-selectin have defects in neutrophil recruitment in the context of inflammation as well as defects in homing of naïve lymphocytes to secondary lymphoid organs.
PROTEOGLYCANS WITH C-TYPE LECTIN DOMAINS
The CTLD occurs in several proteoglycans (lecticans or hyalectins) that lack TMs and reside in the extracellular matrix (ECM) (group I; see Figure 34.3). These include aggregan, brevican, versican, and neurocan. Like the selectins, each of these core proteins contains a CTLD, an EGF-like domain, and a CCP domain, but their domain order is different and they are located in the carboxyl terminus of the protein. A large region containing attachment sites for chondroitin sulfate (CS) and keratan sulfate (KS) is proximal to the lectin domain. A more complete discussion of the proteoglycans is provided in Chapter 17. The exact functions of the CTLD in these proteins are still unknown. The CTLD of rat aggrecan is important for Ca++-dependent binding to the fibronectin type II repeats 3–5 of rat tenascin-R. The CTLDs in other lecticans are probably also responsible for protein–protein interactions with other receptors, including tenascin-R, tenascin-C, and other tenascins, which are ECM glycoproteins highly expressed in the nervous system. Interestingly, the protein–protein interactions between rat fibronectin and the CTLD of rat aggregan bear resemblance to the protein–protein interactions seen in P-selectin binding to PSGL-1. Tenascin-R is one of the main carriers of the unusual glycan antigen HNK-1, which was named after its identification on human NK cells. Brevican, a lectican found in the nervous system, binds to the HNK-1-containing glycosphingolipids. Thus, the CTLD in lecticans may represent a versatile structural feature that can be used for both protein–protein and protein–glycan interactions. Interestingly, several aggrecanopathies (inherited defects in aggrecan production/function) are associated with mutations in the exon encoding the CTLD domain of aggrecan.
OTHER PROTEINS WITH C-TYPE LECTIN DOMAINS
A number of proteins with CTLDs have been identified in the pancreas and kidney, but whether these proteins bind glycans is unclear. PKD1, one of two ADPKD gene products associated with autosomal-dominant polycystic kidney disease (ADPKD), has been implicated in cell–cell and cell–matrix interactions. The PKD1 gene encodes polycystin-1 (PC1), a very large protein (4293 amino acids) with a single CTLD near its amino terminus. Carboxy-terminal proteolysis of PC1 during its secretion releases a cytoplasmic portion that becomes nuclear and regulates cell signaling pathways. Interestingly, the CTLD of PC1 appears to bind collagen in a Ca++-dependent fashion that is inhibitable by sugars, including dextrans, but the precise nature of the interaction is still unclear.
Some small proteins with CTLDs include Reg3α and PSP (pancreatic stone protein), which are in the REG group 7 of CTLs, and are essentially isolated CTLDs that are preceded by a signal sequence. Reg3α (also known as HIP/PAP) is a secreted CTL that an amino-terminal signal sequence and a 16 kDa CTLD. Reg3α is an anti-inflammatory protein with scavenging activity toward ROS, and can bind peptidoglycan and directly kill Gram-positive, but not Gram-negative, bacteria. Reg3α kills bacteria by first binding to peptidoglycan and then binding to membrane phospholipids and forms a hexameric membrane-permeabilizing oligomeric pore.
Lower vertebrates, invertebrates, and some viruses also contain CTLDs. The galactose-specific lectin from Crotalus atrox binds a variety of galactose-containing glycolipids in a Ca++-dependent fashion. A number of related venom proteins inhibit platelet function and/or the coagulation cascade. Alboaggregin A from the white-lipped pit viper (Trimeresurus albolabris) binds to the platelet GPIb-V-IX complex and stimulates platelet agglutination, but the potential role of glycan recognition in this process is unclear. In contrast, the gp42 protein of Epstein–Barr virus (EBV) has a CTLD that is NK-receptor like, and ectodomain of the poxvirus (Poxviridae) protein A33, a target of subunit vaccine development, has two CTLDs in dimeric form, and has resemblance to the CTLDs of the NK lectins. But, neither of these domains in these viral proteins binds either Ca++ or glycans, and may have evolved by convergent evolution.
ACKNOWLEDGMENTS
The authors acknowledge the helpful comments of Robert Sackstein.
FURTHER READING
- Gesner BM, Ginbsurg V. 1964. Effect of glycosidases on the fate of transfused lymphocytes. Proc Natl Acad Sci 52: 750–755. [PMC free article: PMC300341] [PubMed: 14212553]
- Ashwell G, Morell AG. 1974. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol 41: 99–128. [PubMed: 4609051]
- Rosen SD, Singer MS, Yednock TA, Stoolman LM. 1985. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science 228: 1005–1007. [PubMed: 4001928]
- Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A, McEver RP. 1992. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol 118: 445–456. [PMC free article: PMC2290037] [PubMed: 1378449]
- Norgard-Sumnicht KE, Varki NM, Varki A. 1993. Calcium-dependent heparin-like ligands for L-selectin in nonlymphoid endothelial cells. Science 261: 480–483. [PubMed: 7687382]
- McMahon SA, Miller JL, Lawton JA, Kerkow DE, Hodes A, Marti-Renom MA, Doulatov S, Narayanan E, Sali A, Miller JF, Ghosh P. 2005. The C-type lectin fold as an evolutionary solution for massive sequence variation. Nat Struct Mol Biol 12: 886–892. [PubMed: 16170324]
- Brown GD. 2006. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat Rev Immunol 6: 33–43. [PubMed: 16341139]
- Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. 2006. Surfactant proteins SP-A and SP-D: Structure, function and receptors. Mol Immunol 43: 1293–1315. [PubMed: 16213021]
- Ludwig IS, Geijtenbeek TBH, van Kooyk Y. 2006. Two way communication between neutrophils and dendritic cells. Curr Opin Pharmacol 6: 408–413. [PubMed: 16750420]
- Sperandio M. 2006. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J 273: 4377–4389. [PubMed: 16956372]
- Uchimura K, Rosen SD. 2006. Sulfated L-selectin ligands as a therapeutic target in chronic inflammation. Trends Immunol 27: 559–565. [PubMed: 17049924]
- Zhou T, Chen Y, Hao L, Zhang Y. 2006. DC-SIGN and immunoregulation. Cell Mol Immunol 3: 279–283. [PubMed: 16978536]
- Gupta G, Surolia A. 2007. Collectins: Sentinels of innate immunity. BioEssays 29: 452–464. [PubMed: 17450595]
- Trinchieri G, Sher A. 2007. Cooperation of toll-like receptor signals in innate immune defence. Nat Rev Immunol 7: 179–190. [PubMed: 17318230]
- Gurr W. 2011. The role of REG proteins, a family of secreted C-type lectins, in islet regeneration and as autoantigens in type 1 iabetes. In Type 1 diabetes—Pathogenesis, genetics and immunotherapy (ed. D Wagner., editor. ). InTech, Rijeka, Croatia.
- Zarbock A, Ley K, McEver RP, Hidalgo A. 2011. Leukocyte ligands for endothelial selectins: Specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118: 6743–6751. [PMC free article: PMC3245201] [PubMed: 22021370]
- Grewal PK, Aziz PV, Uchiyama S, Rubio GR, Lardone RD, Le D, Varki NM, Nizet V, Marth JD. 2013. Inducing host protection in pneumococcal sepsis by preactivation of the Ashwell–Morell receptor. Proc Natl Acad Sci 110: 20218–20223. [PMC free article: PMC3864324] [PubMed: 24284176]
- Mukherjee S, Zheng H, Derebe MG, Callenberg KM, Partch CL, Rollins D, Propheter DC, Rizo J, Grabe M, Jiang QX, Hooper LV. 2014. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 505: 103–107. [PMC free article: PMC4160023] [PubMed: 24256734]
- Richardson MB, Williams SJ. 2014. MCL and Mincle: C-type lectin receptors that sense damaged self and pathogen-associated molecular patterns. Front Immunol 5: 288. [PMC free article: PMC4066366] [PubMed: 25002863]
- Dambuza IM, Brown GD. 2015. C-type lectins in immunity: Recent developments. Curr Opin Immunol 32: 21–27. [PMC free article: PMC4589735] [PubMed: 25553393]
- Drickamer K, Taylor ME. 2015. Recent insights into structures and functions of C-type lectins in the immune system. Curr Opin Struc Biol 34: 26–34. [PMC free article: PMC4681411] [PubMed: 26163333]
- D'Souza AA, Devarajan PV. 2015. Asialoglycoprotein receptor mediated hepatocyte targeting—Strategies and applications. J Controlled Release 203: 126–139. [PubMed: 25701309]
- Geijtenbeek TBH, Gringhuis SI. 2015. C-type lectin receptors in the control of T helper cell differentiation. Nat Rev Immunol 16: 433–448. [PubMed: 27291962]
- McEver RP. 2015. Selectins: Initiators of leucocyte adhesion and signaling at the vascular wall. Cardiovasc Res 107: 331–339. [PMC free article: PMC4592324] [PubMed: 25994174]
- Telen MJ, Wun T, McCavit TL, De Castro LM, Krishnamurti L, Lanzkron S, Hsu LL, Smith WR, Rhee S, Magnani JL, Thackray H. 2015. Randomized phase 2 study of GMI-1070 in SCD: Reduction in time to resolution of vaso-occlusive events and decreased opiod use. Blood 125: 1656–1664. [PMC free article: PMC4408290] [PubMed: 25733584]
- Hansen SWK, Ohtani K, Roy N, Wakamiya N. 2017. The collectins CL-L1, CL-K1 and CL-P1, and their roles in complement and innate immunity. Immunobiol 221: 1058–1067. [PubMed: 27377710]
- Kedmi R, Peer D. 2017. Zooming in on selectins in cancer. Sci Transl Med 8: 345fs11. [PubMed: 27358495]
- Sackstein R. 2017. Fulfilling Koch's postulates in glycoscience: HCELL, GPS and translational glycobiology. Glycobiology 26: 560–570. [PMC free article: PMC4847618] [PubMed: 26933169]
- DISCOVERY OF C-TYPE LECTINS AND COMMON STRUCTURAL MOTIFS
- DIFFERENT SUBFAMILIES OF C-TYPE LECTINS
- THE ASHWELL–MORELL RECEPTOR
- OTHER ENDOCYTIC C-TYPE LECTINS
- THE COLLECTINS
- THE MYELOID C-TYPE LECTINS
- THE SELECTINS
- PROTEOGLYCANS WITH C-TYPE LECTIN DOMAINS
- OTHER PROTEINS WITH C-TYPE LECTIN DOMAINS
- ACKNOWLEDGMENTS
- FURTHER READING
- Review C-Type Lectins.[Essentials of Glycobiology. 2022]Review C-Type Lectins.Cummings RD, Chiffoleau E, van Kooyk Y, McEver RP. Essentials of Glycobiology. 2022
- Review C-type Lectins.[Essentials of Glycobiology. 2009]Review C-type Lectins.Cummings RD, McEver RP. Essentials of Glycobiology. 2009
- Review C-type lectins: their network and roles in pathogen recognition and immunity.[Histochem Cell Biol. 2017]Review C-type lectins: their network and roles in pathogen recognition and immunity.Mayer S, Raulf MK, Lepenies B. Histochem Cell Biol. 2017 Feb; 147(2):223-237. Epub 2016 Dec 20.
- Identification of C-type lectin-domain proteins (CTLDPs) in silkworm Bombyx mori.[Dev Comp Immunol. 2015]Identification of C-type lectin-domain proteins (CTLDPs) in silkworm Bombyx mori.Rao XJ, Shahzad T, Liu S, Wu P, He YT, Sun WJ, Fan XY, Yang YF, Shi Q, Yu XQ. Dev Comp Immunol. 2015 Dec; 53(2):328-38. Epub 2015 Jul 14.
- Review Targeting C-type lectin receptors with multivalent carbohydrate ligands.[Adv Drug Deliv Rev. 2013]Review Targeting C-type lectin receptors with multivalent carbohydrate ligands.Lepenies B, Lee J, Sonkaria S. Adv Drug Deliv Rev. 2013 Aug; 65(9):1271-81. Epub 2013 May 30.
- C-Type Lectins - Essentials of GlycobiologyC-Type Lectins - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

