NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.012

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsPlasma membrane proteins are either peripheral proteins or integral membrane proteins. The latter include proteins that span the lipid bilayer once or several times, and another class that are covalently attached to lipids. Proteins attached to glycosylphosphatidylinositol (GPI) via their carboxyl termini are generally found in the outer leaflet of the lipid bilayer and face the extracellular environment. The GPI membrane anchor may be conveniently thought of as an alternative to the single transmembrane domain of type-1 integral membrane proteins. This chapter reviews the discovery, distribution, structure, biosynthesis, properties, and suggested functions of GPI membrane anchors and related molecules.
BACKGROUND AND DISCOVERY
The first data suggesting the existence of protein-phospholipid anchors appeared in 1963 with the finding that crude bacterial phospholipase C (PLC) selectively releases alkaline phosphatase from mammalian cells. Phosphatidylinositol-protein anchors were first postulated in the mid-1970s based on the ability of highly purified bacterial phosphatidylinositol-specific PLC enzymes to release certain proteins, such as alkaline phosphatase and 5′-nucleotidase, from mammalian plasma membranes. By 1985, these predictions were confirmed by compositional and structural data from studies on Torpedo acetylcholinesterase, human and bovine erythrocyte acetylcholinesterase, rat Thy-1, and the sleeping sickness parasite Trypanosoma brucei variant surface glycoprotein (VSG). The first complete glycosylphosphatidylinositol (GPI) structures, which were those for T. brucei VSG and rat Thy-1, were solved in 1988 (Chapter 1, Figure 1.3).
DIVERSITY OF PROTEINS WITH GPI ANCHORS
To date, hundreds of GPI-anchored proteins (GPI-APs) have been identified in many eukaryotes, ranging from protozoa and fungi to humans (Online Appendix 12A). The range of described GPI-APs and the distribution of putative GPI biosynthesis genes suggests that (1) GPI anchors are almost ubiquitous among eukaryotes; (2) GPI-APs are functionally diverse and include hydrolytic enzymes, adhesion molecules, complement regulatory proteins, receptors, protozoan coat proteins, and prion proteins; and (3) in mammals, alternative messenger RNA (mRNA) splicing may lead to the expression of transmembrane and/or soluble and GPI-anchored forms of the same gene product. These variants may be developmentally regulated. For example, neural cell adhesion molecule (NCAM) exists in GPI-anchored and soluble forms when expressed in muscle and in GPI-anchored and two transmembrane forms when expressed in brain.
STRUCTURE OF GPI ANCHORS
The substructure Manα1-4GlcNα1–6myo-inositol-1-P-lipid is a universal hallmark of GPI anchors and related structures. All but one (Online Appendix 12B) protein-linked GPI anchors share a larger common core structure (Figure 12.1). The structural arrangements of GPI anchors are unique among protein–carbohydrate associations in that the reducing terminus of the GPI oligosaccharide is not attached to the protein. Rather, the reducing terminal glucosamine residue is α1-6 linked to the D-myo-inositol head group of a phosphatidylinositol (PI) moiety. A distal, nonreducing mannose residue is attached to the protein via an ethanolamine phosphate (EtNP) bridge between the C-6 hydroxyl group of mannose and the α-carboxyl group of the carboxy-terminal amino acid. GPIs are one of the rare instances in which glucosamine is found without either an N-acetyl or N-sulfate moiety (as in proteoglycans) (Chapter 17).

FIGURE 12.1.
General structure of GPI anchors. All characterized GPI anchors share a common core consisting of ethanolamine-PO4-6Manα1-2Manα1-6Manα1-4GlcNα1-6myo-inositol-1-PO4-lipid. Heterogeneity in GPI anchors is derived from various (more...)
Beyond the common core, the structures of mature GPI anchors are quite diverse, depending on both the protein to which they are attached and the organism in which they are synthesized (Figure 12.1 and Online Appendix 12B). Modifications to the core include additional EtNP and a wide variety of linear and branched glycosyl substituents of largely unknown function.
There is considerable variation in the PI moiety. Indeed, GPI is a rather loose term because, strictly speaking, PI refers specifically to D-myo-inositol-1-P-3(sn-1,2-diacylglycerol) (i.e., diacyl-PI), whereas many GPIs contain other types of inositolphospholipids, such as lysoacyl-PI, alkylacyl-PI, alkenylacyl-PI, and inositolphosphoceramide (Online Appendix 12B). Another variation, termed inositol acylation, is characterized by the presence of an ester-linked fatty acid attached to the C-2 hydroxyl of the inositol residue. The presence of this modification makes the anchor inherently resistant to the action of bacterial PI-specific PLC. The available lipid structural data suggest that (1) inositolphosphoceramide-based protein-linked GPIs are only found in “lower” eukaryotes, such as Saccharomyces cerevisiae, Aspergillus niger, Dictyostelium discoideum, and Trypanosoma cruzi; (2) the lipid structures of GPIs generally do not reflect those of the general cellular PI or inositolphosphoceramide pool; and (3) the lipid structures of some (e.g., trypanosome) GPI-APs are under developmental control.
The factors that control the synthesis of a mature GPI anchor found on a given protein appear to be similar to those for other posttranslational modifications such as N- and O-glycosylation. Thus, primary control is at the cellular level, whereby the levels of specific biosynthetic and processing enzymes dictate the final repertoire of structures. Secondary control is at the level of the tertiary/quaternary structure of the protein bearing the GPI anchor, which affects accessibility to processing enzymes. Examples of primary control include (1) differences in GPI glycan side chains in human versus porcine membrane dipeptidase and brain versus thymocyte rat Thy-1 and (2) differences in glycan side chains and lipid structure when T. brucei VSG is expressed in bloodstream and insect life-cycle stages of the parasite. An example of secondary control is the difference in VSG glycan side chains when VSGs with different carboxy-terminal sequences are expressed in the same trypanosome clone.
Non-Protein-Linked GPI Structures
In mammalian cells, some free GPIs (GPI-anchor biosynthetic intermediates) are found at the cell surface, but their functional significance is unknown. On the other hand, several protozoa (particularly trypanosomatids) express high numbers (>107 copies per cell) of free GPIs on their cell surface as metabolic end products. These include the so-called glycoinositol phospholipids (GIPLs) and lipophosphoglycans (LPGs) of the Leishmania. Some protozoan (type-1) GIPLs conform to the Manα1-6Manα1-4GlcNα1-6PI sequence common to protein-linked GPIs, whereas others contain a (type-2) Manα1-3Manα1-4GlcNα1-6PI motif, and still others are hybrid structures containing the branched motif (Manα1-6)Manα1-3Manα1-4GlcNα1-6PI.
IDENTIFICATION OF GPI-APs
The presence of a GPI anchor may be inferred by the identification of an amino-terminal signal peptide and a carboxy-terminal GPI signal peptide from the predicted primary amino acid sequence of a given gene (Figure 12.2). Such predictions can be verified by structural analysis, decribed below, or by indirect methods: the shift of proteins from the pellet to the supernatant after treatment of whole cells with PI-PLC is one such simple procedure. Variations on this theme use Triton X-114 (a nonionic detergent) phase separation whereby GPI-APs partition into the detergent-rich phase before, but not after, PI-PLC treatment. An additional criterion is the appearance of an epitope known as the “cross-reacting determinant” following PI-PLC cleavage. Some GPI anchors are acylated at C-2 of inositol and therefore resistant to PI-PLC but all are sensitive to serum GPI-phospholipase D (GPI-PLD). However, GPI-PLD cleavage generally requires detergent solubilization of the substrate and does not generate a “cross-reacting determinant.” The GPI-PLD reaction also leaves one fatty acid attached to the protein (the inositol acyl group) and, depending on the protein, this may prevent complete Triton X-114 phase separation after GPI-PLD digestion. Finally, GPI anchors may be labeled biosynthetically with [3H]myo-inositol. Certain pore-forming bacterial toxins such as aerolysin have been shown to bind to GPI anchors, and these may be used to probe one- and two-dimensional gel western blots.
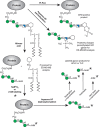
FIGURE 12.2.
Chemical and enzymatic reactions of glycosylphosphatidylinositol (GPI) anchors. Phosphatidylinositol-specific phospholipase C (PI-PLC) releases the lipid portion of the GPI anchor and generates a myo-inositol-1,2-cyclic phosphate group that is part of (more...)
THE CHEMISTRY OF GPI ANCHORS
GPI anchors are complex molecules that include amide, glycosidic, phosphodiester, and hydroxyester linkages between their various components and the challenge of their organic synthesis has been met by several groups. Analogs of GPI substructures have been instrumental in probing the comparative enzymology of GPI biosynthesis in “lower” and “higher” eukaryotes and methods to ligate synthetic GPIs to proteins, to make fluorescent GPI membrane probes and GPI glycan microarrays are now well established.
The GPI-anchor structure lends itself to selective cleavage by several chemical and enzyme reagents. These were originally used to help determine GPI structures and are now applied to confirm the presence of a GPI anchor and/or obtain partial structural information from native or [3H]mannose-, [3H]glucosamine-, [3H]inositol-, or [3H]fatty-acid-radiolabeled GPI-APs or GPI-biosynthetic intermediates. Some of these reactions and their applications for GPI structure analysis are illustrated in Figure 12.2. A key reaction is nitrous acid deamination of the glucosamine residue. This gentle (room temperature, pH 4.0) reaction is dependent on the free amino group of the glucosamine residue and gives a highly selective cleavage of the glucosamine-inositol glycosidic bond. The reaction liberates the PI moiety, which can be isolated by solvent partition and analyzed by mass spectrometry, and generates a free reducing terminus on the GPI glycan in the form of 2,5-anhydromannose. This reducing sugar can be reduced to [1-3H]2,5-anhydromannitol (AHM) by sodium borotritide reduction to introduce a radiolabel, or it may be attached to a fluorophore such as 2-aminobenzamide (2-AB) by reductive amination. Once the GPI glycan is radioactively or fluorescently labeled and dephosphorylated with aqueous hydrogen fluoride, the glycan can often be conveniently sequenced using exoglycosidases. Partial structural information may also be obtained by tandem mass spectrometry of GPI-peptides generated by trypsin, proteinase K, or Pronase digestion of GPI-APs, or by tandem mass spectrometry of GPI glycans released by aqueous hydrogen fluoride dephosphorylation and permethylated before analysis.
GPI BIOSYNTHESIS AND TRAFFICKING
The biosynthesis of GPI anchors occurs in three stages: (1) preassembly of a GPI precursor in the endoplasmic reticulum (ER) membrane, (2) attachment of the GPI to newly synthesized protein in the lumen of the ER with concomitant cleavage of a carboxy-terminal GPI-addition signal peptide, and (3) lipid remodeling and/or carbohydrate side-chain modifications in the ER and after transport to the Golgi.
Analysis of GPI precursor biosynthesis was first made possible by the development of a cell-free system in T. brucei. Each trypanosome has 1×107 molecules of GPI-linked VSG on its surface. Therefore, enzymes and intermediates in the GPI-biosynthetic pathway are relatively abundant in microsomal membrane preparations produced from this organism. The sequence of events underlying GPI biosynthesis has been studied in T. brucei, T. cruzi, Toxoplasma gondii, Plasmodium falciparum, Leishmania major, Paramecium, S. cerevisiae, Cryptococcus neoformans, and mammalian cells. The emphasis on eukaryotic microbes reflects the abundance of GPI-APs in these organisms and the potential of GPI inhibition for chemotherapeutic intervention. This notion has been genetically validated in the bloodstream form of T. brucei, in yeast, and in Candida albicans.
The essential events in GPI precursor biosynthesis are, like the core structure, highly conserved. There are, however, variations on the theme, and T. brucei and mammalian cell GPI pathways are used here to represent these differences (Figure 12.3). In all cases, GPI biosynthesis involves the transfer of N-acetylglucosamine from UDP-GlcNAc to PI to give GlcNAc-PI via an ER-membrane-bound multiprotein complex (Figure 12.4, Table 12.1,). This step occurs on the cytoplasmic face of the ER, as does the second step of the pathway, the de-N-acetylation of GlcNAc-PI to GlcN-PI. Notable differences between the T. brucei and mammalian GPI-biosynthetic pathways occur from GlcN-PI onward. Thus, inositol acylation (the transfer of fatty acid to the C-2 hydroxyl group of the D-myo-inositol residue) of GlcN-PI strictly follows the action of the first mannosyltransferase in T. brucei, whereas these steps are temporally reversed in mammalian cells. In the mammalian pathway, inositol acylation and inositol deacylation are discrete steps that occur only at the beginning and end of the pathway, respectively, whereas in T. brucei these reactions occur on multiple GPI intermediates. Furthermore, in some mammalian cells such as human erythroblasts, the inositol-linked fatty acid is never removed and the mature GPI protein retains three fatty chains (Online Appendix 12B, panel C). Fatty-acid remodeling in T. brucei occurs at the end of the pathway, but before transfer to VSG protein, and involves exchanging the sn-2 fatty acids (a mixture of C18–C22 species) and the sn-1 fatty acid (C18:0) exclusively for C14:0 myristate. In contrast, the lipid remodeling in mammalian cells is more complex. Many protein-linked GPIs contain sn-1-alkyl-2-acyl-PI with two saturated fatty chains, whereas major cellular PI is predominantly sn-1-stearoyl-2-arachidonoyl-PI (i.e., with C18:0 and C20:4 fatty acids and few, if any, alkyl or alkenyl species). Two processes are involved in these structural changes. First, remodeling from the diacyl-PI to the 1-alkyl-2-acyl form having unsaturated fatty acid at the sn-2-position occurs in the ER to GlcN-aPI. The reaction that mediates this remodeling is yet to be determined. Second, fatty-acid remodeling occurs after GPI is transferred to proteins, the inositol-linked acyl chain is removed, and GPI-APs are transported to the Golgi. This is accomplished by exchanging the unsaturated sn-2 fatty acid with saturated fatty acid, mainly stearate (C18:0). Lipid remodeling of GPIs in yeast also involves two processes but they occur in the ER. The first is fatty-acid remodeling that exchanges the unsaturated sn-2 fatty acid with the C26:0 chain. The second process involves the exchange of diacylglycerol for ceramide on many, but not all, GPI proteins.
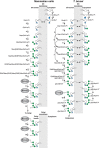
FIGURE 12.3.
Glycosylphosphatidylinositol (GPI)-biosynthetic pathways of mammalian cells and Trypanosoma brucei. These examples show that, despite the highly conserved core structure of GPI anchors, some diversity in GPI-anchor biosynthesis exists. In particular, (more...)

FIGURE 12.4.
Predicted topologies of the components of glycosylphosphatidylinositol (GPI) biosynthesis in mammalian cells. Components within boxes belong to multisubunit complexes. The step numbers refer to those in Figure 12.3 and Table 12.1. The topologies of the (more...)
TABLE 12.1.
Components of the mammalian glycosylphosphatidylinositol (GPI)-biosynthetic machinery
The identification of GPI pathway genes has been principally by expression cloning using GPI-deficient mutants of mammalian cells and temperature-sensitive yeast mutants. More recently, epitope tagging/pull-down/proteomic approaches have been used to identify GPI pathway-associated components. The known mammalian and yeast components and their respective topologies in the ER membrane are shown in Table 12.1 and Figure 12.4.
The transfer of the preassembled GPI precursor to protein occurs via a multisubunit transamidase complex with a cysteine-protease-like catalytic subunit. The reaction involves two complex substrates: the GPI precursor and the carboxyl terminus of a partially folded nascent protein (Figure 12.5). The carboxy-terminal GPI-addition signal peptide (GPIsp) has three domains: (1) three relatively small amino acids (Ala, Asn, Asp, Cys, Gly, or Ser) located at ω, ω+1, and ω+2, where ω is the amino acid attached to the GPI anchor and where ω+1 and ω+2 are the first two residues of the cleaved peptide; (2) a relatively polar domain of typically five to 10 residues; and (3) a hydrophobic domain of typically 15–20 hydrophobic amino acids. These GPIsp sequences do not have a strict consensus, but they are easily identified by eye and by automated algorithms. The final hydrophobic stretch of amino acids often resembles a transmembrane domain, but it is the absence of positively charged and polar residues immediately after it that makes a GPIsp easy to spot. Like N-glycosylation sequons, a GPIsp will only be functional if the protein is translocated into the ER. Essentially all GPI-APs have an amino-terminal signal peptide as well.
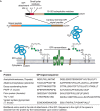
FIGURE 12.5.
(A) Features of glycosylphosphatidylinositol (GPI)-anchored proteins and their processing by GPI transamidase. GPI-anchored proteins have an amino-terminal signal peptide and a carboxy-terminal GPI-addition signal peptide (top) that is removed and directly (more...)
Apart from the final glycan structures (Online Appendix 12B), little is known about the genes and enzymes involved in adding carbohydrate side chains to the conserved GPI core. The only exceptions are the mannosyltransferase (encoded by SMP3/PIGZ) that adds the fourth αMan residue to yeast and mammalian GPI anchors during GPI precursor assembly in the ER, and a Gal- and GlcNAc-transferase involved in elaborating the T. brucei procyclin GPI anchor in the Golgi.
The transport of GPI-APs from the ER to the Golgi is mediated by coat protein II (COPII)-coated transport vesicles. Packaging of GPI-APs into the vesicles requires a transmembrane cargo receptor, a complex of four p24 family proteins, that link luminally oriented GPI to COPII components on the cytoplasmic side.
MEMBRANE PROPERTIES OF GPI-APs
GPI-APs with two long hydrocarbon chains (i.e., those containing diacylglycerol, alkylacylglycerol, alkenylacylglycerol, or ceramide) provide a stable association with the lipid bilayer. It follows that inositol-acylated GPI proteins with three fatty-acid chains should be more stably associated. On the other hand, a GPI-related structure with only a single C24:0 alkyl chain (e.g., the lipophosphoglycan of Leishmania) has a half-life of only minutes at the cell surface and is secreted intact into the medium. The thermodynamics of bilayer interactions also depend on the nature of the fatty-acid chains (length and degree of saturation). In this regard, the saturated nature of most (but not all) mammalian GPI anchors (Online Appendix 12B, panels C and D) is thought to explain why GPI-APs associate with “lipid rafts.” The current model for mammalian lipid rafts is that of transient liquid-ordered nanoclusters of membrane components. These are dependent on dynamic cortical actin asters that, through adaptor proteins, cluster phosphatidylserine (PS) phospholipids on the inner face of the plasma membrane bilayer. Compared with most other phospholipid classes, PS has long saturated fatty acids and bringing these together in a cluster provides a nucleating center to cocluster other long-chain membrane components in the outer leaflet of the membrane, including GPI-APs and glycosphingolipids. This coupling effect occurs because the long saturated lipid chains from inner leaflet PS overlap and interact with those of GPI-APs and glycosphingolipids in the middle of the bilayer. A compelling aspect of this model is that, although actin can organize molecules in the outer leaflet into nanoclusters, the clustering of GPI-APs in the outer leaftlet (with antibodies or multivalent ligands) can also, conversely, organize the cortical actin and its associated machinery. This provides a possible explanation for the perplexing, but well-characterized, ability of GPI-APs to transduce signals across the plasma membrane.
There are many examples of transmembrane signaling via the cross-linking of GPI-APs with antibody and clustering with a second antibody on various cells, particularly leukocytes. Cellular responses include an increase in intracellular Ca++, tyrosine phosphorylation, proliferation, cytokine induction, and oxidative burst. These antibody-induced signaling events are clearly dependent on the presence of a GPI anchor and might be due to the induction and coalescence of lipid raft nanoclusters. Despite the plethora of GPI-protein cross-linking/signal-transduction examples, it should be noted that there are no receptor/ligand pairs of established physiological relevance that signal in a GPI-dependent way. Thus, GPI-APs known to be involved in transmembrane signaling, such as the glial-cell-(line-)derived neurotrophic factor receptor-α (GDNFR-α), need to be associated with transmembrane β coreceptors to transmit their signals. Similarly, GPI-anchored CD14 (the LPS/LPS-binding protein receptor) functions equally well with a GPI anchor or with a spliced transmembrane domain, and the signal-transducing partner for CD14 is the transmembrane Toll-like receptor-4.
GPI ANCHORS AS TOOLS IN CELL BIOLOGY
The replacement of carboxy-terminal transmembrane domains of type-1 integral membrane proteins by GPI-addition signal peptides allows their expression on the plasma membrane of transfected mammalian cells in GPI-anchored form. This can be a useful way to produce soluble forms of membrane proteins. For example, the T-cell receptor could not be expressed in a soluble form by simply deleting the transmembrane domain, but it could be expressed in GPI-anchored form and then rendered soluble by the action of bacterial PI-PLC. In addition, purified GPI-APs can be used to coat hydrophobic surface plasmon resonance chips, thus providing a convenient way of orienting and presenting proteins for binding studies. It is clear that purified GPI-APs will spontaneously insert into lipid bilayers. The physiological significance of direct GPI-protein exchange between membranes is still uncertain, particularly because all mammals express potent GPI-PLD activity in serum that can remove the lipid (phosphatidic acid) component of the anchor and, therefore, prevent GPI-protein reinsertion. The reinsertion property of GPI-APs has been exploited experimentally to “paint” exogenous proteins onto cell surfaces.
BIOLOGICAL FUNCTIONS OF GPI ANCHORS
GPI anchors are essential for life in some, but not all, eukaryotic microbes. In the yeast S. cerevisiae, and probably most fungi, the presence of a GPI anchor is used to target certain mannoproteins for covalent incorporation into the β-glucan cell wall. Cross-linking occurs via a transglycosylation reaction, whereby a mannose residue within the GPI-anchor core is transferred to the β-glucan polymer. Defects in cell wall biosynthesis are known to be detrimental to yeast and this may be why GPI biosynthesis is essential to this organism. Gene knockout studies have shown that GPI biosynthesis is also essential for the bloodstream form of T. brucei, even in tissue culture. This may be due to nutritional stress because this parasite uses an essential GPI-anchored transferrin receptor. On the other hand, surprisingly, GPI biosynthesis and/or transfer to protein are not essential for the insect-dwelling forms of T. brucei or Leishmania. The availability of GPI-deficient mammalian cell lines shows that GPI-APs are not essential at a cellular level. However, mouse knockouts and tissue-specific conditional knockouts of the PIGA gene (the catalytic subunit of the UDP-GlcNAc:PI α1-6 N-acetylglucosaminyltransferase) clearly show that GPI-APs are essential for early embryo and tissue development, respectively. In the plant Arabidopsis, GPI biosynthesis is required for cell wall synthesis, morphogenesis, and pollen tube development. GPI anchors impart to their attached proteins the ability to be shed in soluble form from the cell surface through the action of cellular or serum GPI-cleaving enzymes. Mammalian sperm acquire an ability to fuse with oocytes after GPI-anchored TEX101 is released by a sperm-associated GPI-cleaving enzyme tACE (testis form angiotensin converting enzyme). Certain proliferating motor neurons initiate differentiation after a GPI-anchored proteinase inhibitor RECK is released by a GPI-cleaving enzyme GDE2. When the activity of ADAM10 metalloproteinase suppressed by RECK is expressed, Notch-ligand is degraded by ADAM10, resulting in Notch signaling termination and a switch from proliferation to differentiation. In “lower” eukaryotes, GPI anchors may be useful for assembling particularly dense cell-surface protein coats, such as the VSG coat of T. brucei. In this case, each parasite expresses five million VSG dimers on the cell surface to protect it against complement-mediated lysis. If each VSG monomer had, instead of a GPI anchor, a single transmembrane domain, there would be little room for other integral membrane proteins such as hexose and nucleoside transporters. Generally, GPI-APs do recycle through intracellular compartments but, compared with typical transmembrane proteins, they reside in higher proportion on the cell surface and have longer half-lives. There are several examples of the exchange of GPI-APs from one cell surface to another. Some GPI-APs are incorporated into exosomes, suggesting a possibility of exosome-mediated cell-to-cell transfer. Sperm acquire some GPI-APs such as CD52 from epididymis most likely mediated by exosomes.
GPI ANCHORS AND DISEASE
Paroxysmal nocturnal hemoglobinuria (PNH) is a human disease in which patients suffer from hemolytic anemia. The condition arises from loss of expression of several GPI-APs that protect their blood cells from lysis by the complement system (e.g., decay accelerating factor and CD59). The defect in PNH cells is a somatic mutation in the X-linked PIGA gene and appears to occur in a bone marrow stem cell. Unlike other enzymes in the pathway, which are encoded by autosomal genes, PNH caused by PIGA mutations is thought to arise at a higher frequency because of X inactivation. Both in male and female stem cells, somatic mutation in the one active allele of PIGA results in the complete loss of a functional UDP-GlcNAc:PI α1–6 N-acetylglucosaminyltransferase (Chapter 46).
Inherited GPI deficiencies (IGDs) are caused by germline mutations in genes involved in GPI biosynthesis, protein transfer, and remodeling. Because complete GPI deficiency causes embryonic lethality, mutations in IGDs are hypomorhic, causing partial deficiency. Mutations in genes involved in GPI remodeling such as PGAP1 can be null and cause GPI-APs with abnormal structure. Patients with IGDs caused by mutations in 14 genes in the GPI-biosynthetic pathway have been reported. Most of these mutations were identified by whole exome sequencing of patients’ cells. Major symptoms of IGDs are neurological problems such as developmental delay/intellectual disability, seizures, cerebral and/or cerebellar progressive atrophy, hearing loss, and visual impairment. Other symptoms include hyperphosphatasia; brachytelepharangy; typical facial features such as hypertelolism and tented mouth; cleft palate; anorectal, renal, and heart anomalies; and Hirschsprung disease (Chapter 45).
GPI biosynthesis and transfer to protein are essential for yeast, for pathogenic fungi, and for the African sleeping sickness parasite T. brucei as mentioned above. Several key surface molecules of the apicomplexan parasites Plasmodium (malaria), Toxoplasma, and Cryptosporidium are GPI-anchored, and it is thought that the GPI pathway is likely to be essential to these pathogens. Thus, pathogen-specific GPI pathway inhibitors are being actively sought as potential drugs. In addition, there is evidence that some parasite GPI anchors have a direct role in modulating the host immune response to infection.
Like other glycoconjugates, GPI-APs can be exploited by pathogens. For example, the GPI anchors themselves are receptors for hemolytic pore-forming toxins such as aerolysin from Aeromonas hydrophilia, which causes gastroenteritis, deep wound infections, and septicemia in humans. In addition, the GPI-AP CD55/DAF is the principal cell-surface ligand for enterovirus and several echoviruses. Finally, the endogenous prion protein is GPI-anchored and it is thought that the conformational changes that it undergoes to become the aberrant spongiform-encephalopathy (“mad cow disease” or scrapie in sheep)-causing form may be associated with a clathrin-independent endocytic pathway followed by GPI-anchored prion protein in neurons.
ACKNOWLEDGMENTS
The authors appreciate helpful comments and suggestions from Vered Padler-Karavani, Kristian Saied-Santiago, and Daniel Varon Silva.
FURTHER READING
- Ferguson MA, Williams AF. 1988. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem 57: 285–320. [PubMed: 3052274]
- Ferguson MA, Homans SW, Dwek RA, Rademacher TW. 1988. Glycosyl-phosphatidylinositol moiety that anchors Trypanosoma brucei variant surface glycoprotein to the membrane. Science 239: 753–759. [PubMed: 3340856]
- Guha-Niyogi A, Sullivan DR, Turco SJ. 2001. Glycoconjugate structures of parasitic protozoa. Glycobiology 11: 45R–59R. [PubMed: 11358874]
- de Macedo CS, Shams-Eldin H, Smith TK, Schwarz RT, Azzouz N. 2003. Inhibitors of glycosylphosphatidylinositol anchor biosynthesis. Biochimie 85: 465–472. [PubMed: 12770785]
- Maeda Y, Ashida H, Kinoshita T. 2006. CHO glycosylation mutants: GPI anchor. Methods Enzymol 416: 182–205. [PubMed: 17113867]
- Levental I, Grzybek M, Simons K. 2010. Greasing their way: Lipid modifications determine protein association with membrane rafts. Biochemistry 49: 6305–6316. [PubMed: 20583817]
- Nikolaev AV, Al-Maharik N. 2011. Synthetic glycosylphosphatidylinositol (GPI) anchors: How these complex molecules have been made. Nat Prod Rep 28: 970–1020. [PMC free article: PMC5496678] [PubMed: 21448495]
- Tsai YH, Liu X, Seeberger PH. 2012. Chemical biology of glycosylphosphatidylinositol anchors. Angew Chem Int Ed Engl 51: 11438–11456. [PubMed: 23086912]
- Guo Z. 2013. Synthetic studies of glycosylphosphatidylinositol (GPI) anchors and GPI-anchored peptides, glycopeptides, and proteins. Curr Org Synth 10: 366–383. [PMC free article: PMC4063365] [PubMed: 24955081]
- Raghupathy R, Anilkumar AA, Polley A, Singh PP, Yadav M, Johnson C, Suryawanshi S, Saikam V, Sawant SD, Panda A, Guo Z, Vishwakarma RA, Rao M, Mayor S. 2015. Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell 161: 581–594. [PMC free article: PMC4651428] [PubMed: 25910209]
- Kinoshita T, Fujita M. 2017. Biosynthesis of GPI-anchored proteins: Special emphasis on GPI lipid remodeling. J Lipid Res 57: 6–24. [PMC free article: PMC4689344] [PubMed: 26563290]
- Muñiz M, Riezman H. 2017. Trafficking of glycosylphosphatidylinositol anchored proteins from the endoplasmic reticulum to the cell surface. J Lipid Res 57: 352–360. [PMC free article: PMC4767001] [PubMed: 26450970]
- BACKGROUND AND DISCOVERY
- DIVERSITY OF PROTEINS WITH GPI ANCHORS
- STRUCTURE OF GPI ANCHORS
- IDENTIFICATION OF GPI-APs
- THE CHEMISTRY OF GPI ANCHORS
- GPI BIOSYNTHESIS AND TRAFFICKING
- MEMBRANE PROPERTIES OF GPI-APs
- GPI ANCHORS AS TOOLS IN CELL BIOLOGY
- BIOLOGICAL FUNCTIONS OF GPI ANCHORS
- GPI ANCHORS AND DISEASE
- ACKNOWLEDGMENTS
- FURTHER READING
- Review Glycosylphosphatidylinositol Anchors.[Essentials of Glycobiology. 2009]Review Glycosylphosphatidylinositol Anchors.Ferguson MAJ, Kinoshita T, Hart GW. Essentials of Glycobiology. 2009
- Review Glycosylphosphatidylinositol Anchors.[Essentials of Glycobiology. 2022]Review Glycosylphosphatidylinositol Anchors.Komath SS, Fujita M, Hart GW, Ferguson MAJ, Kinoshita T. Essentials of Glycobiology. 2022
- Review The Glycosylphosphatidylinositol Anchor: A Linchpin for Cell Surface Versatility of Trypanosomatids.[Front Cell Dev Biol. 2021]Review The Glycosylphosphatidylinositol Anchor: A Linchpin for Cell Surface Versatility of Trypanosomatids.Borges AR, Link F, Engstler M, Jones NG. Front Cell Dev Biol. 2021; 9:720536. Epub 2021 Nov 1.
- Synthetic analogues of glycosylphosphatidylinositol-anchored proteins and their behavior in supported lipid bilayers.[J Am Chem Soc. 2007]Synthetic analogues of glycosylphosphatidylinositol-anchored proteins and their behavior in supported lipid bilayers.Paulick MG, Wise AR, Forstner MB, Groves JT, Bertozzi CR. J Am Chem Soc. 2007 Sep 19; 129(37):11543-50. Epub 2007 Aug 23.
- Review Biosynthesis of GPI-anchored proteins: special emphasis on GPI lipid remodeling.[J Lipid Res. 2016]Review Biosynthesis of GPI-anchored proteins: special emphasis on GPI lipid remodeling.Kinoshita T, Fujita M. J Lipid Res. 2016 Jan; 57(1):6-24. Epub 2015 Nov 12.
- Glycosylphosphatidylinositol Anchors - Essentials of GlycobiologyGlycosylphosphatidylinositol Anchors - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

