NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-.
The modulation of immune response activity is one of the major goals in the development of novel therapeutics for human immune or inflammatory diseases. The innate system resides at the intersection of the pathways of microbial recognition, inflammation, and cell death, thereby offering various therapeutic targets. In this context, NOD1 and NOD2 are of particular interest, since they recognize distinct structures derived from bacterial peptidoglycans and directly activate the NF-κB pathway, which controls the production of proinflammatory molecules. Mutations in the NOD1 and NOD2 genes are associated with a number of human inflammatory disorders, including Crohn's disease (CD), Blau syndrome, early-onset sarcoidosis, and atopic diseases, which characteristically cause constitutive NF-κB activation. Thus, small molecule inhibitors of NOD1 would provide powerful research tools for elucidating the roles of these proteins in modulating immune response in primary cultured cells from humans and in animal models. This probe report describes ML146 (CID-5310346), a second specific inhibitor of the NOD1-mediated activation of NF-κB of a new chemical class (purine-2,6-dione) compared to the previously submitted NOD1 specific inhibitor, ML130 (CID-1088438). While this probe is not as potent or selective as the first probe, CID-1088438, it does meet probe criteria and represents a second bona fide scaffold for a NOD1 selective probe.
Probe project: NOD1 pathway selective inhibitors
Assigned Assay Grant #: 1 R03 MH084844-01
Screening Center Name & PI: Burnham Center for Chemical Genomics & John C. Reed
Chemistry Center Name & PI: Burnham Center for Chemical Genomics & John C. Reed (renamed Conrad Prebys Center for Chemical Genomics at Sanford|Burnham Medical Research Institute in 2010)
Assay Submitter & Institution: Dr John C Reed & Burnham Institute for Medical Research (renamed Sanford|Burnham Medical Research Institute in 2010)
PubChem Summary Bioassay Identifier (AID): AID-1575
Probe Structure & Characteristics
This probe report describes a 2nd specific inhibitor of the NOD1-mediated activation of NF-κB of a new chemical class (purine-2,6-dione) compared to the previously submitted NOD1 specific inhibitor CID1088438, (ML130, imidazol-2-amine).

| CID | Target Name | IC50/EC50 (nM) [SID, AID] | Anti-target Name(s) | IC50/EC50 (μM) [SID, AID] | Selectivity | Secondary Assay(s) Name: IC50/EC50 (nM) [SID, AID] |
|---|---|---|---|---|---|---|
| CID5310346 ML146 | NOD1 | 1536 nM IC50 SID-87225488, AID-2333 & AID-2466 | NOD2 TNFα HEK293 cytotoxicity | 12.66 μM IC50
SID-87225488, AID-2334
& AID-2475 44.61 μM IC50 SID87225488 AID-2337& AID-2483 >20 μM IC50 SID-87225488, AID-2335 & AID-2469 | > 8- fold Vs NOD2 >29- fold vs TNFα | NOD1 mediated IL-8 secretion: Assay provider’s 3430 nM
IC50
SID-87225488, AID-2505 NOD2 mediated IL-8 secretion: Assay provider’s >25,000 nM IC50 SID-87225488, AID-2503 TNFα mediated IL-8 secretion: Assay provider’s >25,000 nM IC50 SID-87225488, AID-2504 NF-κB selectivity γ-tri-DAP: Assay provider 14,430 nM IC50 SID-87225488, AID-2793 NF-κB selectivity DOX: Assay provider >25000 nM IC50 SID-87225488, AID-2789 NF-κB selectivity PMA/Iono: Assay Provider >25000 nM IC50 SID-87225488, AID-2792 |
Recommendations for the scientific use of this probe
Mutations in the NOD1 and NOD2 genes are associated with a number of human inflammatory disorders, including Crohn’s disease (CD), Blau syndrome, early-onset sarcoidosis, and atopic diseases, which characteristically cause constitutive NF-κB activation. Small molecule inhibitors of NOD1 would provide powerful research tools for elucidating the roles of these proteins in primary cultured cells from humans and in animal models.
1. Scientific Rationale for Project
Specific Aims
The main objective of this study was to identify small molecule inhibitors of NF-κB activity induced by NOD1. This was achieved by generating and validating a cell-based, Luciferase reporter gene assay for use in high throughput screening (HTS) to identify small molecule inhibitors of NOD-dependent NF-κB activation. The primary HTS assay was used for NOD1 driven NF-κB-activation which induced an integrated NF-κB dependent luciferase expression cassette. Various downstream counter-screens and secondary assays were employed to further characterize the selectivity of the hits, setting the stage for subsequent structure-activity relationship (SAR) studies which led to the optimization of chemical probe.
Background and Significance
NOD1 and NOD2 are members of the NOD-like receptor (NLR) family, which share structural similarity with a subset of plant disease-resistance (R) proteins involved in the hypersensitive response against plant pathogens. The NLR proteins display (a) a C-terminal leucine-rich repeat (LRR) domain that is involved in recognition of conserved microbial patterns or other ligands; (b) a centrally located nucleotide-binding NACHT domain that mediates self-oligomerization and is essential for NLR activation, and (c) a N-terminal effector domain, which is responsible for the interaction with adaptor molecules that result in signal transduction. Based on the nature of their N-terminal domains, the NLRs have been divided into three subgroups: the Nods (NOD1 and NOD2) and IPAF possess a caspase recruitment domain (CARD); the NALPs (NALP1–14) display a pyrin domain (PYD); and NALP presents a baculovirus inhibitor of apoptosis protein repeat domain (BIR) (1–4).
Both NOD1 and NOD2 are cytoplasmic proteins that detect muropeptides derived from peptidoglycan (PG). PG is a major component of the Gram-positive bacterial cell wall, while in Gram-negative bacteria it is found as a thin layer in the periplasmic space. NOD2 detects muramyl dipeptide (MDP), a motif that is present in the PGs of both Gram-positive and Gram-negative bacteria and is also a major component of many immunoadjuvants. In contrast, the recognition of bacterial PG by NOD1 is dependent on the presence of the meso-DAP, an amino acid characteristic of most Gram-negative and some Gram-positive bacteria, such as Listeria monocytogenes and Bacillus spp. (5–7). Recent work has shown that meso-DAP itself can activate human epithelial cells through NOD1 to secrete antibacterial factors and cytokines (8). The minimal structure detected by NOD1 is the dipeptide D-Glu-meso-diaminopimelic acid (tri-DAP) (5, 9).
NOD1 and NOD2 physically associate with RICK (Ripk2/Rip2/CARDIAK), a CARD-containing protein kinase, through homophilic CARD-CARD interactions. Once RICK is recruited, it interacts with the IKK subunit IKKγ (also called NEMO), promoting its modification with lysine 63-linked polyubiquitin chains (which are not substrates for the proteasome), resulting in activation of the IκB kinases (IKKs) that phosphorylate the NF-κB inhibitor IκBα, targeting it for lysine 48-linked polyubiquitination and proteasome dependent degradation (10–12). After IκBα is degraded, free NF-κB translocates into the nucleus, where it drives the transcription of κB-containing genes (13, 14). Over-expression of NOD1, NOD2, or RICK is able to induce NF-κB activation (15–17).
Mutations in NOD1 and NOD2 are associated with a number of human inflammatory disorders, including Crohn’s disease (CD), Blau syndrome, early-onset sarcoidosis, and atopic diseases, which cause NF-κB constitutive activation (18, 19). In diseases such as asthma or inflammatory bowel disease, there is a change of NOD1 expression to certain splice variant isoforms, which lead to abnormal inflammation (18). In addition, intestinal macrophages of CD patients overproduce NF-κB targets, including the pro-inflammatory cytokines tumor necrosis factor α (TNFα) and the interleukins IL-1β and IL-6 (20, 21). Notably, the fact that NOD2 has been identified as the first susceptibility gene for Crohn’s disease (21, 22) suggests intriguing interconnections between bacterial sensing and chronic inflammatory diseases.
The modulation of immune response activity is one of the major goals in the development of novel therapeutics for human immune or inflammatory diseases. The innate system resides at the intersection of the pathways of microbial recognition, inflammation, and cell death, thereby offering various therapeutic targets (23). In this context, NOD1 and NOD2 are of particular interest, since they recognize distinct structures derived from bacterial peptidoglycans and directly activate NF-κB pathway, which controls the production of proinflammatory molecules. Access to chemical inhibitors of NODs will empower research on defining the roles of these proteins in numerous acute and chronic inflammatory diseases, as well as in normal host-defense mechanisms.
In this probe report, we describe the discovery and optimization of an inhibitor that specifically inhibits the NOD1 pathways to NF-κB activation with selectivity over other pathways. A cell-based HTS assay is described that utilizes an NF-κB-driven luciferase reporter gene stimulated with γ-tri-DAP as a measure of NOD1 activity. An analogous companion HTS reporter assay to measure NOD2-dependent activation was used as an initial counterscreen on the entire MLSMR to establish and confirm selectivity of inhibitors for NOD1. Additional selectivity of active compounds against TNFα stimulated activation of NF-κB and any confounding Cytotoxicity were also established and utilized. Secondary assays to confirm compound selectivity towards NOD1 activity on the endogenous NF-κB target gene were done by measuring their effect on the authentic downstream effect of NF-κB activation, production of interleukin-8 (IL-8) in MCF-7 cells.
2. Project Description
a. Original goal for probe characteristics
The original goal was to find compounds that had NOD1 selective inhibitors of NF-κB activation with an IC50 of ≤ 1 µM, hill slopes between 0.5–1.4 that inhibit NOD1 induced IL-8 secretion by < 1.0 µM and are selective for the NF-κB pathway induced by NOD1. Target selectivity over NOD2 is 5X. Target selectivity over TNFα is 10X.
b. Information for each Assay Implemented and Screening Run
i. PubChem Bioassay Name(s), AID(s), Assay-Type (Primary, DR, Counterscreen, Secondary)
Table 1PubChem Assay Summaries for Probe project
| PubChemBioAssay Name | AID | Probe Type | Assay Type | Assay Format | Assay Detection & well format |
|---|---|---|---|---|---|
| Summary assay for the identification of compounds that inhibit NOD1 [Summary] | 1575 | Inhibitor | Summary | N/A | N/A |
| uHTS luminescence assay for the identification of compounds that inhibit NOD1 [Confirmatory] | 1578 | Inhibitor | Primary | Cell- based | luminescence & 1536 |
| uHTS luminescence assay for the identification of compounds that inhibit NOD2 [Confirmatory] | 1566 | Inhibitor | Counterscreen for NOD1 (also NOD2 Primary) | Cell- based | luminescence & 1536 |
| HTS assay for identification of inhibitors of TNFα-specific NF-κB induction | 1852 | Inhibitor | Counterscreen | Cell- based | luminescence & 1536 |
| uHTS Fluorescence assay for the identification of cytotoxic compounds among compounds active in NOD1 cell inhibition assay [Confirmatory] | 1849 | Inhibitor | Cytotoxicity Counterscreen | Cell- based | luminescence & 1536 |
| uHTS luminescence assay for the identification of compounds that inhibit NOD2 in MDP treated cells. [Confirmatory] | 2001 | Inhibitor | Counterscreen | Cell- based | luminescence & 1536 |
| SAR analysis of compounds that inhibit NOD1 [Confirmatory] | 2333 | Inhibitor | SAR | Cell- based | Luminescence & 384 |
| SAR analysis of compounds that inhibit NOD1 – Set 2 [Confirmatory] | 2466 | Inhibitor | SAR | Cell- based | Luminescence & 384 |
| SAR analysis of compounds that inhibit NOD2 [Confirmatory] | 2334 | Inhibitor | SAR | Cell- based | Luminescence & 384 |
| SAR analysis of compounds that inhibit NOD2 – Set 2 [Confirmatory] | 2475 | Inhibitor | SAR | Cell- based | Luminescence & 384 |
| SAR analysis of inhibitors of TNFα specific NF- κB induction [Confirmatory] | 2337 | Inhibitor | SAR Counterscreen | Cell- based | Luminescence & 1536 |
| SAR analysis of inhibitors of TNFα specific NF- κB induction – Set 2 [Confirmatory] | 2483 | Inhibitor | SAR Counterscreen | Cell- based | Luminescence & 1536 |
| SAR analysis of compounds that are cytotoxic to HEK293 [Confirmatory] | 2335 | Inhibitor | SAR Cytotoxicity Counterscreen | Cell- based | luminescence & 1536 |
| SAR analysis of compounds that are cytotoxic to HEK293 – Set 2 [Confirmatory] | 2335 | Inhibitor | SAR Cytotoxicity Counterscreen | Cell- based | luminescence & 1536 |
| SAR analysis of muramyl dipeptide (MDP) induced IL-8 secretion in MCF-7/NOD2 cells.– Set 2 [Confirmatory]] | 2503 | Inhibitor | Secondary Assay for specificity | Cell- based | Absorbance (at 450 nm) & 96 |
| SAR analysis of tumor necrosis factor alpha (TNFαlpha) induced IL-8 secretion in MCF- 7/NOD1 cells – Set 2 [Confirmatory] | 2504 | Inhibitor | Secondary Assay for specificity | Cell- based | Absorbance (ELISA) of cell extracts & 96 |
| SAR analysis of GM-Tri-DAP induced IL-8 secretion in MCF-7/NOD1 cells – Set 2 [Confirmatory] | 2505 | Inhibitor | Secondary Assay for specificity | Cell- based | Absorbance (ELISA) of cell extracts & 96 |
| SAR analysis of NF-κB dependent luciferase using DAP as an inducer – Set 2 [Confirmatory] | 2793 | Inhibitor | Secondary Assay for specificity | Cell- based | luminescence & 96 |
| SAR analysis of NF-κB dependent luciferase using PMA/Ionomycin as an inducer – Set 2 [Confirmatory] | 2792 | Inhibitor | Secondary Assay for specificity | Cell- based | luminescence & 96 |
| SAR analysis of NF-κB dependent luciferase using Doxorucibin as an inducer – Set 2 [Confirmatory] | 2789 | Inhibitor | Secondary Assay for specificity | Cell- based | luminescence & 96 |
ii. Assay Rationale & Description
This primary screen assay measured the luciferase activity induced in the cell line 293T-kB-LV-LUC upon exposure to Ala-γ-Glu-diaminopimelic acid (γ-tri-DAP), which acts through the NOD1 signaling pathways to activate NF-κB, thus inducing an integrated NF-κB dependent luciferase expression cassette. The cell-based HTS assay utilized NF-κB-mediated luciferase reporter gene activity as a measure of NOD1 modulation. The assay used a luminescent readout.
The primary screening protocol is described below.
Assay materials
- HEK-293-T NF-κB-Luc cell line obtained from the assay provider’s laboratory.
- γ-tri-DAP (Ana Spec cat #60774) obtained from assay provider’s laboratory.
- SteadyGlo (Promega)
Table 2Reagents used for the uHTS experiments
| Reagent | Vendor |
|---|---|
| Human Embryonic Kidney Cells stably transduced with a 5X NF-κB RE (response elements) upstream of a firefly luciferase cassette | Cell stocks from AP, scale up by BCCG |
| Ala-γ-Glu-diaminopimelic acid – inducer of NOD1 pathway to activation of NF-κB | Ana Spec Cat #60774 (initial lot donated by AP) |
| Commercial lumigenic Luciferase substrate $1,992.00/L (Britelite™) | Perkin-Elmer |
The following uHTS protocol was implemented at single point concentration confirmation:
Day1
- Harvest HEK-293-T NF-κB-Luc at 100% confluency
- Dispense 3 uL (6000 cells)/well to every well of a 1536 TC-treated white plate (Corning # 3727).
- Spin down plates at 1000 rpm for 1 min in an Eppendorf 5810 centrifuge.
- Using a HighRes biosolution pintool equipped with V&P Scientific pins, stamp 10nl of 2mM compounds in DMSO (col 5–48) and 10nl DMSO controls (col 1–4) to plates
- Lid Plates. Incubate cells for 1 hour at room temp.
- Dispense 2 uL/well of γ-tri-DAP (1.875 ug/mL) in assay media containing 1.375% DMSO to columns 3–48.
- Spin down plates for 30 sec in an Eppendorf 5810 centrifuge.
- Lid Plates. Incubate overnight (16 hours) in 37°C 5% CO2 incubator
Day 2
- Equibrate plates to room temperature for 10 mins.
- Add 3 uL SteadyGlo well with Multidrop
- Spin plates for 10 secs in a Velocity11 VSpin, shake for 30 secs.
- Incubate plates for 20 mins at room temperature.
- Read luminescence on Perkin Elmer ViewluxTM.
The average Z’ for the screen was 0.6, the signal to background was 11.1, signal to noise was 78.6 and signal to window was 6.0.
Rationale for confirmatory, counter and selectivity assays
Past experience with cell-based assays for NF-κB and pilot LOPAC screen of NOD1 and with NOD2, BCCG projected a substantial number of initial hits for NOD1 (~6800 hits for NOD1 and ~1400 hits for NOD2 inhibitors. Therefore, PubChem comparisons for existing NF-κB firefly luciferase data, as well as promiscuous and generally toxic compounds filters were used before any retests of compounds.
Confirmation assays
The initial confirmatory screens were obtained from full dose-response of compounds from solvated MLSMR compounds to confirm activity seen first in test agents from screening library. The criteria was to have NOD1 active IC50s below 10 µM with at least a 5-fold selectivity over NOD2. For NOD2, IC50s would have to fall below 10 µM with at least a 5-fold selectivity over NOD1. For dual activity we were looking for comparable potency (IC50 within 2 to 3-fold) in NOD1 and NOD2 below 10 µM. Compounds that did met these criteria and showed well-behaved plots with Hill slopes between 0.7 and 1.4 were progressed to next stage. NOD1 second level confirmatory screens were obtained from full dose-response of compounds from dry powders in NOD1 and NOD2. Compounds fulfilling the above mentioned criteria were advanced to secondary assays.
Counterscreen assays
Counterscreens consist of an Alamar Blue(tm) cytotoxicity filter and a dose response assay to identify hits specific to tumor necrosis factor alpha (TNFα modulated NF-κB. A positive in a cytotoxicity assay invalidates as false positive a positive from the same compound in the NOD and/or TNFα assays. Since multiple cellular stimuli acting through various pathways lead to NF-κB induction, the TNFα assay is designed to identify hits specific to TNFα modulated pathways (non-NOD modulated).
Secondary Assays
Secondary assays performed by the Assay Provider’s lab (Dr. Ricardo Correa) to establish that 1) the compounds do actually inhibit the biologically relevant downstream effectors of NOD1 stimulated pathway (IL-8 secretion) and are not just the reporter pathway, and 2) selectively inhibit the NOD1 dependent pathway to NF-κB activation in other cell lines. The AIDs for these assays are summarized in Table 3 below:
Table 3
Summary of the secondary assays used in NOD1 studies.
NOD1: IL-8 secretion (AID-2505): γ-tri-DAP induction of human breast cancer epithelial cell lines MCF-7 expressing NOD1, combined with small doses of cycloheximide (CHX), specifically induces IL-8 production and release (26,27). NOD1 specifically detects γ-Tri-DAP, a tripeptide motif found in Gram-negative bacterial peptidoglycan, resulting in activation of the transcription factor NF-κB pathway (5).
NOD2: IL-8 secretion (AID-2503): muramyl dipeptide (MDP) induction of human breast cancer epithelial cell lines MCF-7 over-expressing NOD2 combined with small doses of cycloheximide (CHX), specifically induces IL-8 production and release (26,27). Nod2 is a general sensor of peptidoglycan through the recognition of muramyl dipeptide (MDP), the minimal bioactive peptidoglycan motif common to all bacteria (5).
TNFα: IL-8 secretion (AID-2504): The assay uses tumor necrosis factor alpha (TNFα), a canonical NF-κB inducer, and is designed for identification of hits specific to TNFα-modulated pathways in MCF-7/NOD1 cells (5). NOD1 specific inhibitors are not expected to affect this pathway (i.e. IL-8 secretion). In all cases secreted IL-8 was quantified with 96-well ELISA kit for IL-8 (BD Biosciences) using a SpectraMax 190 to measure absorbance at 570 nm.
iii. Summary of Results
The following flowchart summarizes the compound triage and decision tree for advancement of compounds:
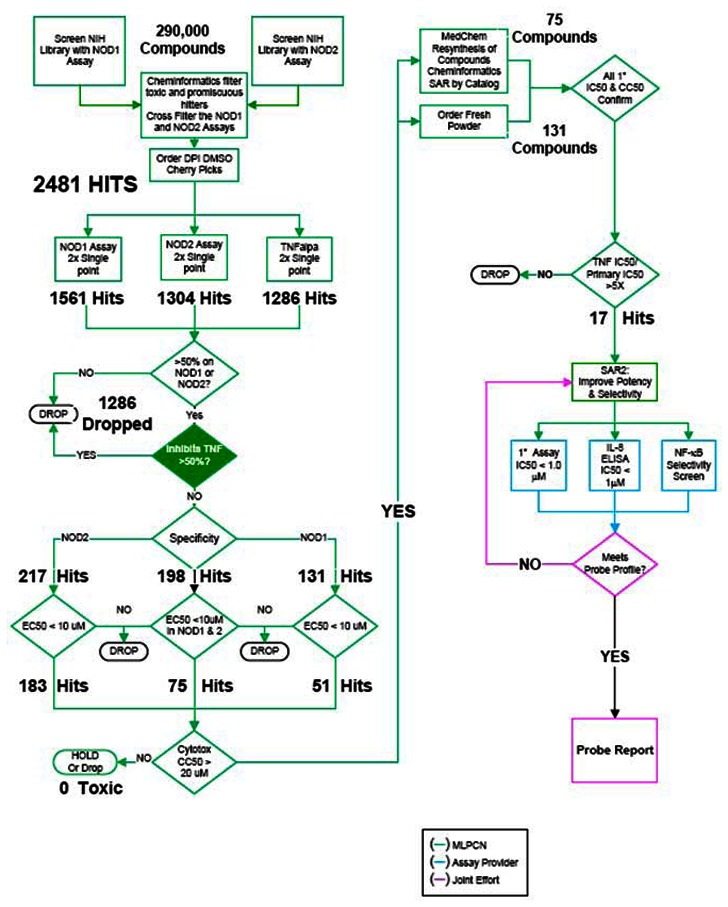
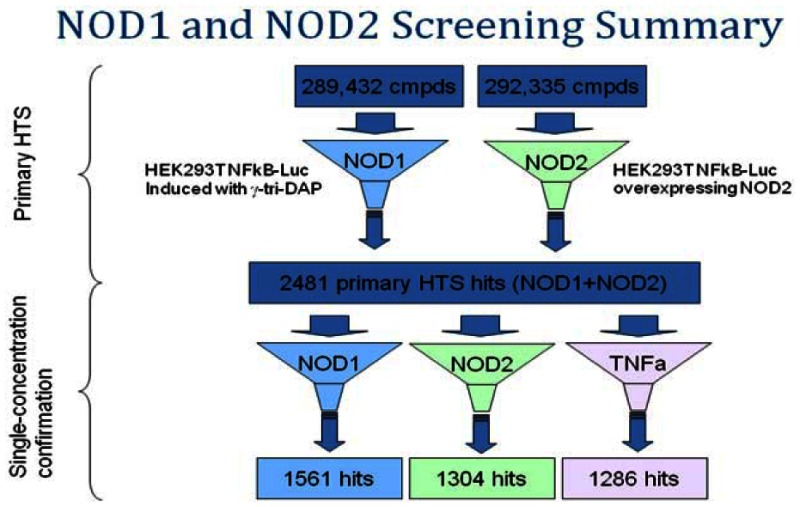
A library of approximately 290,000 compounds was interrogated in 2 assays: NOD1 and a NOD2-selective reporter assay. After further in silico screening by cheminformatics to eliminate historically promiscuous bioactives, 2481 hits with activity >50% at a single concentration point of 4 µM in either NOD1 or NOD2 were identified. Of these primary screening hits, 1536 were NOD1 hits 1304 were NOD2 hits.
MLSMR compounds were subsequently ordered for reconfirmation in single dose and dose response. The compounds were first confirmed in 4µM single-point duplicate in the NOD1, NOD2 and TNFα assays. TNFα was used as a third filter assay to identify hits specific to TNFα mediated NF-kB activation, which is putatively not NOD-mediated.
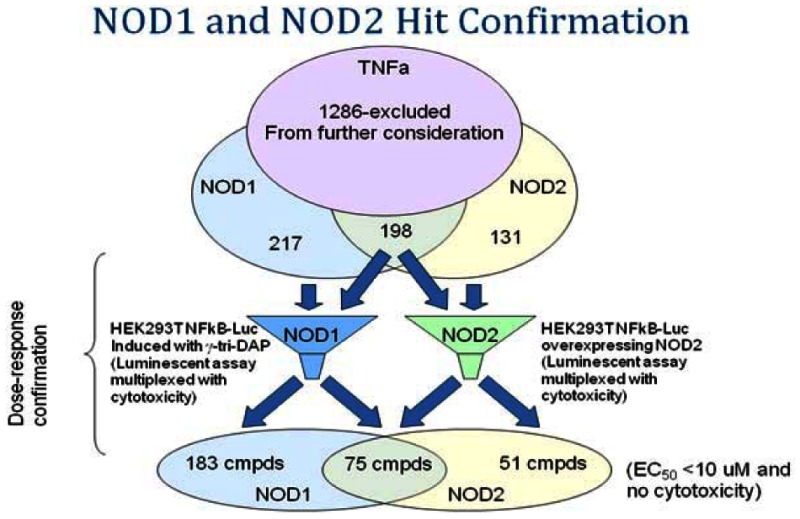
Hit totals for reconfirmation in duplicate retests at a single concentration were 217, 131 and 198 actives for NOD1, NOD2 and NOD1/2 respectively. A total of 1236 compounds were identified as hits in the TNFα assay (>50% activity at 4 µM) and these were excluded from further consideration.
Reconfirmed MLSMR NOD1 and NOD2 actives were further assayed in dose response. To be considered active, compounds would fall into one of 3 bins: For a NOD1 active, IC50s would have to fall below 10 µM with at least a 5-fold selectivity over NOD2. For NOD2, IC50s would have to fall below 10 µM with at least a 5-fold selectivity over NOD1. For dual activity we were looking for comparable potency (within 2 to 3-fold) in NOD1 and NOD2 below 10 µM. All would have to show a clean cytotoxicity profile in the Alamar™ Blue assay (< 20 µM).
The total number of hits was further reduced upon testing in dose response to 183, 51 and 75 for NOD1, NOD2 and NOD1/2 respectively. At this stage, the Alamar Blue™ cytotoxicity assay was multiplexed in dose response with the NOD assays.
Chemistry and cheminformatics resources were then employed in the selection of both novel and chemically tractable molecules to pursue for a NOD1, NOD2 and NOD1/2 selective probe. Structures of interest and analogs thereof were either purchased as dry powders or, where unavailable, synthesized by BIMR. In total, 75 structures were synthesized and 131 ordered though outside vendors. These constituted the SAR driving chemistries from which the NOD1 probe candidate and thirteen analogs emerged.
SAR testing of re-constituted powders encompassed dose response testing of compounds in four assays: NOD1, NOD2, TNFα, and Alamar Blue(tm) cytotoxicity. At this stage, the Alamar Blue™ cytotoxicity assay was multiplexed in dose response with the TNFα assay. Final probe selection, however, rested on the outcome of testing in a separate, biologically relevant functional assay, interleukin-8 (IL-8) secretion ELISA and on further selectivity testing in reporter assays using additional NF-κB pathway inducers (doxorubicin and PMA alongside the canonical NOD1 inducer γ-tri-DAP) to eliminate these as possible targets of our testing agents. Testing is still ongoing on the analogs of the NOD1 probe nominee but we have repeatedly confirmed dose dependent inhibition of IL-8 secretion and inactivity of the probe in TNFα, PMA and doxorubicin induced NF-kB as well as inactivity in MDP induced (NOD2) mediated IL-8 release.
c. Probe Optimization
i. Description of SAR & chemistry strategy (including structure and data) that led to the probe
Cheminformatics and Medicinal Chemistry Analysis
Probe optimization/SAR
Compound 1 CID5310346 (MLS-0012325) (entry 1, Table 4) was identified through a high-throughput screening campaign involving 290,000 compounds as an active and NOD1-selective scaffold. After confirmation of the initial results, the hit-to-probe process was initiated by both analog-by-catalog approach and internal medicinal chemistry effort. The structure-activity relationship data is presented in Table 4 below.
Table 4
SAR Analysis for NOD1 Selective Xanthine Scaffold(Medicinal Chemistry & Cheminformatics Analysis).
It is observed that the bioactivity of these compounds is dependent on the nature of the substituent R1. The number of carbons, branching pattern and degree of unsaturation of substituent R1 influences the activity of these compounds (entries 1–6, Table 4).
Compound 5 (CID 647508, MLS-0018316) with a pentyl group as R1 substituent maintains equivalent activity as the hit compound 1 (CID 5310346, MLS-0012325). Reducing the carbon chain length from five (compound 5, CID 647508, MLS-0018316) to three and introducing a double bond (compound 2, CID 2989653, MLS-0061481) decreases the compound potency by 10-fold. The presence of branching in carbon chain of substituent R1 (compound 3, CID 3111693, MLS-0059529) also decreases the potency by 13-fold. The presence of a heteroatom such as oxygen in R1 (compound 4, CID 652751, MLS-0013570) results in complete loss of activity.
Substituent “R2” as the thioalkyl group on the xanthine ring is imperative as replacement results in loss of NOD1 versus NOD2 selectivity (entries 1,5 versus 13 – 15, Table 4). The absence of the thioalkyl group in compound 13 (CID 666267, MLS-0004183) and compound 15, (CID5389756, MLS-0008596) results in loss of selectivity. Compound 12 (CID 646940, MLS-0082272) with a carbonyl group in substituent R2 results in complete loss of activity. Compound 14 (CID 909915, MLS-0298349) with R2 as the NO2 group maintains equivalent activity as compounds 1 (CID5310346, MLS-0012325) and 5 (CID 647508, MLS-0018316) but exhibits complete loss of NOD1 versus NOD2 selectivity.
Substituent “R3” as H provides the most active compounds from the series (i.e. entry 1,5 vs. 10 – 11, 13 and 15 Table 4). The presence of R3 as a methyl group in compounds 10 – 11, 13 and 15 results in complete loss of NOD1 versus NOD2 selectivity. Ultimately, the final probe molecule 1 (CID 5310346, MLS-0012325) was selected based on assay potency data (IC50 values) and fulfillment of agreed probe criteria including secondary assays (IL-8 ELISA, NF-kB luciferase assay).
In summary, a new class of potent inhibitors of NOD1 induced NF-kB activation based on the xanthine scaffold has been identified. The presence of thioalkyl group as the R2 and R3=H are crucial for high inhibitory activity (compound 1, CID 5310346, MLS-0012325). The number of carbons, branching pattern and degree of unsaturation of substituent R1 (i.e. butenyl, entry 1, vs i-propyl, entry 3) also influence the inhibitory activity of these compounds.
3. Probe
a. Chemical name of probe compound
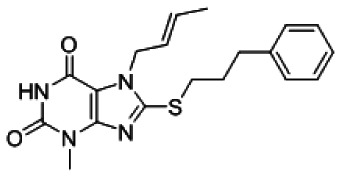
7-[(E)-but-2-enyl]-3-methyl-8-(3-phenylpropylsulfanyl)purine-2,6-dione [ML146]
b. Probe chemical structure including stereochemistry
c. Structural Verification Information of probe SID
The probe SID is SID-87225488
Purity
>96% (HPLC)
Mass Spec
ESI m/z 371 [M+H].

NMR Purity
95% 1H NMR (400 MHz, DMSO-d6) δ 11.03 (s, 1H), 7.26 – 7.14 (m, 5H), 5.54 – 5.51 (m, 2H), 4.68–4.67 (m, 2H), 3.26 (s, 3H), 3.19 (t, J = 8Hz, 2H), 2.67 (t, J = 8 Hz, 2H), 1.96 (quint, J = 8 Hz, 2H), 1.58 (d, J= 4 Hz,3H).

d. PubChem CID (corresponding to the SID)
PubChem CID is CID-5310346
e. Availability from a vendor
Compound tested was originally purchased from Life Chemicals (F0381-3333) and has been provided to the MLSMR. BCCG is currently synthesizing additional materials.
f. MLS#'s of probe molecule and five related samples that were submitted to the SMR collection
Table 5Submission of Probe and 6 analogs to the MLSMR (MLSMR)
| Probe/Analog | MLS# (MLSMR) | MLS-# BCCG | CID | SID | Source | Amt (mg) | Date |
|---|---|---|---|---|---|---|---|
| Probe [ML146] | MLS002699436 | 012325 | 5310346 | 87225488 | InterBioScreen | 50 | 2/17/2010 |
| Analog 1 | MLS002699437 | 004183 | 666267 | 87544812 | InterBioScreen | 20 | 2/17/2010 |
| Analog 2 | MLS002699438 | 008596 | 5389756 | 87544811 | InterBioScreen | 20 | 2/17/2010 |
| Analog 3 | MLS002699439 | 013615 | 5761203 | 87544795 | Asinex | 20 | 2/16/2010 |
| Analog 4 | MLS002699440 | 013818 | 653676 | 87544794 | Asinex | 20 | 2/16/2010 |
| Analog 5 | MLS002699441 | 018316 | 647508 | 87544796 | Asinex | 20 | 2/16/2010 |
| Analog 6 | MLS002699442 | 342317 | 814607 | 87544799 | Asinex | 20 | 2/16/2010 |
g. Mode of action for biological activity of probe
The probe molecule CID5310346 selectively (> 8-fold) inhibits NOD1 dependent activation of NF-κB pathways as ascertained through γ-tri-DAP stimulated luciferase signaling in a NF-κB-linked reporter assay in HEK293T cells containing endogenous NOD1 levels with submicromolar potency (1.54 µM IC50), while not inhibiting MDP stimulated (NOD2-dependent) signaling in both reporter cell lines containing both low and overexpressed NOD2 proteins. The probe molecule is also selective over the non-NOD stimulated pathways (TNFα stimulation) of NF-κB in these reporter assays. While this probe is not as potent or selective as our first probe, CID3238219, it does meet probe criteria and represents a second bonafide scaffold for a NOD1 selective probe.
Furthermore, the probe molecule and closely related analogs (additionally, see section 5 below), appear also to selectively inhibit the biologically relevant terminal effect of NOD1 (γtri-DAP) dependent NF-κB activation, namely IL-8 secretion, but not NOD2 dependent, nor TNFα dependent IL-8 secretion in biologically relevant MCF-7 cells as determined by IL-8 ELISA kits of cell culture supernatants.
h. Detailed synthetic pathway for making probe and analogs
The general 2-step reaction schema for the purine-2,6-dione probe and related analogs are shown below:
Step 1:

Step 2:

Specific experimental for example analog CID3123424
Procedure
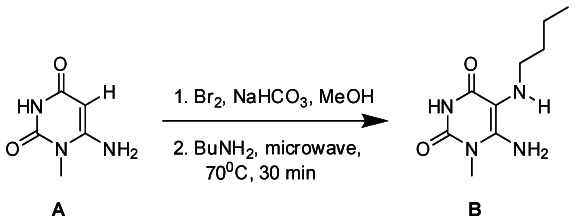
Bromine (183uL, 3.5 mmol) was added to a suspension of 6-amino-1-methyluracil (A) (500.0 mg, 3.5 mmol) and sodium bicarbonate (298 mg, 3.5 mmol) in methanol (20 mL) under vigorous stirring at 0 °C. The resulting mixture was stirred at room temperature for 2 h. The solution was then cooled to 4 °C, and the white precipitate was collected by filtration. The white solid was stirred in water (10 mL) at 4 °C, filtered, washed with cold water, and dried under high vacuum to yield 6-amino-5-bromo-1-methyluracil (450 mg, 57.7%). 6-amino-5-bromo-1-methyluracil (100.0 mg, 0.45 mmol) was transferred to a 10 mL microwave reaction tube with butyl amine (0.27 mL, 2.73 mmol) and was irradiated using CEM microwave synthesizer at 70 °C for 30 minutes. The resultant brown solution was cooled down to room temperature and 5 mL of diethyl ether was added which led to precipitation of a solid that was filtered and washed with 10 ml of diethyl ether and water and dried under vacuum to yield 6-amino-5-(butylamino)-1-methylpyrimidine-2,4(1H,3H)-dione (B) as an off-white solid (50mg, 51.8%). 1H NMR (500 MHz, DMSO) δ 10.46 (s, 1H), 6.35 (s, 2H), 3.22 (s, 3H), 2.72 (s, 1H), 2.62 (s, 2H), 1.40 (dd, J = 14.9, 7.7 Hz, 2H), 1.31 (dd, J = 15.1, 7.4 Hz, 2H), 0.88 (t, J = 7.3 Hz, 3H).
Procedure
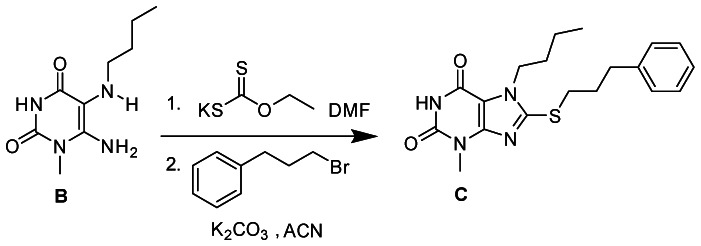
A mixture of 6-amino-5-(butylamino)-1-methylpyrimidine-2,4(1H,3H)-dione (B) (40.0 mg, 0.19 mmol) and potassium ethyl xanthogenate (241.7 mg, 1.5 mmol) in anhydrous DMF (2 mL) was capped and irradiated on a CEM microwave synthesizer at 120°C for 20 minutes. The resultant dark brown solution was cooled down to room temperature followed by addition of 5 mL of water to give a clear solution which was acidified with 2 N HCl solution to pH 4–5. The resulting precipitate was filtered, washed with cold water, diethyl ether and dried under vacuum to give 7-Butyl-8-mercapto-3-methyl-1H-purine-2,6(3H,7H)-dione as an off-white solid. 7-Butyl-8-mercapto-3-methyl-1H-purine-2,6(3H,7H)-dione (40.0mg, 0.16 mmol) was dissolved in 2 ml anhydrous acetonitrile, followed by addition of 1-bromo-3-phenylpropane (53.2 mg, 0.27 mmol) and potassium carbonate (37.0 mg, 0.27 mmol). The reaction mixture was stirred at room temperature for 12h After 12 hrs the solid was filtered and the solvent was removed from the filtrate under reduced pressure. The final product C was purified via column chromatography using 30% ethylacetate-hexane to yield 7-butyl-3-methyl-8-(3-phenylpropylthio)-1H-purine-2,6(3H,7H)-dione (C) (34.2 mg, 48.7%). 1H NMR (500 MHz, MeOD) δ 7.38 – 7.07 (m, 5H), 4.23 (t, J = 7.3 Hz, 2H), 3.48 (s, 3H), 3.31 (d, J = 7.4 Hz, 2H), 2.81 (dd, J = 19.8, 12.5 Hz, 2H), 2.18 – 2.05 (m, 2H), 1.85 – 1.71 (m, 2H), 1.47 – 1.31 (m, 2H), 1.03 – 0.88 (m, 3H). MS (ESI) calculated C19H24N4O2S m/z = 372.16, found m/z = 373.08 [M+H].
i. Summary of probe properties (solubility, absorbance/fluorescence, reactivity, toxicity, etc.)
The probe compound CID5310346 [ML146] (MLS-0012325) exhibited low solubility and high permeability at the three pH levels tested. It exhibits high plasma protein binding (both human and mouse). It has high stability in both human and mouse plasma. It shows low stability in the presence of mouse microsomes but moderate stability in human microsomes (determined by exploratory pharmacology group). The probe compound has a LD50 >50µM towards Fa2N-4 immortalized human hepatocytes.
Table 6Summary of in vitro ADMET/PK Properties of NOD1 Inhibitor Probe
| Probe CID Probe ML# BCCG MLS-# | Aqueous Solubility (μg/mL)a (@ pH) | PAMPA Pe (x10−6 cm/s)b (@ pH) | Plasma Protein Binding (% Bound) | Plasma Stabilityc Human Mouse/ | Hepatic Microsome Stabilityd Human/Mouse | Hepatic Toxicitye LC50 (μM) | |
|---|---|---|---|---|---|---|---|
| Human 1μM/10μM | Mouse 1μM/10μM | ||||||
| CID5310346 | 0.57 (5.0) | 1269 (5.0) | 99.41/97.38 | 98.81/97.20 | 100/100 | 8.82/0.86 | >50 |
| ML146 | 0.66 (6.2) | 1516 (6.2) | |||||
| MLS-012325 | 0.62 (7.4) | 1344 (7.4) | |||||
- a
in aqueous buffer, pH’s 5.0/6.2/7.4
- b
in aqueous buffer; Donor compartment pH’s 5.0/6.2/7.4; Acceptor compartment pH 7.4
- c
% remaining at 3 hr
- d
% remaining at 1 hr
- e
towards Fa2N-4 immortalized human hepatocytes
j. Probe properties
Table 7Properties computed from Structure
| CID5310346 ML146 MLS-012325 | |
|---|---|
| Molecular Weight | 370.46858 |
| Molecular formula | C19H22N4O2S |
| XLogP3-AA | 3.5 |
| H-Bond Donor | 1 |
| H-Bond Acceptor | 3 |
| Rotatable Bond Count | 7 |
| Tautomer Count | 3 |
| Exact Mass | 370.146347 |
| MonoIsotopic Mass | 370.146347 |
| Topological Polar Surface Area | 67.2 |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 536 |
| Isotope Atom Count | 0 |
| Defined Atom StereoCenter Count | 0 |
| Undefined Atom StereoCenter Count | 0 |
| Defined Bond StereoCenter Count | 1 |
| Undefined Bond StereoCenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
4. Comparative data showing probe specificity for target in biologically relevant assays
Table 8 provides the data from the secondary assay performed by the Assay Provider’s lab to support the text in sec. 3g above)
Table 8
Comparative data for probe specificity.
The probe molecule and a few closely related analogs selectively inhibit the biologically relevant terminal effect, IL-8 secretion, in an NOD1 selective manner.
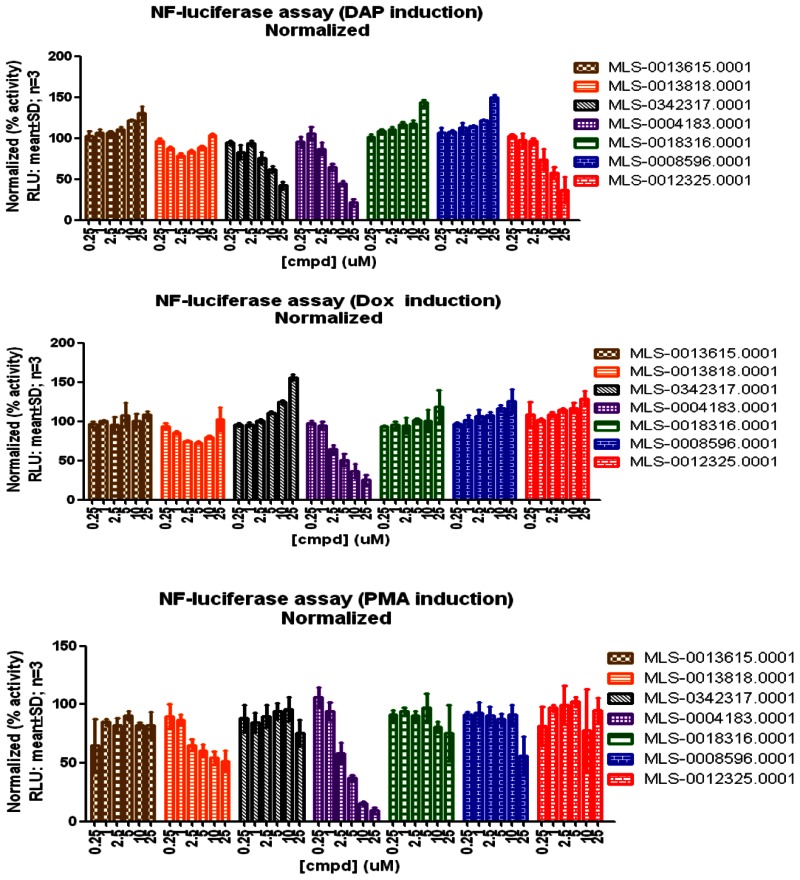
Finally, the probe molecule (CID5310346, MLS-0012325,) and one analog (CID814607, MLS-0342317) also are selective for NOD1 dependent activation of NF-κB as they do not inhibit doxorubicin (DNA damage) and PMA/ionomycin (phorbol ester/ionophore) induced pathways (see three panels of figure below and refer to Table 3 for structures). One compound, (CID666267, MLS-004183), appeared to be non-selective for NF-κB activation among the three pathways different pathways to NF-κB activation. Estimated potencies are summarized in Table 9 below.
Table 9
NF-kB Activation Pathway Selectivity of Probe and analogs.
5. Bibliography
- 1.
- Inohara Chamaillard, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. [PubMed: 15952891]
- 2.
- Carneiro LA, Travassos LH, Girardin SE. Nod-like receptors in innate immunity and inflammatory diseases. Ann Med. 2007;39:581–593. [PubMed: 18038361]
- 3.
- Murray PJ. NOD proteins: an intracellular pathogen-recognition system or signal transduction modifiers? Curr Opin Immunol. 2005;17:352–358. [PubMed: 15950446]
- 4.
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. [PubMed: 17967410]
- 5.
- Girardin SE, Travassos LH, Herve M, Blanot D, Boneca IG, Philpott DJ, Sansonetti PJ, Mengin-Lecreulx D. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–41708. [PubMed: 12871942]
- 6.
- Tattoli I, Travassos LH, Carneiro LA, Magalhaes JG, Girardin SE. The Nodosome: Nod1 and Nod2 control bacterial infections and inflammation. Semin Immunopathol. 2007;29:289–301. [PubMed: 17690884]
- 7.
- Viala J, Sansonetti P, Philpott DJ. Nods and ‘intracellular’ innate immunity. C R Biol. 2004;327:551–555. [PubMed: 15330254]
- 8.
- Uehara A, Fujimoto Y, Fukase K, Takada H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol Immunol. 2007;44:3100–3111. [PubMed: 17403538]
- 9.
- Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, Valvano MA, Foster SJ, Mak TW, Nunez G, Inohara N. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. [PubMed: 12796777]
- 10.
- Abbott DW, Wilkins A, Asara JM, Cantley LC. The Crohn’s disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–2227. [PubMed: 15620648]
- 11.
- Yoo NJ, Park WS, Kim SY, Reed JC, Son SG, Lee JY, Lee SH. Nod1, a CARD protein, enhances pro-interleukin-1beta processing through the interaction with pro-caspase-1. Biochem Biophys Res Commun. 2002;299:652–658. [PubMed: 12459189]
- 12.
- Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Nunez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. Embo J. 2007 [PMC free article: PMC2234345] [PubMed: 18079694]
- 13.
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. [PubMed: 12360211]
- 14.
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. [PubMed: 17183360]
- 15.
- Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, Carrio R, Merino J, Liu D, Ni J, Nunez G. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J Biol Chem. 1999;274:14560–14567. [PubMed: 10329646]
- 16.
- Bertin J, Nir WJ, Fischer CM, Tayber OV, Errada PR, Grant JR, Keilty JJ, Gosselin ML, Robison KE, Wong GH, Glucksmann MA, DiStefano PS. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-kappaB. J Biol Chem. 1999;274:12955–12958. [PubMed: 10224040]
- 17.
- Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. [PubMed: 11087742]
- 18.
- Carneiro L, Magalhaes J, Tattoli I, Philpott D, Travassos L. Nod-like proteins in inflammation and disease. J Pathol. 2008;214:136–148. References Cited Page 46 Principal Investigator/Program Director (Last, first, middle): Reed, John, C. [PubMed: 18161746]
- 19.
- Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Nunez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. [PubMed: 17944960]
- 20.
- Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nunez G, Keshav S. Crohn’s disease and the NOD2 gene: a role for paneth cells. Gastroenterology. 2003;125:47–57. [PubMed: 12851870]
- 21.
- Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. [PubMed: 15692052]
- 22.
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. [PubMed: 11385576]
- 23.
- Ulevitch RJ. Therapeutics targeting the innate immune system. Nat Rev Immunol. 2004;4:512–520. [PubMed: 15229470]
- 24.
- Pfeifer A, Ikawa M, Dayn Y, Verma IM. Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc Natl Acad Sci U S A. 2002;99:2140–2145. [PMC free article: PMC122332] [PubMed: 11854510]
- 25.
- Pfeifer A, Verma IM. Gene therapy: promises and problems. Annu Rev Genomics Hum Genet. 2001;2:177–211. [PubMed: 11701648]
- 26.
- da Silva Correia J, Miranda Y, Leonard N, Hsu J, Ulevitch RJ. Regulation of Nod1-mediated signaling pathways. Cell Death Differ. 2007;14:830–839. [PubMed: 17186025]
- 27.
- da Silva Correia J, Miranda Y, Austin-Brown N, Hsu J, Mathison J, Xiang R, Zhou H, Li Q, Han J, Ulevitch RJ. Nod1-dependent control of tumor growth. Proc Natl Acad Sci U S A. 2006;103:1840–1845. [PMC free article: PMC1413646] [PubMed: 16446438]
- 28.
- Hahn JS. Regulation of Nod1 by Hsp90 chaperone complex. FEBS Lett. 2005;579:4513–4519. [PubMed: 16083881]
- 29.
- Goetz MP, Toft DO, Ames MM, Erlichman C. The Hsp90 chaperone complex as a novel target for cancer therapy. Ann Oncol. 2003;14:1169–1176. [PubMed: 12881371]
- 30.
- Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Hannein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of Caspase-1 activation. Molecular Cell. 2007;25:713–724. [PubMed: 17349957]
- PMCPubMed Central citations
- PubChem BioAssay for Chemical ProbePubChem BioAssay records reporting screening data for the development of the chemical probe(s) described in this book chapter
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review High Throughput Screening Assays for NOD1 Inhibitors - Probe 1.[Probe Reports from the NIH Mol...]Review High Throughput Screening Assays for NOD1 Inhibitors - Probe 1.Magnuson G, Khan P, Yuan H, Brown B, Divlianska DB, Stonich D, Peddibhotla M, Su Y, Dad S, Sergienko E, et al. Probe Reports from the NIH Molecular Libraries Program. 2010
- Review The Nodosome: Nod1 and Nod2 control bacterial infections and inflammation.[Semin Immunopathol. 2007]Review The Nodosome: Nod1 and Nod2 control bacterial infections and inflammation.Tattoli I, Travassos LH, Carneiro LA, Magalhaes JG, Girardin SE. Semin Immunopathol. 2007 Sep; 29(3):289-301. Epub 2007 Aug 10.
- Review Nod1 and Nod2 in innate immunity and human inflammatory disorders.[Biochem Soc Trans. 2007]Review Nod1 and Nod2 in innate immunity and human inflammatory disorders.Le Bourhis L, Benko S, Girardin SE. Biochem Soc Trans. 2007 Dec; 35(Pt 6):1479-84.
- Discovery and characterization of 2-aminobenzimidazole derivatives as selective NOD1 inhibitors.[Chem Biol. 2011]Discovery and characterization of 2-aminobenzimidazole derivatives as selective NOD1 inhibitors.Correa RG, Khan PM, Askari N, Zhai D, Gerlic M, Brown B, Magnuson G, Spreafico R, Albani S, Sergienko E, et al. Chem Biol. 2011 Jul 29; 18(7):825-32.
- Identification of Inhibitors of NOD1-Induced Nuclear Factor-κB Activation.[ACS Med Chem Lett. 2011]Identification of Inhibitors of NOD1-Induced Nuclear Factor-κB Activation.Khan PM, Correa RG, Divlianska DB, Peddibhotla S, Sessions EH, Magnuson G, Brown B, Suyama E, Yuan H, Mangravita-Novo A, et al. ACS Med Chem Lett. 2011 Oct 13; 2(10):780-785. Epub 2011 Aug 5.
- High Throughput Screening Assays for NOD1 Inhibitors - Probe 2 - Probe Reports f...High Throughput Screening Assays for NOD1 Inhibitors - Probe 2 - Probe Reports from the NIH Molecular Libraries Program
Your browsing activity is empty.
Activity recording is turned off.
See more...
