NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.025

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsThis chapter focuses on the nematode (roundworm) Caenorhabditis elegans as an example of the phylum Nematoda. C. elegans provides a powerful genetic system for studying glycans during embryological development and in primitive organ systems.
DEVELOPMENTAL BIOLOGY OF C. ELEGANS
C. elegans is transparent, and individual cells can be easily visualized in the living organism through all stages of development. Basically, the worm is a tube within a tube (Figure 25.1). A cuticle composed of a collagenous, multilayered, protective exoskeleton surrounds the worm. The “mouth” at the anterior end connects to a tubular intestinal system, which is composed of a muscular pharynx and an intestine. The gonad occupies most of the body cavity. In the hermaphrodite, the gonad is bilobed, with each lobe connecting via an oviduct and spermatheca to a shared midventral vulva and uterus. The worm exists as two sexes, hermaphrodite or male (no female organisms exist). Eggs pass through the spermatheca where fertilization takes place by stored sperm, and the eggs begin to develop inside the mother. During sexual reproduction, males fertilize hermaphrodites. The male sperm is also stored in the spermatheca and is preferentially used during fertilization.
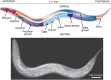
FIGURE 25.1.
Caenorhabditis elegans. A composite diagram (upper panel) and photograph (lower panel) of the adult hermaphrodite with labeled body parts. (Photograph kindly provided by Dr. Ian D. Chin-Sang at Queen's University, Kingston, Ontario.) For additional details (more...)
Gastrulation begins before egg laying; at this stage, the embryo contains about 30 cells (Figure 25.2). Proliferation results in an embryo of 558 relatively undifferentiated cells. Following this, organogenesis/morphogenesis begins, terminal differentiation occurs, and the embryo hatches. The animal normally passes through four larval stages, termed L1, L2, L3, and L4 (Figure 25.2). The end of each larval stage is marked by molting, when the cuticle is shed. In L1 larvae, the nervous system, the reproductive system, and the digestive tract begin to develop, and this is completed by the L4 stage. Mature adults develop ∼45–50 h after hatching. Mature hermaphrodites contain 959 somatic cells, including 302 neurons, and 95 body-wall muscle cells. At this time, the hermaphrodite can lay its first eggs, thus completing the 3.5 d life cycle. The adult hermaphrodite produces oocytes for ∼4 d, resulting in about 300 progeny; afterward, the animal lives for another 10–15 d. Overcrowding or starvation results in the formation of dauer larvae, a dormant stage, which are easily distinguished from other developmental stages by morphology and behavior.

FIGURE 25.2.
Life cycle of Caenorhabditis elegans. For additional details on the biology of C. elegans, see the WormAtlas.
GLYCANS IN C. ELEGANS
Considering the large number of glycomic studies on C. elegans since 2001, it is probably not an exaggeration to state that this anatomically simple worm has one of the most varied and unusual glycomes of any invertebrate organism studied to date. Although there are a number of conserved elements in its glycans, there are many notable differences between the types of glycans made by C. elegans and those in “higher” animals. There are, for example, no sialic acids or other anionic moieties on its N-glycans, but it expresses a wide range of fucosylated structures. The N-glycans are often truncated, but contain modifications very different from those found in “higher” animals. Moreover, worm O-glycans and glycolipids, which also lack sialic acids, have different core structures. Although some predictions regarding the types of oligosaccharides in the worm can be made from the range of glycosylation-relevant genes in its genome, glycan analyses continue to surprise as to the glycomic potential of this organism.
The N-glycans of C. elegans are synthesized by the common eukaryotic pathway involving generation of the 14-sugar precursor Glc3Man9GlcNAc2-P-P-Dol and transfer of the glycan to asparagine residues within the sequon -Asn-X-Ser/Thr- of nascent polypeptides in the endoplasmic reticulum (ER) (Chapter 9). These glycans can be trimmed to Man9GlcNAc2-Asn and further to Man5GlcNAc2-Asn by α-glucosidases I and II and α-mannosidases I and II. The Man5GlcNAc2-Asn can be modified by the addition of N-acetylglucosamine to generate GlcNAc1Man5GlcNAc2-Asn, which is then further processed in a unique way in C. elegans (Figure 25.3). Subsequent trimming and modification by Golgi β-hexosaminidases, α-mannosidases, and α-fucosyltransferases generate truncated N-glycans with three or fewer mannose residues (so-called paucimannosidic forms) and two types of core α1-3 fucose residue, in addition to the common vertebrate modification with α1-6 fucose. Although truncated N-glycans are common in C. elegans, the organism also generates more complex branched structures. An interesting modification is the addition of galactose to core fucose residues and to a bisecting position of the core β-mannose in a significant population of the N-glycans (Figure 25.3). Many hybrid and complex N-glycans in C. elegans contain phosphorylcholine linked to outer-chain N-acetylglucosamine residues (a modification that appears to be a recurring feature of nematode glycoproteins) and hexose and fucose residues of N- and O-linked glycans can be methylated.
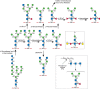
FIGURE 25.3.
Biosynthesis of paucimannosidic and core fucosylated N-glycans in Caenorhabditis elegans.
The “mucin-type” O-glycans of C. elegans contain core-1 structures common to vertebrates (Chapter 10), but they also include extended core-1 O-glycans containing β-glucose and glucuronic acid residues, α1-2 fucose residues, and 2-O-methylated fucose (Figure 25.4). As in mammals and insects, the C. elegans genome encodes multiple UDP-GalNAc: polypeptide α-N-acetylgalactosaminyltransferases (ppGalNAcTs) that modify Ser/Thr residues of mucin core polypeptides. C. elegans also contains a gene encoding the relevant β1-3 galactosyltransferase (T-synthase), which generates the core 1 O-glycan structure.
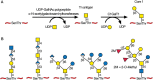
FIGURE 25.4.
Biosynthesis of core-1 O-glycan in Caenorhabditis elegans (A) and some O-glycans proposed to occur in adult worms (B).
Similar to mammals, plants, and insects, C. elegans synthesizes O-GlcNAc on Ser/Thr residues of cytoplasmic and nuclear proteins, using a highly conserved O-GlcNAc transferase (OGT); the worm also has the O-GlcNAcase that removes O-GlcNAc (Chapter 19). Interestingly, deletion of OGT, although fatal to vertebrate cells, is not lethal to C. elegans but is accompanied by a phenotype that resembles human insulin resistance. A related phenotype is also produced by deletion of the O-GlcNAcase.
Glycosphingolipids (Chapter 11) in C. elegans have a core consisting of GlcNAcβ1-3Manβ1-4Glcβ1-Cer, which is based, as in insects, on the arthroseries Manβ1-4Glcβ1-Cer core rather than the common Galβ1-4Glcβ1-Cer core found in vertebrates. In addition, C. elegans has genes encoding the enzymes for synthesis of GPI-anchored glycoproteins, but the GPI anchor structures are not yet defined (Chapter 12).
C. elegans has all of the enzymes for synthesizing the glycosaminoglycans (GAGs) and chondroitin and heparan sulfate (HS) but not those for keratan sulfate (KS) or dermatan sulfate (DS). However, C. elegans only generates unsulfated chondroitin chains because it lacks the relevant sulfotransferases and epimerase present in vertebrates (Chapter 17). In contrast, C. elegans does have the sulfotransferases and epimerase for generating HS, and the overall structure is similar to the chains elaborated by vertebrates. C. elegans does not make hyaluronan (Chapter 16).
As judged by the characterization of peptide-O-fucosyltransferase POFUT1 and POFUT2 homologs, C. elegans also synthesizes glycoproteins with O-fucose (Fucα1-Ser/Thr) on epidermal growth factor (EGF)-like domains and thrombospondin type-1 repeats (TSRs), in the context of precise consensus sequences (Chapter 13). Regarding elongation of this developmentally relevant modification, there is no evidence as yet for the necessary Fringe-related β1-3GlcNAcT, but a homolog of the β1-3-glucosyltransferase which modifies fucosylated TSRs is predicted in the worm genome. Another TSR-modifying enzyme, the C-mannosyltransferase is encoded by the dpy-19 gene. On the other hand, genomic data would suggest that O-mannosylation of dystroglycan is absent from C. elegans.
GLYCOSYLTRANSFERASE GENES IN C. ELEGANS
The C. elegans genome encodes homologs of many of the enzymes for glycoconjugate biosynthesis that are found in “higher” animals and humans, including enzymes for the synthesis of O-GalNAc (mucin-type) glycans, O-GlcNAc, N-glycans, GAGs, glycosphingolipids (GSLs), and glycosylphosphatidylinositol (GPI) anchors. In contrast to vertebrates (Chapter 15), the worm lacks sialic acids and any enzymes associated with sialic acid biosynthesis or utilization. Additionally, on the basis of the structures of its N- and O-glycans, glycolipids, and GAGs, C. elegans is predicted to express a wide assortment of enzymes involved in glycoconjugate metabolism. Indeed, the genome of C. elegans appears to encode about 300 carbohydrate-active enzymes (CAZy database; http://www.cazy.org/), including glycosyltransferases, glycosidases, epimerases, polysaccharide lyases, and carbohydrate esterases (Chapter 52). The glycosyltransferase family contains more than 240 genes. To date, most of these putative enzymes are not characterized, and very little is known overall about their expression or function in C. elegans. Some information has arisen from phenotypes generated by mutagenesis, as discussed below. Together, these studies have identified some interesting differences and similarities between C. elegans and vertebrates.
The C. elegans genome contains some extremely large classes of glycosyltransferase homologus, including 11 putative ppGalNAcTs, 32 α-fucosyltransferase homologs (5 α1-3FucTs, 26 α1-2FucTs, and 1 α1-6FucT), and more than 20 β-N-acetylglucosaminyltransferase homologs. Relatively few glycan-modifying enzymes from C. elegans have been shown to be functional; in only a few cases has their acceptor specificity been characterized. Of the peptide-modifying glycosyltransferases, eleven of the nematode ppGalNAcTs have been prepared as recombinant proteins, but only five of them are active toward mammalian peptide acceptors. The single C. elegans cytosolic or nuclear O-GlcNAc transferase (OGT) and the GAG-initiating O-xylosyltransferase homologs as well as its POFUT1 and 2 O-fucosyltransferases have been characterized at the biochemical and genetic levels.
In terms of Golgi modifications of N-glycans, C. elegans contrasts with humans and most other vertebrates in that it has three genes, rather than just one, encoding the β1-2 N-acetylglucosaminyltransferase (GlcNAcT-1) that catalyzes formation of GlcNAcβ1-2Man5GlcNAc2-Asn (see Figure 25.3). This product is then acted on by a single α-mannosidase II to generate GlcNAcβ1-2Man3GlcNAc2-Asn. As in many invertebrates, the antennal GlcNAc is normally absent from the final glycan products and indeed C. elegans has two relevant β-hexosaminidases (HEX-2 and HEX-3), which cleave GlcNAcβ1-2Man3GlcNAc2-Asn to generate the paucimannose structure Man3GlcNAc2-Asn, a reaction not found in vertebrates. On the other hand, there is no evidence for galactosylation of antennal GlcNAc and the closest C. elegans homolog to the human β1-4 galactosyltransferase is actually a β1-4 N-acetylgalactosaminyltransferase generating the LacdiNAc sequence (GalNAcβ1-4GlcNAc-R). C. elegans can synthesize the canonical core tetrasaccharide of GAGs, GlcAβ1-3Galβ1-3Galβ1-4Xylβ1-Ser, and can extend this to generate chondroitin and HS. As mentioned above, the glycolipids in C. elegans have the unusual arthroseries core structure, and the organism lacks a gene encoding the β1-4 galactosyltransferase that would synthesize the more familiar Galβ1-4Glcβ1-Cer glycolipid core.
Expression of the known glycosyltransferases in C. elegans has not been systematically mapped at the cellular level during development or in the adult organism. Many of the studies are based on promoter analyses using GFP as a reporter. Therefore, the promoter region for a gene of interest (usually 0.5–1.5 kb upstream of the gene and sometimes including the upstream elements and the first few exons of the gene) is ligated to the cDNA encoding GFP. Transgenic animals are then produced by direct injection of DNA into the hermaphrodite gonad. Newly developing animals take up this DNA and become transgenic. Thus, one can observe promoter utilization in the different stages of development (see Figure 25.2). Using this approach, it has been found that some glycosyltransferase genes are widely expressed, including the T-synthase (the β1-3 galactosyltransferase generating core 1 O-glycans; Figure 25.4), the SQV-2 galactosyltransferase that adds the second galactose residue to the core tetrasaccharide of GAGs (Figure 25.5), and the two protein O-fucosyltransferases POFUT1 and POFUT2. In contrast, expression of the six individual core-2 N-acetylglucosaminyltransferases with sequences related to core-2 enzymes in vertebrates (see Chapter 10) occur in selective tissues. One gene, gly-15, is expressed only in two gland cells. Similarly, among the 26 α1-2FucTs that are predicted in C. elegans, one of them (CE2FT-1; FUT-2) is expressed in a single cell in embryos and exclusively in 20 intestinal cells of larval stages L1–L4 and adult worms. Thus, in large gene families, individual members may be expressed in a localized fashion and have unique activities toward certain substrates, whereas single gene families appear to be expressed in all cells.
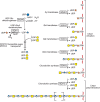
FIGURE 25.5.
Biosynthesis of chondroitin in Caenorhabditis elegans. Mutations in individual steps were identified as squashed vulva mutants, as described in the text.
FUNCTIONAL ANALYSIS OF GLYCOCONJUGATES
Different methods exist to genetically manipulate C. elegans and many of these approaches have yielded important information about the functions of glycosyltransferases, their glycan products and lectin-binding proteins. Several dozen genes involved in glycosylation pathways have been shown to be developmentally important in C. elegans or important in resistance or susceptibility to pathogens in the innate immunity of the worm. Only some of the highlights of this work are described here. More details are available in the literature cited at the end of the chapter.
N-Glycans and O-Glycans on Glycoproteins
Glycoproteins in C. elegans have both N- and O-glycans, as discussed above. In vertebrates, interference of the early steps in N-glycosylation or O-glycosylation causes embryonic lethality or results in severe developmental phenotypes. As in vertebrates, interference of the later steps in N- and O-glycan biosynthesis in C. elegans does not cause developmental problems. As mentioned above, the genome of C. elegans contains three genes (gly-12, gly-13, and gly-14) that encode GlcNAcT-I-like enzymes, whereas mammals have a single GlcNAcT-I gene. Promoter analyses show that gly-12 and gly-13 are expressed in all cells beginning in embryogenesis, whereas gly-14 is expressed only in intestinal cells from L1 to adults. Unexpectedly and in contrast to mammals, deletion of any of these genes singly or in combination does not affect C. elegans development, but has a profound effect on the N-glycome. The triple-knockout worms do not generate paucimannosidic Man2-3GlcNAc2 N-glycans (with and without core α1-6-fucose), but generate Man5GlcNAc2-Asn as the major structure. On the other hand, core α1-3-fucosylation is not abolished in this mutant.
C. elegans contains three α-mannosidase activities: one α-mannosidase II/IIx-like activity involved in N-glycan processing and encoded by the aman-2 (F58H1.1) gene, one lysosomal enzyme, and a third Co++-dependent enzyme. A mutant harboring a large deletion in the F58H1.1 gene generates largely Man5GlcNAc2-Asn, GlcNAc1Man5GlcNAc2-Asn-R, and fucosylated and phosphorylcholine-modified Man5GlcNAc2-Asn, but lacks the paucimannosidic structures. In addition, the mutant has reduced levels of the core α1-3 fucose antigen associated with antibodies to horseradish peroxidase. Interestingly, promoter analyses show that the F58H1.1 gene is expressed in most cells of the organism, but there is no obvious developmental phenotype in the aman-2 mutant.
Two of the largest gene families in C. elegans are the ppGalNAcT and the α-fucosyltransferase families. To date, however, there have been no developmental phenotypes associated with the loss of any members of these families. Although some ppGalNAcT isoforms may be redundant, most fucosyltransferase homologs have no known function. However, three core fucosyltransferases have been characterized and the glycomes of the corresponding mutants analyzed. Although the fut-8 gene encodes an enzyme with the same specificity as the mammalian FUT8 α1-6-fucosyltransferases, two α1-3-fucosyltransferases (FUT-1 and FUT-6) with unusual substrate preferences transfer fucose to the proximal and distal core GlcNAc residues; whereas FUT-1, unlike plant and insect core α1-3-fucosyltransferases, cannot transfer to glycans with a β1-2GlcNAc on the α1-3-mannose, the action of FUT-6 is blocked by the presence of α1-6-mannose. Also, activity of recombinant forms of two α1-2-fucosyltransferases as well as of a fucose-modifying β1-4-galactosyltransferase (GALT-1) have been reported. However, there are still many holes in our knowledge as glycosyltransferases required for many glycosidic bonds are still to be identified or we have many other glycosyltransferase homologs for which no corresponding activity is known.
The biosynthesis of all fucosylated ligands requires the precursor GDP-fucose and its transport into the Golgi apparatus by nucleotide sugar transporters (Chapter 5). Interestingly, the human disease leukocyte-deficiency type II (LAD II) is caused by a defect in the transport of GDP-fucose and the subsequent loss of fucosylated ligands important in leukocyte trafficking and recognition by selectins (Chapter 34). A search of the C. elegans genome for putative nucleotide sugar transporters led to the identification of several candidates, one of which complemented the transport and fucosylation defect in LAD II fibroblasts and led to the identification of the defect in these patients.
C. elegans is also an interesting model system to study infection and innate immunity. The organism may be colonized by different bacterial pathogens, including Pseudomonas aeruginosa, Yersinia pestis, and Yersinia pseudotuberculosis. The two Yersinia species generate a sticky biofilm (an exopolysaccharide matrix encasing a community of bacteria) on the exterior of the worm's head that impairs viability. P. aeruginosa, in contrast, colonizes the intestinal tissues. Another bacterium, Microbacterium nematophilum, sticks to the anus of the animals and induces an irritation in the underlying hypodermal tissue. Bacillus thuringiensis (Bt) infection leads to destruction of the intestine, which is discussed in more detail below in regard to glycolipids. Mutations in the worm, some of them affecting glycosylation processes, have been found that affect colonization by these bacteria.
An especially interesting set of mutations are the srf mutants (altered surface antigenicity mutants). Some of the srf mutants were identified by altered antibody or lectin binding to the cuticle, indicating that loss of cuticle components exposed new antigens. srf-3 mutants are resistant to infection by M. nematophilum. srf-3 encodes a nucleotide sugar transporter that can transport both UDP-galactose and UDP-N-acetylglucosamine, suggesting that altered sugar composition of the cuticle resulting from mutations in this transporter confer resistance to M. nematophilum. Interestingly, there are 18 putative nucleotide sugar transporters in the genome of C. elegans, which is a considerably larger number than the known nucleotide sugars (UDP-galactose, UDP-glucose, UDP-N-acetylglucosamine, UDP-N-acetylgalactosamine, UDP-xylose, GDP-mannose, and GDP- fucose), suggesting possible functional overlap in these transporters. srf-3 mutants are deficient in glycosylation, in particular, they lack O-linked glycoconjugates containing glucuronic acid and galactose and they also have reduced levels of N-glycans and fucose.
C. elegans, like other metazoa, possesses two protein O-fucosyltransferase genes, homologous to the POFUT1 and POFUT2 in humans and Drosophila, and thus has the capacity to generate O-fucose–containing glycoproteins, which in animals are usually linked to serine/threonine residues within cysteine-rich domains such as the EGF-like repeats and TSRs (Chapter 13). This modification is essential for signaling pathways in development and Notch signaling; RNAi studies suggest that at least the worm's POFUT2 (encoded by the pad-2 gene) is required for normal morphogenesis.
Proteoglycans and Glycosaminoglycans
During egg laying, fertilized eggs must pass through the vulva, which is a simple tubular structure that links the gonads with the external cuticle. During postembryonic development, vulva morphogenesis arises through the invagination of a single layer of epithelial cells. Using mutagenesis, several mutations that perturb invagination of the vulva were identified (designated sqv or squashed vulva). In the original screen, 25 mutations were identified in eight genes named sqv-1 through sqv-8. All of the mutations produced a similar phenotype: that is, partial collapse of vulval invagination, elongation of the central vulval cells, hermaphrodite sterility associated with maternal-effect lethality, and cytokinesis defects in the early embryo. All eight sqv genes show homology with vertebrate enzymes that are involved in the biosynthesis of GAGs (Figure 25.5). sqv-1, sqv-4, and sqv-7 encode proteins that have roles in nucleotide sugar metabolism and transport. The SQV-7 nucleotide transporter was the first example of a carrier that could import more than one nucleotide sugar into the Golgi (Chapter 5). SQV-4 and SQV-1 proteins represent sequential enzymes involved in the formation of UDP-glucuronic acid and UDP-xylose, respectively, showing that the sqv mutations most likely affect GAG synthesis. Biochemical analysis of sqv-6, sqv-3, sqv-2, and sqv-8 showed that they encode worm orthologs of the vertebrate transferases required for the assembly of the linkage region tetrasaccharide common to HS and chondroitin. Finally, characterization of sqv5 showed that it encodes the chondroitin synthase. Thus, the various phenotypes (failed invagination of the epithelial layer that forms the vulva, maternal-effect lethality, and cytokinesis defects) result from defective chondroitin formation.
The requirement for chondroitin assembly in seemingly disparate systems may result from biophysical changes in the lumen of the vulva or between the eggshell and the embryo. One idea is that the high negative charge imparted by the glucuronic acids in chondroitin attracts counterions that raise the local osmolarity, causing a swelling pressure. Another possibility is that the chondroitin acts as a physical scaffold bound to the cell membrane or eggshell. Interestingly, the sqv screen did not detect mutations affecting genes that encode proteoglycan core proteins on which the chondroitin chains assemble. Inspection of the C. elegans genome also did not reveal any homologs of vertebrate chondroitin sulfate proteoglycan core proteins. Proteomic analysis subsequently led to the identification of nine novel chondroitin proteoglycan (CPG) core proteins that contain chondroitin chains (Figure 25.6). Two of these (CPG-1 and CPG-2) contain chitin-binding domains that presumably allow the proteoglycans to interact with chitin in the eggshell, thus positioning the proteoglycans between the eggshell and the plasma membrane of the embryo, in which they could serve as spacers or osmotic regulators. Silencing cpg-1 and cpg-2 expression by RNAi recapitulates the cytokinesis defect observed in sqv mutants, suggesting that these are the relevant proteoglycans. The proteoglycans involved in epithelial invagination have not yet been determined.
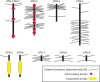
FIGURE 25.6.
Chondroitin proteoglycans (CPGs) of Caenorhabditis elegans.
HS biosynthesis in C. elegans follows the same pattern observed in vertebrate systems (Chapter 17). Mutations in the pathway for HS biosynthesis are lethal in C. elegans. Two of the key genes involved in this pathway are rib-1 and rib-2, homologs of the vertebrate genes Ext2 and Ext1, respectively, which catalyze the polymerization of the backbone of HS chains (GlcAβ1-4GlcNAcα1-4) (Chapter 17). Mutants in rib-2, the worm homolog of Ext1, have defects in development and egg laying. The worm genome also contains a single gene for glucuronic acid C-5 epimerase (hse-5) and five genes for sulfotransferase activities (GlcNAc N-deacetylase/N-sulfotransferase [Ndst], hst-1; uronyl 2-O-sulfotransferase, hst-2; 3-O-sulfotransferases, hst-3.1 and hst-3.2; and 6-O-sulfotransferase, hst-6), all of which are homologs of vertebrate genes involved in HS synthesis. In contrast, vertebrates contain four Ndsts, three 6-O-sulfotransferases, and seven 3-O-sulfotransferases. Although mutations in the epimerase (hse-5) and the sulfotransferases (hst-6, hst-2) do not affect viability, they cause defects in specific cell migration, axonal outgrowth and/or neurite branching. Consistent with this finding, inactivation of the cell surface HS proteoglycan syndecan (sdn-1) affects neural migration and axonal guidance. C. elegans also produces two GPI-anchored HS proteoglycans. LON-2, a member of the glypican family, negatively regulates a bone morphogenetic protein-like signaling pathway that controls body length in C. elegans. Worms also contain a homolog of the vertebrate basement membrane proteoglycan perlecan (encoded by unc-52). At least three major classes of UNC-52 isoforms are produced through alternative splicing, and distinct spatial and temporal expression patterns occur throughout development. In keeping with the “uncoordinated” phenotype, unc-52 mutants affect myofilament assembly in body-wall muscle during embryonic development. Thus, as in vertebrates, HS proteoglycans mediate many fundamental processes during development and in the adult animal.
Glycolipids
The arthroseries glycolipids in C. elegans have a unique core structure composed of GlcNAcβ1-3Manβ1-4Glcβ1-Cer (Figure 25.7). Some of these glycolipids also contain phosphorylcholine modifications on N-acetylglucosamine residues. Recent studies on the resistance of C. elegans to bacterial toxins have led to interesting insights into the structures and functions of glycolipids. Bt toxins are used in both transgenic and organic farming because of their ability to kill insect pests. Bt is toxic to C. elegans, but following mutagenesis, Bt-resistant strains were identified and classified as bre-1 through bre-5. None of the strains showed altered development, but they were highly resistant to Bt. Unexpectedly, the Bt-resistant mutants had truncated glycolipids, which turned out to be the ligands for Bt in the intestinal epithelium. The C. elegans glycolipids are shown in Figure 25.7, along with the genetic steps associated with different bre mutants.
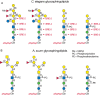
FIGURE 25.7.
Examples of nematode glycolipids. (A) Structures of glycolipids from Caenorhabditis elegans. Mutations that result in truncated glycolipids have been identified as bre mutants, as described in the text. (B) Structures of glycolipids from Ascaris suum (more...)
GLYCAN-BINDING PROTEINS IN C. ELEGANS
Although the C. elegans genome encodes a number of predicted glycan-binding proteins (GBPs), only a few of them have been characterized biochemically or explored by genetic manipulation. The first GBP found in C. elegans was a galectin (Chapter 36), which was isolated by affinity-chromatography and sequenced in 1992. This was surprising because until this observation, galectins were thought to be expressed only in vertebrates. Amazingly, the C. elegans genome encodes 28 putative galectins, nearly twice as many as in humans. Only two of these proteins have been studied in detail: a tandem-repeat 32-kDa galectin (LEC-6) and a prototypical 16-kDa galectin (LEC-1). Both galectins can bind to galactose-containing ligands.
Some 283 clec genes have been identified in the C. elegans genomes that encode proteins with C-type lectin domains (CTLDs) (Chapter 34) identified in the C. elegans genome are contained within 135 proteins (some proteins have multiple CTLDs), but only 19 of these CTLDs have sequences predicting carbohydrate recognition. The functional roles of the CTLDs have not yet been studied in detail in C. elegans. In contrast to the CTLD-containing proteins in vertebrates, most of the proteins with CTLDs in C. elegans have signal sequences and no transmembrane domains, indicating that they are secreted proteins. Expression of several of these CTLDs is up-regulated upon challenge of the animal with nematocidal Bt strains and other pathogenic bacteria, pointing to a role of these lectins in the innate immune system of C. elegans.
GLYCOBIOLOGY OF OTHER NEMATODES
Studies in the nonparasitic nematode C. elegans have been incredibly rewarding because of the ease of genetic manipulation and culture. Much less is known about parasitic nematodes, which cause tremendous death and suffering in animals and people throughout the world. It might be expected that C. elegans and other nematodes share much in common in terms of glycoconjugate structures and biosynthesis, but each nematode has differences in glycans compared with C. elegans. These may pertain to their virulence and parasitic requirements.
Some of the major parasitic nematodes that have been studied in terms of glycoconjugates include Ascaris suum, Trichinella spiralis, Dictyocaulus viviparous, Haemonchus contortus, Onchocerca volvulus, Necator americanus, Dirofilaria immitis, Oesphagostomum dentatum, Toxocara canis, and Toxocara cati (Chapter 43). A. suum is a parasitic intestinal nematode of pigs. Like C. elegans, the A. suum N-glycans are paucimannose-rich and contain phosphorylcholine and core fucose residues. In contrast, the N-glycans of the parasitic nematode of deer Parelaphostrongylus tenuis are extensively terminally modified with galactose and carry the terminal structure Galα1-3Galβ1-4GlcNAc-R. The cattle parasite D. viviparous has N-glycans with Lewis antigens (see Chapter 14), including Lewis x. The sheep parasite H. contortus synthesizes N-glycans containing the fucosylated LacdiNAc antigen GalNAcβ1-4(Fucα1-3)GlcNAc-R (Chapter 43). Nematodes also make unusual glycolipids, and it is likely that each type of nematode synthesizes different glycolipid structures. One of the best-studied nematodes in terms of glycolipids is A. suum. Many of these glycolipids, which also contain the arthroseries, have galactose and fucose modifications, in addition to phosphorylcholine and phosphorylethanolamine. Virtually nothing is known about the genetics regulating glycosylation in these parasitic nematodes, as most studies have focused on the analysis of N- and O-glycans and some glycolipids.
ACKNOWLEDGMENTS
The authors acknowledge contributions to the previous version of this chapter from Jeffrey D. Esko and helpful comments and suggestions from Katharina Paschinger, Sarah Baas Robinson, Kristian Saied-Santiago, and Eillen Tecle.
FURTHER READING
- Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [PMC free article: PMC1213120] [PubMed: 4366476]
- Drickamer K, Dodd RB. 1999. C-Type lectin-like domains in Caenorhabditis elegans: Predictions from the complete genome sequence. Glycobiology 9: 1357–1369. [PubMed: 10561461]
- Oriol R, Mollicone R, Cailleau A, Balanzino L, Breton C. 1999. Divergent evolution of fucosyl-transferase genes from vertebrates, invertebrates, and bacteria. Glycobiology 9: 323–334. [PubMed: 10089206]
- Dodd RB, Drickamer K. 2001. Lectin-like proteins in model organisms: Implications for evolution of carbohydrate-binding activity. Glycobiology 11: 71R–79R. [PubMed: 11425795]
- Hirabayashi J, Arata Y, Kasai K. 2001. Glycome project: concept, strategy and preliminary application to Caenorhabditis elegans. Proteomics 1: 295–303. [PubMed: 11680876]
- Schachter H. 2004. Protein glycosylation lessons from Caenorhabditis elegans. Curr Opin Struct Biol 14: 607–616. [PubMed: 15465323]
- Olson SK, Bishop JR, Yates JR, Oegema K, Esko JD. 2006. Identification of novel chondroitin proteoglycans in C. elegans: Embryonic cell division depends on CPG-1 and CPG-2. J Cell Biol 173: 985–994. [PMC free article: PMC2063922] [PubMed: 16785326]
- Shi H, Tan J, Schachter H. 2006. N-glycans are involved in the response of Caenorhabditis elegans to bacterial pathogens. Methods Enzymol 417: 359–389. [PubMed: 17132514]
- Antoshechkin I, Sternberg PW. 2007. The versatile worm: Genetic and genomic resources for Caenorhabditis elegans research. Nat Rev Genet 8: 518–532. [PubMed: 17549065]
- Barrows BD, Haslam SM, Bischof LJ, Morris HR, Dell A, Aroian RV. 2007. Resistance to Bacillus thuringiensis toxin in Caenorhabditis elegans from loss of fucose. J Biol Chem 282: 3302–3311. [PubMed: 17135259]
- Laughlin ST, Bertozzi CR. 2009. In vivo imaging of Caenorhabditis elegans glycans. ACS Chem Biol 4: 1068–1072. [PMC free article: PMC2807738] [PubMed: 19954190]
- Gravato-Nobre MJ, Stroud D, O'Rourke D, Darby C, Hodgkin J. 2011. Glycosylation genes expressed in seam cells determine complex surface properties and bacterial adhesion to the cuticle of Caenorhabditis elegans. Genetics 187: 141–155. [PMC free article: PMC3018313] [PubMed: 20980242]
- Wohlschlager T, Butschi A, Grassi P, Sutov G, Gauss R, Hauck D, Schmieder SS, Knobel M, Titz A, Dell A, et al. 2014. Methylated glycans as conserved targets of animal and fungal innate defense. Proc Natl Acad Sci 111: E2787–E2796. [PMC free article: PMC4103367] [PubMed: 24879441]
- Yan S, Brecker L, Jin C, Titz A, Dragosits M, Karlsson NG, Jantsch V, Wilson IB, Paschinger K. 2015. Bisecting galactose as a feature of N-glycans of wild-type and mutant Caenorhabditis elegans. Mol Cell Proteomics 14: 2111–2125. [PMC free article: PMC4523199] [PubMed: 26002521]
- Jiménez-Castells C, Vanbeselaere J, Kohlhuber S, Ruttkowski B, Joachim A, Paschinger K. 2017. Gender and developmental specific N-glycomes of the porcine parasite Oesophagostomum dentatum. Biochim Biophys Acta 1861: 418–430. [PMC free article: PMC5201199] [PubMed: 27751954]
- Review Nematoda.[Essentials of Glycobiology. 2009]Review Nematoda.Cummings RD, Esko JD. Essentials of Glycobiology. 2009
- Review Nematoda.[Essentials of Glycobiology. 2022]Review Nematoda.Wilson IBH, Paschinger K, Cummings RD, Aebi M. Essentials of Glycobiology. 2022
- Review The use of Caenorhabditis elegans in parasitic nematode research.[Parasitology. 2004]Review The use of Caenorhabditis elegans in parasitic nematode research.Gilleard JS. Parasitology. 2004; 128 Suppl 1:S49-70.
- Review Mating pheromones of Nematoda: olfactory signaling with physiological consequences.[Curr Opin Neurobiol. 2016]Review Mating pheromones of Nematoda: olfactory signaling with physiological consequences.Leighton DH, Sternberg PW. Curr Opin Neurobiol. 2016 Jun; 38:119-24. Epub 2016 May 21.
- Neuropeptide-like protein diversity in phylum Nematoda.[Int J Parasitol. 2008]Neuropeptide-like protein diversity in phylum Nematoda.McVeigh P, Alexander-Bowman S, Veal E, Mousley A, Marks NJ, Maule AG. Int J Parasitol. 2008 Nov; 38(13):1493-503. Epub 2008 May 25.
- Nematoda - Essentials of GlycobiologyNematoda - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

