NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.010

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsMany glycoproteins carry glycans initiated by GalNAc attached to the hydroxyl of Ser or Thr residues. Mucins are the class of glycoproteins carrying the greatest number of O-GalNAc glycans (also called mucin-type O-glycans), but this posttranslational modification is common among many glycoproteins. The sugars found in O-GalNAc glycans include GalNAc, Gal, GlcNAc, Fuc, and Sia, whereas Man, Glc, or Xyl residues are not represented. Sialic acids may be modified by O-acetylation, and Gal and GlcNAc by sulfation. The length of O-GalNAc glycans may vary from a single GalNAc to more than 20 sugar residues and can include blood group and other glycan epitopes. This chapter describes the structures, biosynthesis, and functions of O-GalNAc glycans in mammals.
MUCIN GLYCOPROTEINS
About 150 years ago, E. Eichwald and E. Hoppe-Seyler noted that highly glycosylated proteins, which they termed mucins, are found throughout the body. Mucins contain hundreds of heterogeneous O-GalNAc glycans attached to the protein scaffold (Figure 10.1) and may also carry a small number of N-glycans. GalNAc O-linked to Ser/Thr is the initiating sugar of O-GalNAc glycans and is usually extended to form one of four common core structures (Table 10.1, Figure 10.1). Each core can subsequently be extended to give a mature linear or branched O-GalNAc glycan.

FIGURE 10.1.
A simplified model of a large secreted mucin. The VNTR (variable number tandem repeat) region rich in Ser, Thr, and Pro is highly O-glycosylated and the molecule assumes an extended “bottle brush” conformation. The cysteine-rich regions (more...)
TABLE 10.1.
O-GalNAc glycan cores and antigenic epitopes of mucins
The great variety, density, and clustering of O-GalNAc glycans on mucins control their chemical, physical, and biological properties. This complexity often makes it very difficult to assign functions to individual O-GalNAc glycans at particular attachment sites. Mucins line the epithelial surfaces of the body, including the gastrointestinal, genitourinary, and respiratory tracts, where they shield epithelial cells against physical and chemical damage and protect against infection. Mucins can be antiadhesive and repel cell-surface interactions or, alternatively, promote adhesion by mediating recognition of glycan-binding proteins via their O-GalNAc glycans. Mucins have multiple effects on the immune system and also serve to maintain cellular homeostasis via signaling mechanisms. Both secreted and membrane-bound mucins are produced in epithelial cells. Mucins in mucus secretions include large and polymeric (gel-forming) mucins and smaller, non-gel-forming monomeric mucins. Goblet cells in the tracheobronchial, gastrointestinal, and genitourinary tracts are specialized for the production of the large, gel-forming mucins. These mucins are stored in granules from which they can be quickly secreted on receiving external stimuli. Mucins also have roles in fertilization. A number of diseases are associated with abnormal mucin gene expression and abnormal mucin O-GalNAc glycans. These include cancer, inflammatory bowel disease, and hypersecretory bronchial and lung diseases. Because O-glycans are hydrophilic and usually negatively charged, they promote binding of water and salts and are major contributors to the viscosity and adhesiveness of mucus. In cystic fibrosis, the abnormally high viscosity of mucus in lungs leads to obstruction, establishment of infections with pathogenic bacteria, and tissue malfunction. Transmembrane mucins have a type I topology, with a single transmembrane domain, an O-glycosylated domain that interacts with the extracellular space, and a short intracellular cytoplasmic tail at the carboxyl terminus that interacts with other proteins. Membrane-associated mucin-like glycoproteins such as P-selectin glycoprotein ligand 1 (PSGL-1) have O-glycosylated regions but are less densely O-glycosylated than mucins.
The first mucin polypeptide gene to be cloned, MUC1, encodes a transmembrane mucin that is ubiquitous in the epithelium. The levels of MUC1 are usually high in tumors, in which its glycosylation is often abnormal. A hallmark of mucins is the presence of numerous “variable number tandem repeat” (VNTR) regions that carry the majority of the O-GalNAc glycans (50%–80% of the weight of the molecule) (Figure 10.1). VNTRs can vary in length and composition and are rich in Pro, Thr, and Ser residues that facilitate O-GalNAc glycosylation. The O-GalNAc glycans expressed on a mucin are the result of the spectrum of glycosyltransferases that are active in the cell type producing the mucin. The dense O-glycosylation in the VNTR regions causes mucin glycoproteins to adopt an extended “bottle brush” conformation (Figure 10.1). The dense glycosylation prevents cleavage by proteases and may also prevent recognition of protein epitopes by antibodies. Humans have about 20 different mucin genes, expressed in a tissue-specific fashion, and having different properties. The mucin genes, their transcripts, the resulting mucin proteins, and the attached O-GalNAc glycans all show extreme variability.
O-GalNAc GLYCAN CORE STRUCTURES
The O-GalNAc glycans of mucins have four major core structures (cores 1 to 4, Table 10.1). Each core can be extended (Figure 10.1) by a variety of sugar residues to give linear or branched chains that resemble those on N-glycans (Chapter 9) and glycolipids (Chapter 11). Blood group determinants are commonly found in mucins at nonreducing termini of O-GalNAc glycans. The extension of O-GalNAc by a β1-3Gal forms the most common (core 1) O-GalNAc glycan. Core 2 is formed by the addition of β1-6GlcNAc to the GalNAc of core 1. Less common core structures are core 3, in which β1-3GlcNAc is added to O-GalNAc, and core 4, in which core 3 is branched by the addition of β1-6GlcNAc (Table 10.1). Core 1 and 2 O-GalNAc glycans are found in glycoproteins and mucins produced in many different cell types. However, core 3 and 4 O-GalNAc glycans are more restricted to mucins and glycoproteins in gastrointestinal and bronchial tissues.
A single GalNAc residue attached to Ser/Thr forms the Tn antigen. The unsubstituted core 1 O-GalNAc glycan forms the Thomsen-Friedenreich (TF or T antigen). Although Tn and T antigens are usually cryptic because they are extended by other sugars, they are found at increased levels in mucins from cancer cells. They can also carry Sia and form sialyl-Tn or sialyl-T antigens.
O-GalNAc cores are often extended to form complex O-GalNAc glycans. Depending on the spectrum of biosynthetic enzymes in a cell producing a glycoprotein, O-GalNAc glycans may include the ABO and Lewis blood group determinants, polysialic acid, the linear i antigen (Galβ1-4GlcNAcβ1-3Gal), and the GlcNAc β1-6-branched I antigens (Table 10.1). Extensions by type 1 (Galβ1-3GlcNAc) or type 2 (Galβ1-4GlcNAc) units can be repeated and provide scaffolds for the attachment of additional sugars or functional groups. The termini of O-GalNAc glycans may contain Fuc and Sia in α-linkages, Gal, GalNAc and GlcNAc in both α- and β-linkages, and sulfate. Many of these terminal sugars are antigenic or recognized by lectins. In particular, the sialylated and sulfated Lewis antigens are ligands for selectins (Chapter 34), and Gal-terminating structures are ligands for galectins (Chapter 36). Some sugar residues, or their modifications, may mask underlying antigens or receptors. For example, O-acetyl groups on the Sia of the sialyl-Tn antigen prevent recognition by anti-sialyl-Tn antibodies. Gut bacteria may actively remove this mask. Dense O-glycosylation of mucin domains provides almost complete protection from protease degradation.
ISOLATION, PURIFICATION, AND ANALYSIS OF MUCIN O-GalNAc GLYCANS
The O-linkage between GalNAc and Ser/Thr residues is labile under alkaline conditions. Thus, O-GalNAc glycans can be released by a reaction termed β-elimination (i.e., treatment with 0.1 M sodium hydroxide). The hemiacetal GalNAc produced will undergo rapid alkali-catalyzed degradation under these conditions (called peeling) but can be reduced with sodium borohydride to yield stable N-acetylgalactosaminitol at the reducing end of the released O-glycan. β-Elimination is the method of choice to release O-glycans from glycoproteins that also have N-glycans because the latter are not susceptible to cleavage under mild conditions. O-GalNAc glycans as well as other Ser/Thr-linked glycans would be released as alditols by β-elimination, but with losses of labile O-acetyl or sulfate esters. An alternative method that preserves the reducing end of O-GalNAc uses ammonia followed by boric acid. O-GalNAc that is not substituted with another sugar can be enzymatically released by an N-acetylgalactosaminidase. Another glycosidase, termed O-glycanase, releases only core 1 (Galβ1-3GalNAc-) from Ser/Thr, provided the disaccharide is not further substituted. Thus, sialidase treatment followed by O-glycanase releases most simple core 1 O-GalNAc glycans. Terminal Sia residues can also be easily removed with mild acid treatment. There are no known enzymes that can release more complex and extended whole O-GalNAc glycans, but mixtures of exoglycosidases can be used to sequentially remove sugars from O-GalNAc glycans on a glycoprotein. Glycoproteins with clusters of sialylated O-GalNAc glycans may be digested by an O-sialoglycoprotein endopeptidase.
Released, intact O-GalNAc glycans may be separated by different chromatographic methods, including high-performance liquid chromatography (HPLC). Chemical derivatization of GalNAc at the reducing end helps in the separation and subsequent analysis of sugar composition and linkages by gas chromatography and mass spectrometry (MS) (Chapter 50). Another tool to isolate O-glycans with specific epitopes is affinity chromatography using lectins. For example, Helix pomatia agglutinin binds to terminal GalNAc, whereas peanut lectin binds to unsubstituted core 1 (Figure 10.2).
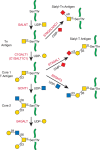
FIGURE 10.2.
Biosynthesis of core 1 and 2 O-GalNAc glycans as described in the text. Green lines are protein.
The structures of O-GalNAc glycans released from mucins and other glycoproteins may be determined by a combination of liquid or gas chromatography, MS, and nuclear magnetic resonance (NMR) spectroscopy. The anomeric linkage of each sugar can be determined using specific glycosidases that distinguish between α- or β-linked sugars and by one- and two-dimensional NMR methods (Chapter 50). The sites of O-GalNAc glycan modification in mucins are difficult to determine directly, but this has been achieved by sensitive MS methods. However, by deleting the gene encoding core 1 β1-3 galactosyltransferase 1 (T synthase; C1GALT1), or its chaperone COSMC (C1GALT1C1), the synthesis of all core 1 and core 2 O-GalNAc glycans is prevented, giving a terminal GalNAc at previous core 1 and core 2 sites. Glycoproteins or tryptic peptides with only GalNAc at Ser/Thr residues may be separated on long lectin columns and analyzed by MS or MS/MS, which reveals each Ser or Thr modified by GalNAc in the glycoproteome.
BIOSYNTHESIS OF O-GalNAc GLYCANS
O-GalNAc glycans are added to protein in the Golgi apparatus. The biosynthetic glycosyltransferases are type II transmembrane proteins with a short cytoplasmic tail at the amino terminus directed toward the cytoplasm, a transmembrane domain, a short stem region, and the catalytic domain in the lumen of the Golgi. The arrangement within Golgi membranes appears to be similar to an “assembly line” with early reactions occurring in the cis-Golgi and late reactions in the trans-Golgi (Chapter 4). Many of the enzymes, however, are diffusely distributed in Golgi compartments. Type II glycosyltransferases are cleaved in the stem domain by signal peptide-like proteases (SPPLs) and secreted from the cell, explaining their presence in the circulation.
The subcellular localization, activity levels, and substrate specificities of glycosyltransferases involved in the assembly of O-GalNAc glycans have a critical role in determining the range of O-glycans synthesized by a cell (Table 10.2 and Figures 10.2 and 10.3). In contrast to the initial reactions of N-glycosylation and O-mannosylation, no lipid-linked intermediates and no glycosidases appear to be involved in O-GalNAc glycan biosynthesis in which donor nucleotide sugars are transported into the Golgi from the cytoplasm, and released nucleotide reaction products are transported out of the Golgi. The glycosyltransferases that are involved solely in the assembly of mucin O-GalNAc glycans are listed in Table 10.2. However, other enzymes that contribute to the synthesis of N-glycans and glycolipids also act on O-glycans, and some of these prefer O-glycans as acceptor substrates (Chapter 14). In vitro assays have shown that the activities of glycosyltransferases are controlled by factors such as metal ions and pH. It is interesting that in vitro assays often do not reflect the efficient synthesis suggested by the dense complex O-glycosylation found in mucins. The reason for this may be that glycosyltransferases in the Golgi exist in a complex with other enzymes in the same pathway, leading to highly efficient synthesis of complex O-glycans.
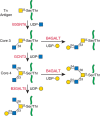
FIGURE 10.3.
Biosynthesis of core 3 and 4 O-GalNAc glycans as described in the text. Green lines are protein.
TABLE 10.2.
Glycosyltransferases that synthesize mucin O-GalNAc glycans
Polypeptide-N-Acetylgalactosaminyltransferases
The first and essential step of O-GalNAc glycosylation is the addition of GalNAc in α-linkage to Ser or Thr by a polypeptide GalNAc-transferase (GALNT) (Table 10.2, Figure 10.2). Humans have 20 genes encoding GALNTs. The large number of GALNTs provides redundancy and also reflects differences in substrate specificity. Studies in the fly indicate that certain GALNTs are required for normal development, but deletion of single GALNTs in mammals have to date given only subtle effects in the adult. The GALNTs are found throughout the animal kingdom but not in bacteria, yeast, or plants. All GALNTs are classified in the GT27 family with a GT-A fold, and most have a lectin (ricin-like) domain at the carboxyl terminus, which is unique among glycosyltransferases. The lectin domain is connected to the catalytic domain by a linker of 10 to 25 amino acids. O-GalNAc residues remote from the Ser/Thr to be glycosylated can be recognized by the lectin domain, which facilitates efficient catalysis at that Ser/Thr. The DXH motif found in all GALNTs appears to be important in metal ion binding.
Immunohistochemistry of submaxillary glands has shown that some GALNTs localize to the cis-Golgi. However, studies in cultured cells suggest that several enzymes of the family are located throughout the Golgi. The first step of O-GalNAc glycosylation is clearly regulated by the amino acid sequence of the protein substrate, although a defined sequence of amino acids with a Ser/Thr that will receive a GalNAc has not been identified. All GALNTs bind UDP-GalNAc as the donor substrate but they may differ in peptide acceptor specificities. In vitro assays show that many GALNTs do not efficiently transfer GalNAc to Ser, but rather to Thr. Pro residues in the vicinity of an O-glycosylation site have a role in creating a suitable acceptor, either by causing exposure of hydroxyls to be glycosylated, or by binding to the enzyme in a Pro-binding motif. An investigation of more than 300 peptide substrates from experimentally established O-GalNAc sites using 10 recombinant GALNTs in in vitro assays revealed substantial overlap in the ability of the GALNTs to transfer O-GalNAc to many peptides. The most ubiquitously expressed GALNT1, GALNT2, and GALNT3 acted on most peptides, whereas those with restricted tissue expression were somewhat more selective. Databases that estimate the likelihood of O-glycosylation at specific sites based on known sequences around O-glycosylation sites of several hundreds of glycoproteins exist (e.g., O-GlycBase and NetOGlyc). However, these predictions do not account for the different GALNTs expressed in the cell type producing a glycoprotein and do not apply to mucins. Because of overlapping localization of GALNTs and the extension enzymes, it is likely that a heterogeneous mixture of different O-GalNAc glycans is present throughout the biosynthetic pathway and on all mature glycoproteins. Also, the presence of O-GalNAc glycans on proteins is likely often missed because of this limited predictive value of amino acid sequence.
Synthesis of O-GalNAc Glycan Cores
O-GalNAc glycan synthesis begins with the transfer of GalNAc from UDP-GalNAc to Ser or Thr catalyzed by a polypeptide GalNAc-transferase (GALNT). Although a single unextended GalNAc linked to Ser or Thr (the Tn antigen) is uncommon in normal mucins, it is often found at increased levels in tumor mucins. This suggests that the extension of O-GalNAc glycans beyond the first sugar is blocked in some cancer cells. Evidence that this blockage facilitates tumorigenesis and/or progression of solid tumors has been obtained by preventing extension of O-GalNAc in precancerous cells. Sia added to GalNAc by the α2-6 sialyltransferase ST6GALNAC1 generates the sialyl-Tn antigen, which is common in advanced tumors. Other sugars are not known to be added to this O-glycan, but it can be O-acetylated by Sia O-acetyltransferase, which prevents detection by anti-sialyl-Tn antibodies.
The addition of one or two neutral sugars to O-GalNAc generates one of the different cores of O-GalNAc glycans (Table 10.1 and Figure 10.2). Core 1 (Galβ1-3GalNAc-O-Ser/Thr) is generated by C1GALT1 (Table 10.2 and Figure 10.2). This activity is present in most cell types but absolutely requires the molecular chaperone C1GALT1C1 (COSMC) during synthesis in the endoplasmic reticulum (ER) to ensure proper folding and activity in the Golgi. Lack of core 1 synthesis in certain cell types can be due to either defective C1GALT1 or the absence of functional C1GALT1C1 and is reflected in high expression of Tn and sialyl-Tn antigens. For example, Jurkat T cells and colon cancer leukemic stem cells (LSCs) lack C1GALT1C1, and thus C1GalT1 activity, and have high expression of Tn and sialyl-Tn antigens.
A GlcNAc β1-6 branch added to the GalNAc residue of core 1 forms core 2 O-GalNAc glycans (Tables 10.1 and 10.2, Figure 10.2). Core 2 O-GalNAc glycans are more cell-type specific than the essentially ubiquitous core 1 O-GalNAc glycans, and their expression is highly regulated during activation of lymphocytes, cytokine stimulation, and embryonic development. Leukemia and cancer cells and other diseased tissues have abnormal amounts of core 2 O-GalNAc glycans. The enzymes responsible for core 2 synthesis are core 2 β1-6 N-acetylglucosaminyltransferases 1, 2, and 3 (C2GnT-1, GCNT1). These glycosyltransferases do not require divalent cations as cofactors and X-ray crystallography shows that positively charged amino acids replace the function of divalent metal ions. There are two different types of core 2 β1-6 N-acetylglucosaminyltransferases. The L-type enzyme (leukocyte type, GCNT1) synthesizes only core 2 O-GalNAc glycans, whereas the M-type enzyme (mucin type, GCNT3) synthesizes core 4 O-GalNAc glycans (Table 10.1, Figure 10.3). The L-type enzyme is active in many tissues and cell types, but the M type is found only in mucin-secreting tissues such as the intestine, stomach, and respiratory tissues. The expression and activity of both the L and M types are altered in certain tumors. The synthesis of core 2 O-GalNAc glycans has been correlated with tumor metastasis, possibly because of selectin ligands that are preferentially assembled on core 2 O-GalNAc glycans and that facilitate egress from the circulation (Chapter 34).
The synthesis of core 3 O-GalNAc glycans appears to be restricted mostly to mucus epithelia from the gastrointestinal and respiratory tracts and to salivary glands. The enzyme responsible is core 3 β1-3 N-acetylglucosaminyltransferase 6 (C3GnT6; B3GNT6) (Table 10.2, Figure 10.3). The enzyme has low in vitro activity but must be highly efficient in vivo because colonic mucins are rich in core 3 O-GalNAc glycans. The expression and activity of B3GNT6 is especially low in colonic tumors and virtually absent from tumor cells in culture. Overexpression of the enzyme in colon cancer cells decreases their ability to metastasize. Mice deficient in B3GNT6 show increased susceptibility to colitis and tumor development. The synthesis of core 4 by the M-type GCNT3 requires the prior synthesis of a core 3 O-GalNAc glycan (Figure 10.3). Transfection of colon cancer cells HCT116 with GCNT3 suppresses cell growth and invasive properties. In a xenograft model in nude mice, transfection with GCNT3 also suppresses tumor growth. Thus, both core 3 and 4 O-GalNAc glycans repress tumor progression, although the mechanisms of repression are not clear.
Synthesis of Complex O-GalNAc Glycans
The elongation of O-GalNAc glycans is catalyzed by families of β1-3 GlcNAc-transferases and β1-3 and β1-4 Gal-transferases to form repeated type 1 and 2 poly-N-acetyllactosamines (Table 10.1). Although most of the extension enzymes act on a number of different glycans, core 3 is a preferred acceptor for β3GalT5 (B3GALT5). In addition, the Galβ1-3 residues of core 1 and 2 O-GalNAc glycans are preferred substrates for the elongation enzyme β1-3 N-acetylglucosaminyltransferase 3 (β3GlcNAcT-3, B3GNT3). Less common elongation reactions are the formation of GalNAcβ1-4GlcNAc (LacdiNAc) and Galβ1-3GlcNAc- sequences. Linear poly-N-acetyllactosamine units can be branched by members of the β1-6 N-acetylglucosaminyltransferase family (e.g., GCNT2), resulting in the I antigen (Table 10.2). Most of these elongation and branching reactions also occur on O- and N-glycans and glycolipids.
Some sialyltransferases and sulfotransferases prefer O-GalNAc glycans as substrates but many of these enzymes have an overlapping specificity and also act on N-glycans. A family of α2-6 sialyltransferases (ST6GALNACI to ST6GALNAC4) with distinct specificities synthesize sialyl-Tn and sialylated core 1 O-GalNAc glycans. A family of α2-3 sialyltransferases is responsible for the synthesis of sialylated O-GalNAc glycans, with ST3GAL1 being mainly involved in the sialylation of the Galβ1-3 residue of core 1 and 2 O-GalNAc glycans. Sialylation blocks the further linear extension of O-glycan chains.
Sulfotransferases are localized in the Golgi and cap O-GalNAc glycans with a sulfate ester linked to the 3-position of Gal or the 6-position of GlcNAc. The sulfate group is transferred from 3′-phosphoadenosine-5′-phosphosulfate (PAPS). This adds a negative charge to O-GalNAc glycans of lung, intestinal, and other mucins that has a considerable effect on the chemical and metal ion binding properties of these glycans. GAL3ST4 is the major sulfotransferase acting on the Gal residue of core 1 O-glycans. Skeletal type keratan sulfate (KS) is also an O-GalNAc-linked highly sulfated polysaccharide (Chapter 17). O-acetyltransferases that add O-acetyl esters to one or more hydroxyl groups of Sia residues remain poorly characterized. Some evidence suggests that the esters can be added to CMP-Sias before transfer.
The α1-2 fucosyltransferases FUT1 and FUT2 synthesize the blood group H determinant of O-GalNAc glycans which can be converted by an α1-3 Gal-transferase to blood group B or by an α1-3 GalNAc-transferase to blood group A (Table 10.1). In addition, α1-3 and α1-3/4 fucosyltransferases synthesize the Lewis antigens (Table 10.1). A number of uncommon and antigenic sugars are also found on O-GalNAc glycans. For example, neuropilin-2 found in the nervous system has core 1 and 2 O-GalNAc glycans that carry polysialic acid residues synthesized by polysialytransferase IV (PST; ST8SIA4). These highly charged glycans play a critical role in the negative regulation of cell adhesion during maturation of the nervous system. An α1-4 GlcNAc-transferase from gastric tissue adds GlcNAc in α1-4 linkage to β1-4 Gal in core 1 and 2 O-GalNAc glycans. The GlcNAc α1-4 glycans appear to inhibit colonization by Helicobacter pylori.
FUNCTIONS OF O-GalNAc GLYCANS
The functions of O-GalNAc glycans are many and varied. Mucins that have hundreds of complex O-GalNAc glycans provide a barrier for a wide range of intestinal microbes. O-GalNAc glycans can contribute to the conformation and exposure of peptide epitopes and the adhesive properties of mucins and glycoproteins. Selectins and galectins recognize O-GalNAc glycans, which play a role in the innate immune system.
Methods to determine O-GalNAc glycan functions include the use of biosynthetic inhibitors, the construction of cell lines that lack or overexpress specific enzymes in O-glycosylation pathways, the use of O-GalNAc glycan-specific lectins or antibodies, or removal of specific sugar residues by glycosidases. For example, a critical role of O-GalNAc glycans in selectin-mediated cell adhesion was revealed by treating cells with GalNAc-O-benzyl. This “O-glycosylation extension inhibitor” is a competitive substrate of core 1 and core 3 synthesis. Thus, treatment with GalNAc-O-benzyl causes a reduction of all O-GalNAc glycans on glycoproteins, which instead are synthesized on the GalNAc-O-benzyl acceptor. Inhibitor-treated cancer cells lose the ability to bind to E-selectin and endothelial cells in vitro. The ligands for certain selectin-mediated interactions between endothelial cells and leukocytes require sialyl Lewis x that is commonly attached to core 2 O-GalNAc glycans (Table 10.1). This selectin–glycan interaction is important for the attachment of leukocytes to the capillary endothelium during homing of lymphocytes or the extravasation of leukocytes during the inflammatory response (Chapter 34). Cancer cells often express sialyl Lewis x and may thus use the selectin-binding properties of their cell surface as a mechanism to invade tissues. O-GalNAc glycans change during lymphocyte activation and are abnormal in leukemic and tumor cells.
Cell lines defective in specific O-glycosylation pathways or cells specially engineered to express altered O-GalNAc glycans, as well as mice with targeted mutations, are excellent models to study roles of O-GalNAc glycosylation. Cells lacking C1GALT1C1 have been developed to identify a partial O-GalNAc proteome (i.e., the specific locations of core 1or core 2 O-GalNAc glycans in the glycoproteome). The same approach has identified roles for core 1 and core 2 O-GalNAc glycans in cell transformation and cancer cell progression. Mice lacking C1GALT1, and thus lacking core 1 and core 2 O-GalNAc glycans, die during embryogenesis because of defective angiogenesis and hemorrhaging, whereas mice lacking core 3 O-GalNAc glycans develop colitis and are also prone to develop colorectal tumors.
Although knocking out a single polypeptide GalNAc transferase does not result in an obvious phenotype in mice, glycosyltransferases specific to O-GalNAc glycans are associated with human disease. For example, GALNT3 is responsible for adding a GalNAc residue to Thr178 in the phosphate-regulating factor FGF23. A deficiency in GALNT3 leads to decreased circulation of FGF23 and aberrant phosphate homeostasis. Plasma lipid levels appear to be regulated by GALNT2 levels, and a deficiency of GALNT2 is associated with cardiovascular disease. Loss of GALNT11 causes heterotaxy because of the loss of O-GalNAc on NOTCH1 leading to alterations in motile versus immotile cilia and affecting laterality.
Because of the association of increased levels of Tn, sialyl-Tn, and T antigens with cancer, vaccines containing these structures on mucin fragments have been synthesized as mimics of cancer cell O-GalNAc glycans, and have shown moderate success. MUC1 is a prime candidate for immunization in cancer as it is overexpressed and has mostly short O-GalNAc glycans in adenocarcinomas. Thus, in breast cancer MUC1, the chains are relatively short and sialylated, with Tn and sialyl-Tn being prominent. However, to be effective, the glycosylation should be the same as in the cancer cells to be immunized against. A fully synthetic linear MUC1 glycopeptide immunogen reported recently is a promising vaccine candidate. The sialyl-Tn antigen has also been shown to play a role in the metastatic process and may therefore be a suitable target antigen to prevent metastasis. O-GalNAc glycans are also potential markers for diagnosis and prognosis of cancers. For example the CA125 antigen is a glycosylated mucin circulating in serum that is used as a diagnostic and prognostic marker, particularly in ovarian cancer. Understanding the full range of O-GalNAc glycans produced and identifying specific O-GalNAc glycan pathway members associated with disease will continue to reveal potential therapeutic targets and useful diagnostic and prognostic tools.
ACKNOWLEDGMENTS
The authors acknowledge the contribution of Harry Schachter to previous versions of this chapter. The authors appreciate helpful comments and suggestions from Harry Schachter, Jenny H.L. Chik, Frederico Alisson da Silva, and Chengcheng Huang.
FURTHER READING
- Fukuda M. 2002. Roles of mucin-type O-glycans in cell adhesion. Biochim Biophys Acta 1573: 394–405. [PubMed: 12417424]
- Hollingsworth MA, Swanson BJ. 2004. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4: 45–60. [PubMed: 14681689]
- Tarp MA, Clausen H. 2008. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta 1780: 546–563. [PubMed: 17988798]
- Brockhausen I. 2010. Biosynthesis of complex mucin-type O-glycans. In Comprehensive natural products. II: Chemistry and biology (ed. Mander L, Lui H-W, Wang PG, editors. ), Vol. 6, pp 315–350. Elsevier, Oxford.
- Jensen PH, Kolarich D, Packer NH. 2010. Mucin-type O-glycosylation—Putting the pieces together. FEBS J 277: 81–94. [PubMed: 19919547]
- Tabak LA. 2010. The role of mucin-type O-glycans in eukaryotic development. Semin Cell Dev Biol 21: 616–621. [PMC free article: PMC2902666] [PubMed: 20144722]
- Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. 2012. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22: 736–756. [PMC free article: PMC3409716] [PubMed: 22183981]
- Brockhausen I, Gao Y. 2012. Structural glycobiology: Applications in cancer research. In Structural glycobiology (ed. Yuriev E, Ramsland PA, editors. ), pp. 177–213. CRC Press, Boca Raton, FL.
- Ju T, Wang Y, Aryal RP, Lehoux SD, Ding X, Kudelka MR, Cutler C, Zeng J, Wang J, Sun X, et al. 2013. Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteomics Clin Appl 7: 618–631. [PMC free article: PMC5808880] [PubMed: 23857728]
- Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, et al. 2013. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J 32: 1478–1488. [PMC free article: PMC3655468] [PubMed: 23584533]
- Radhakrishnan P, Dabelsteen S, Madsen FB, Francavilla C, Kopp KL, Steentoft C, Vakhrushev SY, Olsen JV, Hansen L, Bennett EP, et al. 2014. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci 111: E4066–E4075. [PMC free article: PMC4191756] [PubMed: 25118277]
- Kong Y, Joshi HJ, Schjoldager KT, Madsen TD, Gerken TA, Vester-Christensen MB, Wandall HH, Bennett EP, Levery SB, Vakhrushev SY, et al. 2015. Probing polypeptide GalNAc-transferase isoform substrate specificities by in vitro analysis. Glycobiology 25: 55–65. [PMC free article: PMC4245906] [PubMed: 25155433]
- Review O-GalNAc Glycans.[Essentials of Glycobiology. 2022]Review O-GalNAc Glycans.Brockhausen I, Wandall HH, Hagen KGT, Stanley P. Essentials of Glycobiology. 2022
- Review O-GalNAc Glycans.[Essentials of Glycobiology. 2009]Review O-GalNAc Glycans.Brockhausen I, Schachter H, Stanley P. Essentials of Glycobiology. 2009
- Glycomolecule modifications in the seminiferous epithelial cells and in the acrosome of post-testicular spermatozoa in the alpaca.[Reprod Domest Anim. 2012]Glycomolecule modifications in the seminiferous epithelial cells and in the acrosome of post-testicular spermatozoa in the alpaca.Parillo F, Verini Supplizi A, Mancuso R, Catone G. Reprod Domest Anim. 2012 Aug; 47(4):675-86. Epub 2009 Jan 23.
- Determination of the site-specific oligosaccharide distribution of the O-glycans attached to the porcine submaxillary mucin tandem repeat. Further evidence for the modulation of O-glycans side chain structures by peptide sequence.[J Biol Chem. 2002]Determination of the site-specific oligosaccharide distribution of the O-glycans attached to the porcine submaxillary mucin tandem repeat. Further evidence for the modulation of O-glycans side chain structures by peptide sequence.Gerken TA, Gilmore M, Zhang J. J Biol Chem. 2002 Mar 8; 277(10):7736-51. Epub 2002 Jan 2.
- Structural and Quantitative Characterization of Mucin-Type O-Glycans and the Identification of O-Glycosylation Sites in Bovine Submaxillary Mucin.[Biomolecules. 2020]Structural and Quantitative Characterization of Mucin-Type O-Glycans and the Identification of O-Glycosylation Sites in Bovine Submaxillary Mucin.Kim J, Ryu C, Ha J, Lee J, Kim D, Ji M, Park CS, Lee J, Kim DK, Kim HH. Biomolecules. 2020 Apr 20; 10(4). Epub 2020 Apr 20.
- O-GalNAc Glycans - Essentials of GlycobiologyO-GalNAc Glycans - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

