This is a work of the US government and distributed under the terms of the Public Domain
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Toxicology Program. NTP Technical Report on the Toxicity Studies of Trans-resveratrol (CASRN 501-36-0) Administered by Gavage for Two Weeks or Three Months to F344/NTac Rats, Wistar Han [Crl:WI(Han)] Rats, and B6C3F1/N Mice: Toxicity Report 102 [Internet]. Research Triangle Park (NC): National Toxicology Program; 2021 Dec.
![Cover of NTP Technical Report on the Toxicity Studies of Trans-resveratrol (CASRN 501-36-0) Administered by Gavage for Two Weeks or Three Months to F344/NTac Rats, Wistar Han [Crl:WI(Han)] Rats, and B6C3F1/N Mice](/corehtml/pmc/pmcgifs/bookshelf/thumbs/th-ntptox102-lrg.png)
NTP Technical Report on the Toxicity Studies of Trans-resveratrol (CASRN 501-36-0) Administered by Gavage for Two Weeks or Three Months to F344/NTac Rats, Wistar Han [Crl:WI(Han)] Rats, and B6C3F1/N Mice: Toxicity Report 102 [Internet].
Show detailsData Availability
The National Toxicology Program (NTP) evaluated all study data. Data relevant for evaluating toxicological findings are presented here. All study data are available in the NTP Chemical Effects in Biological Systems (CEBS) database: https://doi.org/10.22427/NTP-DATA-TOX-102.150
Rats
Two-week Study in F344/NTac Rats
All Fischer 344 (F344/NTac) rats survived until study termination following administration of trans-resveratrol (RES) (Table 2). Mean body weights of all dosed groups were within 5% of the vehicle control groups at all measured time points (Table 2; Figure 2). No dose-related clinical observations were noted (Appendix F). There were no significant dose-related differences in organ weights and no dose-related gross or microscopic findings (Appendix F).
Table 2
Summary of Survival and Mean Body Weights of Male and Female F344/NTac Rats in the Two-week Gavage Study of Trans-resveratrol.

Figure 2
Growth Curves for Male and Female F344/NTac Rats in the Two-week Gavage Study of Trans-resveratrol
Dose Selection Rationale for Three-month Studies in Wister Han Rats
The same doses were used in the 3-month study in Wistar Han rats as were used in the 2-week studies in F344/NTac rats due to the absence of observed toxicity at the 2-week study doses. Due to programmatic changes in the NTP testing program, the F344/NTac strain of rat was changed to Wistar Han.119 There was no evidence that there would be differential toxicity in this strain, thus a 2-week study in Wistar Han rats was not conducted.
Three-month Study in Wistar Han Rats (Perinatal Phase)
No significant dose-related differences were observed in pregnancy status, maternal survival, gestation length, or number of Wistar Han dams that littered (Table 3). Maternal mean body weights and body weight gains were lower in all dosed animals except for those in the lowest dose group, 78 mg RES/kg body weight/day (mg/kg/day). Maternal mean body weights were significantly decreased (4%–10% lower) relative to the vehicle control group starting on gestation day (GD) 15 in the 625 and 1,250 mg/kg/day groups, on GD 17 in the 312.5 mg/kg/day group, and on GD 18 in the 156 mg/kg/day group. Body weight gains were significantly decreased relative to the vehicle control group by 19%–35% in all but the 78 mg/kg/day group throughout gestation (GD 6–21). During lactation, there were no significant effects of RES on maternal mean body weights or body weight gains (Table 4). No dose-related clinical observations in pups or dams were noted (Appendix F). No significant differences in total or live litter size, sex ratio, or pup survival at PND 1 or 4 were observed (Table 5).
Table 3
Summary of the Disposition of F0 Female Wistar Han Rats during Perinatal Exposure in the Perinatal and Three-month Gavage Study of Trans-resveratrol.
Table 4
Summary of Mean Body Weights and Body Weight Gains of F0 Female Wistar Han Rats during Gestation and Lactation in the Perinatal and Three-month Gavage Study of Trans-resveratrol.
Table 5
Summary of Mean Litter Size and Survival Ratio of F1 Male and Female Wistar Han Rats during Lactation in the Perinatal and Three-month Gavage Study of Trans-resveratrol.
From PND 4 through weaning, male and female pup mean body weights of RES-dosed groups tended to be lower than those of the vehicle control groups, with a negative trend with dose and pairwise significance in one or more dosed groups (312.5, 625, and 1,250 mg/kg/day). At weaning (PND 21), mean body weights of males and females in the highest dosed group, 1,250 mg/kg/day, were significantly decreased (20%–21%) relative to the vehicle control groups. In other male and female dosed groups on PND 21, the difference from the vehicle control groups ranged from 4% to 12% (Table 6). There were no significant dose-related differences in pup survival from PND 4 through weaning.
Table 6
Summary of Preweaning F1 Male and Female Wistar Han Rat Pup Mean Body Weights Following Perinatal Exposure to Trans-resveratrol.
Internal Dose Assessment (Preweaning)
RES and two metabolites, trans-resveratrol-3-O-B-D-glucuronide (trans-R3G) and trans-resveratrol-3-sulfate (trans-R3S), were quantified using validated analytical methods (Appendix D) in the following samples: Wistar Han maternal plasma and whole fetuses at 30, 60, and 90 minutes following dose administration on GD 18; male and female whole pups following dose administration to dams on PND 4; and male and female pup plasma following the last dose administration to pups on PND 21. Corresponding data are presented in Table 7 and Table 8.
Table 7
Summary of Internal Dose Data for Wistar Han Rats in the Perinatal and Three-month Gavage Study of Trans-resveratrola,b.
Table 8
Summary of Internal Dose Data for Preweaning F1 Male and Female Wistar Han Rats in the Perinatal and Three-month Gavage Study of Trans-resveratrola,b.
In GD 18 maternal plasma, RES, trans-R3G and trans-R3S concentrations increased proportionately with dose at all three time points with some exceptions (Table 7). For RES and both metabolites, the highest dam plasma concentrations were generally seen 90 minutes postdose. Concentrations of trans-R3G and trans-R3S were much higher than those of RES demonstrating significant first pass metabolism of RES following oral administration (Table 7). For example, at the 90-minute time point, trans-R3G and trans-R3S concentrations were 33- to 109-fold and 25- to 39-fold higher, respectively, than RES concentrations at all doses and time points (Table 7). RES and metabolites were detected in pooled fetuses on GD 18 at all doses and time points and, as in maternal plasma, increased with dose and time; the highest concentrations were measured at 90 minutes. Concentrations of RES and metabolites in whole fetuses were approximately 1%–11% of dam plasma concentrations demonstrating that the transfer of RES and metabolites from dams to fetuses is low (Table 7).
Male and female PND 4 pups showed dose-dependent increases in RES, trans-R3G and trans-R3S concentrations (Table 8). Similar to GD 18, the metabolite concentrations in whole pups at PND 4 were higher than RES concentrations. Trans-R3G and trans-R3S concentrations in PND 4 pups were 5- to 12-fold and 4- to 7-fold higher than RES concentrations at all doses, respectively. The detection of RES and its metabolites in pups indicated transfer of RES and conjugated RES forms from the dam to pups during lactation.
RES concentrations in PND 21 male and female pup plasma were lower than concentrations in PND 4 whole pups in all dosed groups except the highest dosed group (1,250 mg/kg/day), whereas trans-R3G and trans-R3S concentrations were higher in PND 21 plasma (Table 8). There was no apparent sex difference in the concentration of RES or metabolites in PND 21 pups.
Three-month Study in Wistar Han Rats (Postweaning Phase)
All animals survived to study termination (Table 9). There were no dose-related clinical observations (Appendix F). Mean body weights of RES-dosed animals in all but the 1,250 mg/kg/day group were within 2%–9% of the vehicle control groups at study termination (Table 9; Figure 3). In the 1,250 mg/kg/day group, mean body weights of male and female Wistar Han rats were 21% and 16% lower, respectively, relative to the vehicle control groups 8 days after weaning (study day [SD] 8) (Appendix F). From SD 15 onward, mean body weights of male and female Wistar Han rats in the 1,250 mg/kg/day group were within 20% of the vehicle control groups. By study termination (~SD 95), female Wistar Han rats in the 1,250 mg/kg/day group had mean body weights similar to those of the vehicle control group; male Wistar Han rats weighed 9% less than the vehicle control group (Table 9).
Table 9
Summary of Survival and Mean Body Weights of Male and Female Wistar Han Rats in the Perinatal and Three-month Gavage Study of Trans-resveratrol.
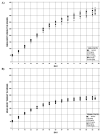
Figure 3
Growth Curves for Male and Female Wistar Han Rats in the Perinatal and Three-month Gavage Study of Trans-resveratrol
At study termination, there were no dose-related effects on clinical chemistry measures (Appendix F). Several significant differences were observed in the female hematology. These differences were small in magnitude, not observed in the males, or within biological variability, and thus were not considered related to dose. Organ weights were not affected by RES administration (Appendix F).
Vaginal cytology and sperm counts were assessed in the 0, 312.5, 625, and 1,250 mg/kg/day groups after 3 months of exposure. Cycle length, number of cycles, and time in each respective stage in RES-dosed Wistar Han rats were similar to the vehicle control group (Appendix F).
Wistar Han rats administered RES did not display any biologically significant changes in testis or epididymis weights, spermatid or spermatozoa counts, or sperm motility (Appendix F). RES did not exhibit the potential to be a male or female reproductive toxicant in the Wistar Han rat.
Internal Dose Assessment (Postweaning)
RES and two metabolites, trans-R3G and trans-R3S, were quantified using a validated analytical method (Appendix D) in male and female Wistar Han rat plasma collected 24 hours after the last dose administration on SD 95. Plasma concentrations of RES and trans-R3S were much lower in offspring at necropsy compared to those in dams on GD 18 or in offspring on PND 21 (Table 7), likely due to the extended time between the last dose and when samples were collected (24 hours) (Table 10).
Table 10
Summary of Internal Dose Data for Postweaning F1 Male and Female Wistar Han Rats in the Perinatal and Three-month Gavage Study of Trans-resveratrola,b.
Histopathology
This section describes the statistically significant or biologically noteworthy changes in the incidences of nonneoplastic lesions of the kidney, jejunum, and Peyer’s patch in male and female Wistar Han rats. No dose-related neoplasms were present. Summaries of the incidences of nonneoplastic lesions mentioned in this section are presented in Appendix F.
Kidney: Nephropathy occurred in all groups of dosed male Wistar Han rats with a positive trend with dose and a significantly increased incidence in the 1,250 mg/kg/day group compared to the vehicle control group (Table 11). This lesion, also known as chronic progressive nephropathy, also occurred in female Wistar Han rats administered ≥156 mg/kg/day, with a positive dose-related trend and a significantly increased incidence in the 1,250 mg/kg/day group compared to the vehicle control group (Table 11). Histologically, this lesion was characterized by focal to multifocal small clusters of tubules within the renal cortex that displayed cytoplasmic basophilia (regeneration), thickened basement membranes, and peritubular mononuclear cell infiltrates.
Table 11
Incidences of Select Nonneoplastic Lesions of the Kidney in Male and Female Wistar Han Rats in the Perinatal and Three-month Gavage Study of Trans-resveratrol.
Renal tubule dilatation occurred in male and female Wistar Han rats with a positive trend with dose and a significantly increased incidence in the 1,250 mg/kg/day groups compared to the vehicle control groups (Table 11). This diagnosis was reserved for those cases with one or two large ectatic tubules within the renal medulla that frequently contained eosinophilic intraluminal material (Figure 4) without histological evidence of lower urinary tract obstruction in the tissue sections evaluated. When the lesion was adjacent to a focus of nephropathy, tubular dilatation was deemed to be part of nephropathy and not diagnosed separately.
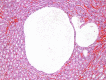
Figure 4
Representative Image of Renal Tubule Dilatation in the Kidney of a Female Wistar Han Rat in the Perinatal and Three-month Gavage Study of Trans-resveratrol (H&E)
The lesion of renal pelvis dilatation occurred with a positive trend for incidence in female Wistar Han rats (Table 11). This lesion was characterized by distention and dilatation of the renal pelvis and occurred without histological evidence of lower urinary tract obstruction in the tissue sections evaluated.
Jejunum: Lymphatic ectasia occurred in the jejunum in the 625 and 1,250 mg/kg/day male groups and in the 1,250 mg/kg/day female group (Table 12). A positive trend with dose was found for the incidence in males. This unique lesion was characterized as central lymphatic dilatation of the villous tips within the jejunum (Figure 5).
Table 12
Incidences of Select Nonneoplastic Lesions of the Small Intestine in Male and Female Wistar Han Rats in the Perinatal and Three-month Gavage Study of Trans-resveratrol.
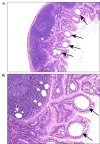
Figure 5
Representative Images of Lymphatic Ectasia in the Jejunum of a Male Wistar Han Rat in the Perinatal and Three-month Gavage Study of Trans-resveratrol (H&E)
Peyer’s patch: Lymphatic ectasia occurred in the Peyer’s patches in the 156, 625, and 1,250 mg/kg/day groups of male Wistar Han rats, and in the 78, 312.5, and 1,250 mg/kg/day groups of female Wistar Han rats (Table 12). This lesion was characterized by a few discrete clear dilated lymphatics within the lymphoid tissue, usually located in the subepithelial dome region (Figure 5).
Mice
Two-week Study in B6C3F1/N Mice
All B6C3F1/N mice survived until study termination, and mean body weights of all dosed groups were within 7% of the vehicle control groups at measured time points (Table 13; Figure 6). No dose-related clinical observations were noted (Appendix F).
Table 13
Summary of Survival and Mean Body Weights of Male and Female B6C3F1/N Mice in the Two-week Gavage Study of Trans-resveratrol.

Figure 6
Growth Curves for Male and Female B6C3F1/N Mice in the Two-week Gavage Study of Trans-resveratrol
A significant increase in relative liver weight was observed in the 2,500 mg/kg/day group of female mice, as well as a positive trend (Table 14). There were no associated dose-related gross or microscopic findings (Appendix F).
Table 14
Summary of Liver Weights and Liver-Weight-to-Body-Weight Ratios for Female B6C3F1/N Mice in the Two-week Gavage Study of Trans-resveratrola,b.
Dose Selection Rationale for Three-month Studies in B6C3F1/N Mice
The same doses were used in the 3-month study in B6C3F1/N mice as were used in the 2-week study due to the lack of observed toxicity at the doses used in the 2-week study.
Three-month Study in B6C3F1/N Mice
All animals survived to study termination (Table 15). Mean body weights of RES-dosed male and female B6C3F1/N mice ranged from within 1%–10% of vehicle control animals and were not significantly different from vehicle control animals at any point during the study (Table 15; Figure 7). No dose-related clinical observations were noted (Appendix F).
Table 15
Summary of Survival and Mean Body Weights of Male and Female B6C3F1/N Mice in the Three-month Gavage Study of Trans-resveratrol.
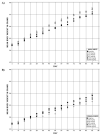
Figure 7
Growth Curves for Male and Female B6C3F1/N Mice in the Three-month Gavage Study of Trans-resveratrol
No effects on hematological parameters were observed with RES exposure (Appendix F).
In male mice, absolute and relative liver weights were significantly increased in the highest dose group (2,500 mg/kg/day) relative to the vehicle control group (Table 16). In female mice, absolute heart weights were significantly decreased in the 1,250 and 2,500 mg/kg/day groups compared to the vehicle control group. Additionally, there were significant increases in relative kidney weights (1,250 and 2,500 mg/kg/day groups) and relative liver weights (625, 1,250, and 2,500 mg/kg/day groups) compared to the vehicle control group (Table 16). No other effects on organ weights were observed.
Table 16
Summary of Select Organ Weights and Organ-Weight-to-Body-Weight Ratios for Male and Female B6C3F1/N Mice in the Three-month Gavage Study of Trans-resveratrola,b.
Reproductive parameters were evaluated in male mice in the 0, 625, 1,250, and 2,500 mg/kg/day groups. There were no significant differences in testis or epididymis weights, testicular sperm count, or percent motile sperm (Table 17). Cauda epididymis sperm counts were 24%, 14%, and 13% lower than the vehicle control group in the 625, 1,250, and 2,500 mg/kg/day groups, respectively, with only the 625 mg/kg/day group attaining statistical significance (Table 17). Histopathological lesions were not observed in either the epididymis or testis.
Table 17
Summary of Reproductive Tissue Evaluations for Male B6C3F1/N Mice in the Three-month Gavage Study of Trans-resveratrola.
Vaginal cytology evaluations were conducted in female mice in the 0, 625, 1,250, and 2,500 mg/kg/day groups. Cycle length and number of cycles in RES-dosed mice were not significantly different from vehicle control mice (Appendix F). The 625 and 2,500 mg/kg/day groups spent less time in estrus and more time in metestrus than the vehicle control group (Appendix F). Extended diestrus was more prevalent in all RES-dosed groups relative to the vehicle control group. Although there were some apparent changes in estrus and metestrus in RES-dosed mice, they were minimal in magnitude and not dose dependent (Appendix F).
Histopathology
This section describes the statistically significant or biologically noteworthy changes in the incidence of nonneoplastic lesions of the nose in female B6C3F1/N mice. No dose-related neoplasms were present. Summaries of the nonneoplastic lesions mentioned in this section are presented in Appendix F.
Nose: Respiratory metaplasia of the olfactory epithelium occurred with a positive dose-related trend and a significantly increased incidence in the 2,500 mg/kg/day female dosed group relative to the vehicle control group (Table 18). This lesion is characterized by focally extensive areas of replacement of olfactory epithelium with columnar ciliated respiratory epithelium. This change was most often present in the dorsal aspect of the nasal cavity at Level II (Figure 8).
Table 18
Incidences of Select Nonneoplastic Lesions of the Nose in Female B6C3F1/N Mice in the Three-month Gavage Study of Trans-resveratrol.
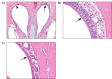
Figure 8
Representative Images of Respiratory Metaplasia of Olfactory Epithelium in the Nose of a Female B6C3F1/N Mouse in the Three-month Gavage Study of Trans-resveratrol (H&E)
Other lesions: There were positive trends with dose in the occurrence of kidney nephropathy in male mice, kidney mineralization in female mice, and mixed cell infiltration in the livers of male and female mice (Appendix F), but the biological significance of these lesions is unknown.
Genetic Toxicology
RES (33 to 3,333 μg/plate) was not mutagenic in Salmonella typhimurium strains TA98, TA100, or TA102 when tested with or without exogenous metabolic activation provided by Aroclor 1254-induced rat liver S9 and cofactors (Table E-1).
In rats, the reticulocyte population is the only red blood cell population that can be accurately assessed for micronucleus frequency in peripheral blood due to efficient splenic scavenging of damaged erythrocytes soon after they emerge from the bone marrow. In both sexes of Wistar Han rats in the 3-month study, there were no significant increases in the frequencies of micronucleated reticulocytes (polychromatic erythrocytes; PCEs) (Table E-2). A positive trend in the percentage of PCEs was observed in female Wistar Han rats; however, the absolute increase (0.38%) in the 1,250 mg/kg/day group compared to the vehicle control group was small and was not considered to be biologically relevant.
No increases in the frequency of micronucleated erythrocytes (either immature or mature) were seen in the peripheral blood of female B6C3F1/N mice in the 3-month study (Table E-3). Significant increases in micronucleated mature erythrocytes (NCEs) were observed for every dosed group in male B6C3F1/N mice. However, the absolute difference in micronucleated NCEs in the dosed groups relative to the vehicle control group ranged from 0.06% to 0.16%. These small increases were not considered biologically relevant.
- Results - NTP Technical Report on the Toxicity Studies of Trans-resveratrol (CAS...Results - NTP Technical Report on the Toxicity Studies of Trans-resveratrol (CASRN 501-36-0) Administered by Gavage for Two Weeks or Three Months to F344/NTac Rats, Wistar Han [Crl:WI(Han)] Rats, and B6C3F1/N Mice
Your browsing activity is empty.
Activity recording is turned off.
See more...