NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Making Healthcare Safer IV: A Continuous Updating of Patient Safety Harms and Practices [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2023 Jul-.

Making Healthcare Safer IV: A Continuous Updating of Patient Safety Harms and Practices [Internet].
Show detailsMain Points
- Active surveillance culturing of asymptomatic individuals is a well-established and widely used patient safety practice in hospitals. However, questions remain about the cost and effectiveness of specific surveillance strategies in reducing clinical infection and transmission events.
- Two new studies of high-risk patients (for Clostridioides difficile and carbapenem-resistant Enterobacterales) found that active surveillance culturing limited to high-risk patient populations could significantly reduce infections. However, these studies compared targeted screening to no screening. The effectiveness of targeted screening compared to universal screening remains unclear.
- Active surveillance culturing of all patients can be labor intensive and consume substantial resources, while limiting screening to specific populations can reduce these burdens. Recent studies provide little evidence of the direct costs or other resources needed to support targeted surveillance.
- Evidence on active surveillance culturing for Candida auris remains sparse, with no effectiveness studies identified. A pilot study provides early evidence for the feasibility of implementing Candida auris surveillance, but a survey of Canadian hospitals and laboratories revealed that most sites were not prepared to implement surveillance programs.
- No recent toolkits are available to support implementation of active surveillance culturing—for all patients or for specific populations—for Clostridioides difficile, methicillin-resistant Staphylococcus aureus, carbapenem-resistant Enterobacterales, or Candida auris.
1. Background and Purpose
AHRQ’s Making Healthcare Safer (MHS) reports consolidate information for healthcare providers, health system administrators, researchers, and government agencies about practices that can improve patient safety across the healthcare system—from hospitals to primary care practices, long-term care facilities, and other healthcare settings. In spring 2023, AHRQ launched its fourth iteration of the MHS Report (MHS IV). Active surveillance culturing as a patient safety practice (PSP) was identified as high priority for inclusion in the MHS IV reports using a modified Delphi technique by a Technical Expert Panel (TEP) that met in December 2022. The TEP included 15 experts in patient safety with representatives of governmental agencies, healthcare stakeholders, clinical specialists, experts in patient safety issues, and a patient/consumer perspective. See the Making Healthcare Safer IV Prioritization Report for additional details.1
Preventing exposure, colonization, and infection of Clostridioides difficile (C. difficile) and multidrug-resistant organisms (MDROs) is a critical patient safety and public health priority for which active surveillance culturing of asymptomatic patients has been advocated and critically evaluated. In the United States, more than 2.8 million antimicrobial-resistant infections occur each year and more than 35,000 people die as a result.2 Clostridioides difficile and MDRO pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant Enterobacterales (CRE), and Candida auris (C. auris), are a particular concern for medically vulnerable persons, resulting in significant patient harm and economic cost.3 These organisms in particular are the focus of multiple frameworks for mitigating the threat of harm due to healthcare-associated infections (HAI), including the National Healthcare Safety Network (NHSN) MDRO module,4 the National Action Plan for Combating Antibiotic-resistant Bacteria (CARB) report,5 along with the Centers for Disease Control and Prevention (CDC) Interim Guidance for a Public Health Response to Contain Novel or Targeted Multidrug-Resistant Organisms (MDROs).6 Owing to these organisms’ increasing prevalence over time, limited treatment options, limited capability to rapidly detect them, and emergence of novel antimicrobial resistance mechanisms they require multifaceted, resource-intense infection prevention and control systems anchored by surveillance programs.4–6 C. difficile and MDRO transmission pathways7 in healthcare settings may involve transmission between patients, providers, and the environment. Prevention and control of C. difficile and MDROs relies upon both traditional infection control approaches, including isolation precautions, hand hygiene, and active surveillance culturing, and newer techniques such as whole genome sequencing, machine learning algorithms, regional MDRO registries, and geospatial mapping.8,9
MHS III examined active surveillance as a PSP within the larger topic of MDROs. Available evidence addressed surveillance for MRSA, CRE, vancomycin-resistant Enterococci (VRE), and general gram-negative bacteria. A separate chapter dedicated to C. difficile infection also reviewed surveillance strategies specific to that organism. The report also noted a lack of consensus regarding surveillance for C. auris.
The TEP prioritization process noted that surveillance and testing topics in MHS III would be subsumed by this rapid response. The rapid response format was selected instead of the more comprehensive and in-depth rapid review format because the evidence base was expected to consist of few new studies and would likely overlap with prior findings.1 Our rapid response subsumes entirely types of surveillance PSPs covered in MHS III but narrows to pathogens that are most burdensome on patient safety, including MRSA, CRE, C. auris, and C. difficile. Additionally, owing to the overlapping findings of the C. difficile testing chapter in MHS III, our rapid response also subsumes the C. difficile surveillance and testing topics as they relate to ASC for asymptomatic patients. Because the publication of updated CDC guidelines for C. difficile testing in 2017 has led to an acceleration in publications evaluating C. difficile testing in symptomatic patients,14,15 and given the concise nature of the rapid response format, we limited our evaluation regarding C. difficile to asymptomatic patients.
1.1. Overview of the Patient Safety Practice
Surveillance is the cornerstone of any C. difficile and MDRO control program, allowing detection of newly emerging pathogens, monitoring epidemiologic trends, and measuring the effectiveness of interventions.10 In healthcare settings, active surveillance can serve a key practical purpose. Patients with clinical infection are only the tip of the iceberg for potential transmission, and implementing infection control procedures (e.g., contact precautions, isolation) for infected patients does not prevent the spread of MDROs from colonized patients. Active surveillance facilitates the identification of asymptomatic colonized patients and the implementation of infection control interventions that can limit further transmission.
Active surveillance culturing (ASC) for C. difficile and MDROs involves the collection and culturing of samples to identify asymptomatic colonization on the skin, mucosal surfaces, or gastrointestinal tract of patients. ASC also requires the systematic collection, analysis, and reporting of data to trend organism burden, identify patient and environmental reservoirs, and measure the impact of infection control interventions to mitigate these harms. Additionally, recent innovations have resulted in new ASC-related approaches. For example, whole genome sequencing surveillance of targeted organisms has identified reservoirs and routes of healthcare transmission that were not apparent using traditional epidemiologic surveillance methods.8,11 Similarly, geospatial mapping techniques combined with genomic data have defined transmission patterns and informed infection control strategies within hospitals and across regional healthcare networks.12,13 Despite these advances, implementing infection surveillance PSPs presents several challenges for hospitals and health systems, including identifying target populations, selecting methods for obtaining and processing ASC specimens, optimizing the timing and frequency of collecting cultures, and evaluating the effectiveness of using ASC on reducing C. difficile and MDRO burden, antimicrobial overuse, HAIs, and cost of care.
1.2. Purpose of the Rapid Review
The overall purpose of this rapid response is to summarize the most relevant and recent literature on the use and utility of active surveillance for detecting asymptomatic colonization with target MDROs, and to highlight how active surveillance can inform infection control interventions to reduce subsequent transmission and risk of clinical infection. The response is organized around the following review questions:
1.3. Review Questions
- What are the frequency and severity of downstream harms associated with asymptomatic colonization due to MRSA, CRE, C. auris, and C. difficile?
- What patient safety measures or indicators have been used to examine the downstream harms associated with asymptomatic colonization due to MRSA, CRE, C. auris, and C. difficile?
- What active surveillance PSPs for MRSA, CRE, C. auris, and C. difficile have been used to prevent or mitigate downstream harms and in what settings have they been used?
- What is the rationale for the active surveillance PSPs for MRSA, CRE, C. auris, and C. difficile that have been used to prevent or mitigate the downstream harms?
- What studies have assessed the effectiveness and unintended effects of active surveillance PSPs for MRSA, CRE, C. auris, and C. difficile and what new evidence has been published since the search was completed for the Making Healthcare Safer (MHS) III report of 2019?
- What are common barriers and facilitators to implementing active surveillance PSPs for MRSA, CRE, C. auris, and C. difficile?
- What resources (e.g., cost, staff, time) are required for implementation of active surveillance PSPs for MRSA, CRE, C. auris, and C. difficile?
- What toolkits are available to support implementation of active surveillance PSPs for MRSA, CRE, C. auris, and C. difficile?
2. Methods
We followed processes proposed by the Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Center (EPC) Program.16 The rapid response is intended to present the end-user with an answer based on the best available evidence, but do not attempt to formally synthesize the evidence into conclusions. While the steps are similar to those of a typical systematic review, the methods are different (i.e., streamlined systematic review methods).
For this rapid response, strategic adjustments were made to streamline traditional systematic review processes and deliver an evidence product in the allotted time. We followed adjustments and streamlining processes proposed by the AHRQ EPC Program. Adjustments include being as specific as possible about the questions, limiting the number of databases searched, modifying search strategies to focus on finding the most valuable studies (i.e., being flexible on sensitivity to increase the specificity of the search), and restricting the search to studies published recently (e.g., since 2019 when the search was done for the Making Healthcare Safer III report) in English, and having each study assessed by a single reviewer. A randomly selected 10 percent sample of excluded references were checked by a second reviewer at the title and abstract screening stage.
Our content expert answered Review Questions 1 and 2 by citing selected references that best answered the questions without conducting a systematic search for all evidence on the targeted harms and related patient safety measures or indicators, in addition to findings identified in Review Question 5 relevant to Review Questions 1 and 2. Our content expert addressed Review Questions 3 and 4 by citing selected references, including explanations of the rationale presented in the studies we found for Review Question 5. Our approach to Review Question 5 is described in detail below in Sections 2.1 through 2.4. For Review Questions 6 and 7, we examined the barriers, facilitators, and required resources reported in the studies we found for Review Question 5, as well as studies identified in our search that provided relevant information but did not meet the eligibility criteria for Question 5. For Review Question 8, we sought to identify publicly available patient safety toolkits developed by AHRQ or other organizations that could help to support implementation of the patient safety practices (PSPs). To accomplish that task, we reviewed AHRQ’s Patient Safety Network (PSNet) (https:/psnet.ahrq.gov) and AHRQ’s listing of patient safety related toolkits (see https://www.ahrq.gov/tools/index.html?search_api_views_fulltext=&field_toolkit_topics=14170&sort_by=title&sort_order=ASC). We also intended to include any toolkits mentioned in the studies found for Review Question 5.
2.1. Eligibility Criteria for Studies of Effectiveness
We searched for original studies and systematic reviews on Review Question 5 according to the inclusion and exclusion criteria presented in Table 1.
Table 1
Inclusion and exclusion criteria.
Active surveillance is designed to inform the use of infection control procedures that can reduce transmission and risk of infection. Additionally, surveillance PSPs are frequently implemented and evaluated as part of multicomponent interventions. Therefore, it can be difficult to examine the independent effect of surveillance on colonization or infection. To reduce the confounding effect of multiple interventions, we excluded studies examining multicomponent bundles that simultaneously introduced surveillance along with other multiple new infection control interventions, unless the study included a mechanism for isolating the effect of surveillance. Conversely, we included studies that used pre-post or cohort designs to evaluate the addition of a new surveillance component to a pre-existing set of infection control strategies.
2.2. Literature Searches for Studies of Effectiveness
We searched PubMed and the Cochrane Library for systematic reviews published since January 1, 2019, that address the review questions. We also conducted searches of PubMed for original studies published since 2019.
2.3. Selection of Studies
To efficiently identify articles that met the eligibility criteria, each title/abstract was reviewed by a single team member. A second team member checked a 10 percent sample of citations to verify that important studies were not excluded. The full text of each potentially eligible article was reviewed by a single team member to confirm eligibility and prepare a summary of the study, including author, year, study design, number of study participants, and main findings relevant to each of the rapid response questions. For Review Question 5, we described the objectives and basic characteristics of studies on the effectiveness of infection surveillance PSPs for methicillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant Enterobacterales (CRE), Candida auris (C. auris), and Clostridioides difficile (C. difficile). A second team member checked a randomly selected 10 percent sample of the excluded citations at full-text screening to verify that important studies were not excluded and confirm the accuracy of extracted data.
2.4. Risk of Bias (Quality) Assessment
For studies that addressed Review Question 5 about the effectiveness of active surveillance PSPs for MRSA, CRE, C. auris, and C. difficile, the primary reviewer used the ROBINS-I tool for assessing the Risk Of Bias In Non-randomized Studies - of Interventions.17 We used specific items in the ROBINS-I tool that assess bias due to confounding, bias in selection of participants into the study, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes, and bias in selection of the reported results. The risk of bias assessments focused on the main outcome of interest in each study.
3. Evidence Base
3.1. Number of Studies
Our search retrieved 610 unique titles and abstracts from which we reviewed 105 full-text articles for eligibility. We found 6 studies that met the inclusion criteria for Review Question 5 (Figure 1).
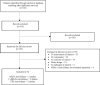
Figure 1
Results of the search and screening. C. difficile = Clostridioides difficile; CRE = carbapenem-resistant Enterobacterales; MRSA = methicillin-resistant Staphylococcus aureus
3.2. Findings for Review Questions
An overview of the studies that met our inclusion criteria for Review Question 5 is presented in Table 2. Our searches identified no eligible systematic reviews or randomized controlled trials. We found six nonrandomized studies since 2019, and we identified no studies that examined surveillance for Candida auris (C. auris).
Table 2
Overview of the included original studies for Review Question 5.
3.2.1. Question 1. What Are the Frequency and Severity of Downstream Harms Associated With Asymptomatic Colonization Due to MRSA, CRE, C. auris, and C. difficile?
Asymptomatic carriage of Clostridioides difficile (C. difficile) and multidrug-resistant organism (MDRO) pathogens methicillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant Enterobacterales (CRE), and C. auris is associated with a substantial risk of subsequent infection and attributable mortality among hospitalized patients. Asymptomatic carriers are an important reservoir of these organisms leading to contamination of the hospital environment and patient transmission events.
Asymptomatic carriage of toxigenic C. difficile strains occurs in 8 to 10 percent of adults residing in hospitals or long-term care facilities and among hospitalized patients may confer a 24-fold increased risk of developing C. difficile disease.24–26 In 2017, the estimated burden of C. difficile infection was 462,100 cases in the United States, and up to 25 percent of patients experience recurrent infection within 30 days of treatment.27,28 In the United States, approximately 15,000 deaths annually are estimated to be directly attributable to C. difficile infections, and more than 80 percent of these deaths occurred among persons aged 65 years or older.
About 2 percent of the general U.S. adult population carry MRSA in their nose. The prevalence of MRSA colonization among persons in U.S. healthcare facilities is higher, estimated at 41.1 per 1,000 hospitalized patients and 22.3 percent among residents of long-term care facilities. While MRSA carriage is a dynamic process associated with gain, loss, or persistence of nasal colonization over time, MRSA colonization is associated with an excess risk of infection and death.29
CRE may colonize a patient’s skin, mucosal surfaces, or gastrointestinal tract. In a 2016 meta-analysis, CRE colonized patients had a 16.5 percent cumulative infection rate.30 In 2017, there were an estimated 12,000 CRE infections in hospitalized patients in the US, 1,100 deaths, and $130 million in attributable healthcare costs.2
Candida auris is an emerging multidrug-resistant fungal pathogen that has spread rapidly in the United States following its first detection in 2016. C. auris carriage most commonly involves the axilla and groin, with most cases of colonization and infection found in high-acuity, post-acute–care facilities. More than 3,000 clinical cases and 7,000 screening cases were identified in the United States by the end of 2021, and clinical cases nearly doubled from 2020 to 2021.31 C. auris is associated with a variety of clinical outcomes, ranging from superficial skin infections to severe bloodstream infections and death.
3.2.2. Question 2. What Patient Safety Measures or Indicators Have Been Used To Examine the Downstream Harms Associated With Asymptomatic Colonization due to MRSA, CRE, C. auris, and C. difficile?
As asymptomatic colonization with C. difficile or MRDOs can lead to healthcare-associated infections (HAIs) due to those organisms, patient-to-patient transmission events, and persistence in the hospital environment, the same infection control interventions are typically applied to patients whether associated with asymptomatic colonization or clinical infection. In the United States, the Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network (NHSN) is the most widely used surveillance system to report and monitor trends in healthcare-associated infections. There are two options for C. difficile and MDRO reporting in NHSN–Laboratory Identified (LabID) Events reporting which uses laboratory-based reporting criteria, and Infection Surveillance which uses clinical-based reporting criteria.4 The Centers for Medicare & Medicaid Serivces (CMS) includes healthcare-onset C. difficile LabID event and MRSA LabID bloodstream infection event data in their Inpatient Quality Reporting Program including the Value-Based Purchasing and Hospital Acquired Conditions payment programs to evaluate acute-care hospital patient safety performance, and makes these data available for consumers on the CMS Hospital Care Compare website.
3.2.3. Question 3. What Active Surveillance PSPs for MRSA, CRE, C. auris, and C. difficile Have Been Used To Prevent or Mitigate Downstream Harms and in What Settings Have They Been Used?
Surveillance for C. difficile and MDROs typically utilize well-established approaches, including monitoring of clinical isolates susceptibility results and incidence-based rates from clinical cultures or clinical infection events, as well as active culture-based surveillance to detect asymptomatic colonization. The population targeted and resources needed for active surveillance vary. Universal active surveillance culturing (ASC) includes screening all patients admitted to an acute care or long-term care facility or unit that is experiencing high-rates of colonization/infection with C. difficile20 or MDRO of interest18,19,23 as well as point-prevalence surveys to estimate the total burden of the target microorganism. Targeted ASC involves screening of specific populations at high risk of C. difficile or MDRO colonization based on factors such as medical condition, admission location (e.g., intensive care unit [ICU]), transfer from a facility with high prevalence of target MDRO (e.g., nursing home or high-acuity post-acute–care facility), recent acute-care hospitalization, or recent travel to a high-risk region.21,22
The timing and interval of both universal and targeted ASC vary widely in practice, but most commonly includes admission testing to detect prevalent colonization or point prevalence surveys.18,20–22 Repeating ASC periodically during prolonged admission to a high-risk unit (e.g., weekly) or on discharge from the hospital or high-risk unit is utilized to detect new incident transmission events.19 The body sites screened for ASC vary depending on the MDRO of interest. For MRSA, culture of the nares is the most commonly used approach,18,19 and addition of wound or perirectal cultures improves sensitivity. For CRE ASC, perirectal or rectal swabs alone or combined with culture of other body sites (e.g., respiratory, inguinal, wounds) are used,21 while a single swab of the axilla and inguinal skin is recommended to detect C. auris colonization. Either perirectal or rectal swabs or stool samples can be used to detect colonization with toxigenic strains of C. difficile in high-risk patients without diarrhea or other evidence of C. difficile disease.20,21 Laboratory methods used for ASC include both rapid, non-culture based molecular tests and conventional culture, often using selective growth media to enhance MDRO recovery but with longer turn-around times than molecular tests. Molecular typing of selected clinical and ASC isolates, increasingly utilizing whole genome sequencing, is used to confirm or identify unsuspected clonal transmission events and to evaluate the impact of infection control interventions.32
3.2.4. Question 4. What Is the Rationale for the Active surveillance PSPs for MRSA, CRE, C. auris, and C. difficile That Have Been Used To Prevent or Mitigate the Downstream Harms?
ASC for C. difficile and MDROs is usually justified by the interaction of four factors: (1) the substantial morbidity, mortality, and costs associated with infection, along with a high and/or growing incidence of these infections in hospital settings; (2) the challenges of treating infections associated with MDROs and widespread concern about growing antibiotic resistance; (3) the availability of infection control measures that can successfully reduce pathogen transmission, such as contact precautions, cleaning and disinfection of the environment and patient-care equipment, and cohorting of patients and staff; and (4) the desire to avoid unnecessary use of these infection control measures due to their costs and burdens. Identification of asymptomatic colonized patients is thus a vital early component that facilitates efficient use of effective infection control strategies. The effectiveness of specific infection control measures has recently been examined in another AHRQ report, Prevention in Adults of Transmission of Infection with Multi-Drug Resistant Organisms.
Focusing ASC PSPs on specific units or settings may be rationalized when a hospital or health system has consistently high levels of colonization or infection or has experienced recent outbreaks.19,20 Targeted surveillance of higher risk populations presents an opportunity to screen more efficiently while also protecting patients at greater risk of harm.21,22,23 Additionally, one recent study described the high costs of universal surveillance as a primary reason for developing an algorithm to identify high-risk patients who could then be prioritized for CRE testing.22
3.2.5. Question 5. What Studies Have Assessed the Effectiveness and Unintended Effects of Active Surveillance PSPs for MRSA, CRE, C. auris, and C. difficile and What New Evidence Has Been Published Since the Search Was Completed for the Making Healthcare Safer (MHS) III Report of 2019?
Six studies meeting the eligibility criteria were published since the completion of MHS III, all of which evaluated the effectiveness of ASC. Two of these studies assessed surveillance for MRSA, two focused on C. difficile, and two examined CRE. Three studies (one MRSA study and both C. difficile studies) were conducted in the United States, while the other three studies were performed in China. Four studies used a pre-post design, one used a stepped wedge design, and one study included retrospective cohort analysis. Four studies were assessed to be at moderate risk of bias, one study was at low risk, and one study was at serious risk. We did not identify any studies that addressed unintended effects of these PSPs.
For MRSA, the two studies included different settings and patient populations, but both found that ASC PSPs did not appear to improve downstream outcomes. One study19 examined patients undergoing cardiovascular surgery at two campuses of an academic medical center in China. Patients at one campus underwent universal nasal screening for any S. aureus colonization prior to surgery, while patients at another campus were not screened. Both campuses implemented identical infection control procedures that included pre-surgical chlorhexidine bathing and prophylactic administration of cefuroxime for all patients irrespective of MRSA screening status. Patients who screened positive for MRSA colonization were treated with mupirocin and vancomycin in addition to cefuroxime, and were placed in contact isolation. Over 4 years, no statistically significant difference was found in MRSA infections (including surgical site, bloodstream, and lower respiratory tract infections) between the campuses. Interestingly, the risk of any S. aureus infection (including methicillin-sensitive infections) was lower in the ASC group. This might be due in part to the low overall number of MRSA infections; only six infections were reported in total (three in each group) out of 2,287 patients.
In another study,18 a U.S. academic medical center examined the effects of discontinuing a policy of universal MRSA nasal screening upon admission in two neonatal ICUs. Infants who tested positive were placed in contact isolation. Comparing the final 3 years during which ASC was performed to the following 3 years that did not include routine screening, no differences were found in overall MRSA infections, MRSA bloodstream infections, or rate of MRSA infections per 1,000 patient days. Given the unique population and characteristics of a neonatal ICU, these findings may not be broadly generalizable to other settings or patient populations. Both studies were assessed to have a moderate risk of bias.
For C. difficile, the results of recent studies were more favorable. Using a stepped-wedge design, a study21 of more than 85,000 patients at four hospitals in a U.S. health system evaluated a targeted surveillance program that conducted polymerase chain reaction (PCR) testing to detect the C. difficile toxin B gene on perirectal swab samples collected from high-risk patients at admission. An algorithm embedded in the hospitals’ electronic health record (EHR) identified patients as high-risk if they had a prior history of C. difficile infection, or if they had been hospitalized in the previous two months or had been in a long-term care facility in the previous 6 months. Other infection control procedures were uniformly present during the entire study period for patients who tested positive for C. difficile. These included contact precautions; use of soap and water for hand hygiene; bleach-based cleaning and ultraviolet light disinfection of patient rooms after discharge; and routine compliance monitoring of these practices. The hospitals did not have an antimicrobial stewardship program. After implementing targeted surveillance, the C. difficile infection rate declined from 6.1 cases/10,000 patient days to 2.9 cases/10,000 patient days. This study was at moderate risk of bias.
Similar success was reported in a narrower pre-post study20 of patients undergoing cystectomy at a U.S. academic medical center. All patients treated after February 2015 were screened for colonization using PCR testing for C. difficile toxin B in stool samples collected immediately before surgery, after sedation was initiated. All patients received 24 hours of preoperative antibiotic prophylaxis with cefoxitin, unless contraindicated. Patients who tested positive for C. difficile colonization were isolated, placed on contract precautions, and treated with intravenous metronidazole. The rate of post-operative C. difficile colitis was reduced from 9.4 percent in patients treated from 2012 through February 2015, to 5.5 percent in patients treated after initiation of screening. However, this study was at serious risk of bias because 21 percent of the patients seen after introduction of the screening program were not actually screened.
One study of a CRE surveillance PSP also provided support for ASC.22 This pre-post study implemented a targeted surveillance program that tested patients identified as high risk for CRE upon admission to two ICUs of a teaching hospital in China. Samples were collected from rectal or perirectal areas or from fecal incontinence bags and tested using PCR. Isolates were tested for blaKPC, blaNDM, and blaIMP carbapenemase genes, and whole genome sequencing was performed for Klebsiella pneumoniae. Risk factors that triggered screening included age, health status, therapeutic treatments, and recent hospitalization. Patients who tested positive were cohorted and placed on contact precautions, and enhanced “education, cleaning and handwashing” was implemented (details of these processes were not reported.) An antibiotic stewardship program was also employed. ASC resulted in reduced rates of CRE colonization and infection. The authors also performed a multivariate regression analysis that determined the surveillance program was associated with a substantial reduction in the risk of CRE infection. This study was assessed to be at low risk of bias.
A second study23 included immunosuppressed patients who were hospitalized while undergoing hematopoietic stem cell transplantation at an academic medical center in China. This is the only study that examined the timing and frequency of surveillance and did not compare ASC to no surveillance. In this pre-post study, the authors compared single testing of stool samples upon admission to weekly surveillance throughout the course of transplant hospitalization. Patients who tested positive for CRE colonization were isolated and placed on contact precautions, and hand hygiene and room disinfection protocols were enhanced. Colonized patients who subsequently developed neutropenic fever received empirical therapy with tigecycline targeting CRE. During the period of one-time screening, 4 patients out of 200 (2.0%) developed CRE infections and two of those patients died. When weekly screening was implemented, 1 of 195 patients (0.5%) was infected, and that patient survived. These differences were not statistically significant, and the study was at moderate risk of bias.
Finally, we did not find any studies that assessed the effectiveness of ASC for C. auris, as this remains an emerging area of research. However, in 2023 the New York State Department of Health reported the results of a pilot study33 that found the use of real-time PCR to screen for C. auris upon admission to three high-risk healthcare units successfully identified colonized patients in a timely manner. Infection control procedures were then implemented to prevent further transmission. Although the downstream effect on colonization or infection was not measured, this study points to the potential benefit of active surveillance for C. auris.
3.2.6. Question 6. What Are Common Barriers and Facilitators to Implementing Active Surveillance PSPs for MRSA, CRE, C. auris, and C. difficile?
The six studies described in Review Question 5 and Table 2 did not discuss factors that either facilitated or presented barriers to implementation of ASC PSPs, with two minor exceptions. As noted in Review Question 4, a study of CRE surveillance22 reported that the cost of universal screening was prohibitive, resulting in development of a targeted and therefore less expensive program. A different type of challenge was described in a study examining C. difficile surveillance in cystectomy patients.20 The authors reported that stool samples were inadequate for screening in 21 percent of included patients, substantially limiting the capacity of the program to identify colonization. We also note that in many hospitals, an important barrier to optimal surveillance is the turnaround time required for laboratory processing and reporting of samples, which can vary substantially between healthcare sites and for each organism.
Our searches also identified three studies that provided insights on barriers and facilitators to implementation of ASC PSPs. A mixed-methods study conducted in the United Kingdom used focus groups, a national survey, and regression modelling to identify factors associated with successful nurse implementation of hospital-based MRSA surveillance programs.34 Several facilitators contributed to nurse adherence to ASC policies. These included integration of ASC into EHR-based admission instructions, routine audit and feedback to nursing staff, leaders who emphasize the value of MRSA screening, and training nurses to understand fully the purpose and significance of surveillance. Barriers included paper-based surveillance systems, EHR-based systems that did not automatically include surveillance instructions in a prominent place, lack of feedback about compliance, and high levels of patient flow.
In addition to the role of staff in implementing ASC PSPs, patient perspectives should also be considered. A qualitative study conducted in the United Kingdom interviewed patients about their experiences undergoing CRE screening.35 Some patients reported discomfort or embarrassment during screening, which could have been related to the use of rectal swabs for sample collection. Patients were typically told that screening was a “norm,” with little information provided to them about the purpose of surveillance or the risks of CRE. The authors concluded that a lack of discussion about the test was problematic, particularly for patients who had a positive test and were then immediately subjected to transmission precautions including isolation. Finally, colonized patients felt as if they were somehow responsible or to blame and endured unfortunate emotional distress.
A third study surveyed Canadian hospitals and laboratories to assess their readiness to implement surveillance for C. auris.36 Survey responses were received from 85 percent (56 out of 66) of hospitals and 84 percent (27 out of 32) of labs. Only 18 percent of hospitals had a C. auris surveillance policy, and just 14 percent tested patients at admission. Only a few labs reported having protocols for C. auris testing, and 15 percent of labs were not confident that they could correctly identify C. auris colonization. Our searches did not identify any similar assessment of U.S. hospitals or labs.
3.2.7. Question 7. What Resources (e.g., cost, staff, time) Are Required for Implementation of Active Surveillance PSPs for MRSA, CRE, C. auris, and C. difficile?
Very limited information was identified regarding resources needed for ASC PSPs. The primary resources associated with any type of surveillance program include staff time to collect test specimen, the cost of swabs and vials, and the time, materials, and costs of laboratory analysis. A targeted program that identifies high-risk patients through an EHR-based algorithm can pose additional costs for development, implementation, and maintenance of the algorithm.37 Universal screening will require more resources than targeted surveillance,21,22 and frequency of testing is a crucial component of cost as well. A study of C. difficile surveillance that targeted high-risk patients reported that approximately one-third of patients were tested on admission based on the risk criteria, and this testing rate “reduc[ed] the cost to an effective level.”21 However, this study did not provide additional information about program costs.
A study18 conducted in two neonatal ICUs that discontinued an active surveillance program for MRSA (consisting of screening on admission followed by weekly testing) reported that the total hospital cost was estimated at $500,000 to $600,000 annually and $448 per patient, but this included the costs of both surveillance and subsequent isolation precautions for colonized patients. The authors also reported the cost of a single MRSA test was $102, which included the purchase price of a swab and the cost for a laboratory to process a culture and report the result.
Finally, we identified one study provided data on the staff time required for MRSA surveillance.38 This study examined how long it took to collect cultures in an operating room before and after surgery in two U.S. hospitals. Cultures were collected from each patient’s nose, axilla, and groin. The authors found that the mean time needed for sampling prior to surgery was 3.39 minutes (standard error: 0.23). After surgery, sampling took a mean of 4.39 minutes (0.25). This did not include time needed to remove materials from transport boxes or return them afterwards. The study also found that inexperienced staff did not need significantly more time to collect cultures than staff with extensive experience.
3.2.8. Question 8. What Toolkits Are Available To Support Implementation of Active Surveillance PSPs for MRSA, CRE, C. auris, and C. difficile?
We did not identify any toolkits published since 2019 to support ASC PSPs for C. difficile or MDRO. However, CDC recently provided some practical guidance for hospitals seeking to establish surveillance for C. auris.39 Additionally, in 2023 updated recommendations for preventing MRSA infections40 were released jointly by the Society for Healthcare Epidemiology of America, the Infectious Diseases Society of America, the Association for Professionals in Infection Control and Epidemiology, the American Hospital Association, and the Joint Commission. Active surveillance strategies are highlighted in five recommendations.
4. Discussion
4.1. Interpretation of Findings
The findings of the rapid response indicate that active surveillance culturing (ASC) may be an effective patient safety practice (PSP) for reducing patient harm, but outcomes can vary by pathogen and surveillance approach. Two new studies of ASC for methicillin-resistant Staphylococcus aureus (MRSA)18,19 found that universal surveillance did not reduce infection risk compared to not using ASC. Conversely, two studies of Clostridioides difficile (C. difficile)20,21 and one study of carbapenem-resistant Enterobacterales (CRE)22 found that ASC significantly reduced infection rates. These results are generally consistent with the findings of Making Healthcare Safer III (MHS III), and we assessed five of the six new studies to be at low or moderate risk of bias, increasing our confidence in the validity of the results. It is unclear if the differences between the studies that reported improved outcomes and those that did not can be attributed to the different types of pathogens, or if they reflect differences in ASC strategies, patient populations, study designs, or other factors.
The heterogeneity and inconsistency of the evidence might be related to the use of other active infection control interventions during the pre-intervention period or in the ASC unexposed cohort. Studies of ASC effectiveness were generally pragmatic in design and unable to account fully for the potential effect of infection prevention interventions occurring in the control groups. This may be especially true for MRSA, where multiple interventions that prevent infections (e.g., targeted surgical prophylaxis, nasal antiseptic decolonization, and topical skin antisepsis with chlorhexidine administration) are administered routinely in hospitals, regardless of whether surveillance is conducted or patient colonization status is known.39 For more recent emerging pathogens, such as CRE, or pathogens with emerging but not established evidence for effectiveness of preventative interventions, such as, C. difficile, surveillance may be a more critical and effective component within infection control bundles.
MHS III concluded that targeted surveillance may perform as well as universal surveillance while using fewer resources, and the newest evidence provides some support for that conclusion. While both studies of MRSA examined universal ASC and did not identify benefit, two of the three studies that reported reduced infection risk (for C. difficile and CRE, respectively), used targeted surveillance. However, both studies compared risk-based targeted surveillance to no surveillance, and we did not identify any studies that directly compared targeted surveillance to universal surveillance. Finally, the lack of studies of Candida auris (C. auris) highlights a critical evidence gap.
4.2. Limitations
This rapid response has several limitations. First, rapid responses use streamlined processes to complete the effort in a narrow timeline. In this review, we limited the studies to articles published in English since 2019. Second, the search allowed for inclusion of studies conducted during the Coronavirus disease 19 (COVID-19) pandemic. Many patient care practices were affected by the COVID-19 pandemic and may impact any studies conducted during this timeframe. Third, we focused on studies that directly compared ASC to another surveillance strategy or to no surveillance. This excluded numerous studies that described the experiences and results associated with implementation of an ASC program within a hospital or health system, but did not provide a comparator. Fourth, studies rely frequently on control groups that are subject to “regular” or “standard” infection control and treatment procedures, but descriptions of these routine practices are often cursory and lack sufficient detail to account for possible confounding factors. Finally, for Review Question 5 we excluded studies that did not report clinical outcomes such as infections or patient colonization, or measures of healthcare utilization. Studies that examine the efficacy of emerging strategies or supplemental approaches, such as whole genome sequencing, often report diagnostic performance results or epidemiologic data rather than clinical results. Indeed, we found no studies that described clinical outcomes associated with use of whole genome sequencing.
4.3. Implications and Conclusions
Active surveillance of C. difficile and MDRO pathogens such as MRSA and CRE is a widely used PSP to detect asymptomatic colonization, trigger infection control strategies, and reduce the spread of healthcare-associated infections. A few recent studies confirm that active surveillance for C. difficile and CRE can help prevent infections. The evidence also suggests that both universal and targeted surveillance approaches can be effective.
Substantial gaps and limitations of the evidence base remain largely unaddressed by the most recent research. Active surveillance PSPs for MRSA are widespread, but new research adds to prior uncertainty about the value of such practices. Targeted surveillance PSPs for any pathogen increasingly appear to be valuable, but published studies have usually compared targeted surveillance to no surveillance. Head-to-head comparisons of targeted surveillance to universal surveillance would be optimal, but such direct assessments are lacking. Further, one recent study highlights a growing interest in de-implementing active surveillance, but additional research on the safety of discontinuing surveillance PSPs is needed.
Additionally, active surveillance PSPs are often implemented in the context of multicomponent infection control interventions or quality improvement efforts, and it is difficult to evaluate the effectiveness of surveillance apart from other strategies. Finally, research on surveillance for C. auris is needed, as hospitals lack the evidence, tools, and resources to address this challenge.
5. References
- 1.
- Rosen M, Dy SM, Stewart CM, et al. Final report on prioritization of patient safety practices for a new rapid review or rapid response. Making Healthcare Safer IV. (Prepared by the Johns Hopkins, ECRI, and Southern California Evidence-based Practice Centers under Contract No. 75Q80120D00003). AHRQ Publication No. 23-EHC019-1. Rockville (MD): Agency for Healthcare Research and Quality; 2023 Jul. https:
//effectivehealthcare .ahrq.gov/products /prioritization-patient-safety-practices. Accessed on August 4, 2023. - 2.
- Antibiotic resistance threats in the United States, 2019 [internet]. Atlanta (GA): Centers for Disease Control and Prevention (CDC); 2019. https://www
.cdc.gov/drugresistance /pdf/threats-report /2019-ar-threats-report-508.pdf. Accessed on August 31, 2022. - 3.
- Johnston KJ, Thorpe KE, Jacob JT, et al. The incremental cost of infections associated with multidrug-resistant organisms in the inpatient hospital setting-A national estimate. Health Serv Res. 2019;54(4):782–92. doi: 10.1111/1475-6773.13135. PMID: 30864179. [PMC free article: PMC6606543] [PubMed: 30864179] [CrossRef]
- 4.
- National Healthcare Safety Network (NHSN) patient safety component manual. Atlanta (GA): Centers for Disease Control and Prevention (CDC), National Healthcare Safety Network (NHSN); 2023 Jan. https://www
.cdc.gov/nhsn /pdfs/pscmanual/pcsmanual_current .pdf - 5.
- Federal Task Force on Combating Antibiotic-Resistant Bacteria. National action plan for combating antibiotic-resistant bacteria (CARB) 2020-2025. Washington (DC): U.S. Department of Health and Human Services, Assistant Secretary for Planning and Evaluation; 2020 Oct. https://aspe
.hhs.gov /reports/national-action-plan-combating-antibiotic-resistant-bacteria-2020-2025 - 6.
- Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases. Interim guidance for a public health response to contain novel or targeted multidrug-resistant organisms (MDROs) [internet]. Atlanta (GA): Centers for Disease Control and Prevention (CDC); 2022. https://www
.cdc.gov/hai /pdfs/mdro-guides /Health-Response-Contain-MDRO-508.pdf. Accessed on May 25, 2023. - 7.
- Blanco N, O’Hara LM, Harris AD. Transmission pathways of multidrug-resistant organisms in the hospital setting: a scoping review. Infect Control Hosp Epidemiol. 2019;40(4):447–56. doi: 10.1017/ice.2018.359. PMID: 30837029. [PMC free article: PMC6897300] [PubMed: 30837029] [CrossRef]
- 8.
- Sundermann AJ, Chen J, Kumar P, et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin Infect Dis. 2022;75(3):476–82. doi: 10.1093/cid/ciab946. PMID: 34791136. [PMC free article: PMC9427134] [PubMed: 34791136] [CrossRef]
- 9.
- Lee BY, Bartsch SM, Hayden MK, et al. How introducing a registry with automated alerts for Carbapenem-resistant Enterobacteriaceae (CRE) may help control CRE spread in a region. Clin Infect Dis. 2020;70(5):843–9. doi: 10.1093/cid/ciz300. PMID: 31070719. [PMC free article: PMC7931833] [PubMed: 31070719] [CrossRef]
- 10.
- Murray J, Cohen AL. Infectious disease surveillance. International Encyclopedia of Public Health. Elsevier, Inc.; 2017. https://www
.ncbi.nlm .nih.gov/pmc/articles/PMC7149515/. Accessed on May 25, 2023. - 11.
- Popovich KJ, Aureden K, Ham DC, et al. SHEA/IDSA/APIC Practice Recommendation: Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute-care hospitals: 2022 Update. Infect Control Hosp Epidemiol. 2023;44(7):1–29. doi: 10.1017/ice.2023.102. PMID: 37381690. [PMC free article: PMC10369222] [PubMed: 37381690] [CrossRef]
- 12.
- Lapp Z, Crawford R, Miles-Jay A, et al. Regional spread of blaNDM-1-Containing Klebsiella pneumoniae ST147 in post-acute care facilities. Clin Infect Dis. 2021;73(8):1431–9. doi: 10.1093/cid/ciab457. PMID: 33999991. [PMC free article: PMC8528401] [PubMed: 33999991] [CrossRef]
- 13.
- Lane CR, Brett J, Schultz M, et al. Search and contain: Impact of an integrated genomic and epidemiological surveillance and response program for control of carbapenemase-producing enterobacterales. Clin Infect Dis. 2021;73(11):e3912–e20. doi: 10.1093/cid/ciaa972. PMID: 32663248. [PMC free article: PMC8662772] [PubMed: 32663248] [CrossRef]
- 14.
- McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1–e48. doi: 10.1093/cid/cix1085. PMID: 29462280. [PMC free article: PMC6018983] [PubMed: 29462280] [CrossRef]
- 15.
- Kociolek LK, Gerding DN, Carrico R, et al. Strategies to prevent Clostridioides difficile infections in acute-care hospitals: 2022 update. Infect Control Hosp Epidemiol. 2023;44(4):527–49. doi: 10.1017/ice.2023.18. PMID: 37042243. [PMC free article: PMC10917144] [PubMed: 37042243] [CrossRef]
- 16.
- Evidence-based Practice Centers. Rockville (MD): Agency for Healthcare Research and Quality; 2023. https:
//effectivehealthcare .ahrq.gov/about/epc. Accessed on July 28, 2023. - 17.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. PMID: 27733354. [PMC free article: PMC5062054] [PubMed: 27733354] [CrossRef]
- 18.
- Petersen RY, Hillman NH, Sadiq FH, et al. Effects of discontinuation of weekly surveillance testing on methicillin-resistant Staphylococcus aureus in the NICU. Am J Perinatol. 2023. doi: 10.1055/s-0043-1763481. PMID: 36848933. [PubMed: 36848933] [CrossRef]
- 19.
- Sun J, Qian D, Zhou R, et al. Effects of preoperative Staphylococcus aureus screening and targeted decolonization bundle protocols in cardiac surgery: a nine-year review of a regional cardiovascular center in China. J Thorac Dis. 2022;14(12):4741–50. doi: 10.21037/jtd-22-591. PMID: 36647471. [PMC free article: PMC9840028] [PubMed: 36647471] [CrossRef]
- 20.
- Calaway AC, Jacob JM, Tong Y, et al. A prospective program to reduce the clinical incidence of Clostridium difficile colitis infection after cystectomy. J Urol. 2019;201(2):342–9. doi: 10.1016/j.juro.2018.09.030. PMID: 30218764. [PubMed: 30218764] [CrossRef]
- 21.
- Peterson LR, O’Grady S, Keegan M, et al. Reduced Clostridioides difficile infection in a pragmatic stepped-wedge initiative using admission surveillance to detect colonization. PLoS One. 2020;15(3):e0230475. doi: 10.1371/journal.pone.0230475. PMID: 32191763. [PMC free article: PMC7082001] [PubMed: 32191763] [CrossRef]
- 22.
- Li S, Guo FZ, Zhao XJ, et al. Impact of individualized active surveillance of carbapenem-resistant enterobacteriaceae on the infection rate in intensive care units: a 3-year retrospective study in a teaching hospital of People’s Republic of China. Infect Drug Resist. 2019;12:1407–14. doi: 10.2147/IDR.S201644. PMID: 31213858. [PMC free article: PMC6538007] [PubMed: 31213858] [CrossRef]
- 23.
- Yang TT, Luo XP, Yang Q, et al. Different screening frequencies of carbapenem-resistant Enterobacteriaceae in patients undergoing hematopoietic stem cell transplantation: which one is better? Antimicrob Resist Infect Control. 2020;9(1):49. doi: 10.1186/s13756-020-0706-0. PMID: 32183898. [PMC free article: PMC7077122] [PubMed: 32183898] [CrossRef]
- 24.
- Zacharioudakis IM, Zervou FN, Pliakos EE, et al. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381–90; quiz 91. doi: 10.1038/ajg.2015.22. PMID: 25732416. [PubMed: 25732416] [CrossRef]
- 25.
- Riggs MM, Sethi AK, Zabarsky TF, et al. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis. 2007;45(8):992–8. PMID: 17879913. [PubMed: 17879913]
- 26.
- Baron SW, Ostrowsky BE, Nori P, et al. Screening of Clostridioides difficile carriers in an urban academic medical center: Understanding implications of disease. Infect Control Hosp Epidemiol. 2020;41(2):149–53. doi: 10.1017/ice.2019.309. PMID: 31822302. [PMC free article: PMC7702293] [PubMed: 31822302] [CrossRef]
- 27.
- Guh AY, Mu Y, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382(14):1320–30. doi: 10.1056/NEJMoa1910215. PMID: 32242357. [PMC free article: PMC7861882] [PubMed: 32242357] [CrossRef]
- 28.
- Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18 Suppl 6:21–7. doi: 10.1111/1469-0691.12046. PMID: 23121551. [PubMed: 23121551] [CrossRef]
- 29.
- Gupta K, Martinello RA, Young M, et al. MRSA nasal carriage patterns and the subsequent risk of conversion between patterns, infection, and death. PLoS One. 2013;8(1):e53674. doi: 10.1371/journal.pone.0053674. PMID: 23326483. [PMC free article: PMC3542362] [PubMed: 23326483] [CrossRef]
- 30.
- Tischendorf J, de Avila RA, Safdar N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: A systematic review. Am J Infect Control. 2016;44(5):539–43. doi: 10.1016/j.ajic.2015.12.005. PMID: 26899297. [PMC free article: PMC5262497] [PubMed: 26899297] [CrossRef]
- 31.
- Lyman M, Forsberg K, Sexton DJ, et al. Worsening spread of Candida auris in the United States, 2019 to 2021. Ann Intern Med. 2023;176(4):489–95. doi: 10.7326/M22-3469. PMID: 36940442. [PubMed: 36940442] [CrossRef]
- 32.
- Janezic S, Rupnik M. Development and implementation of whole genome sequencing-based typing schemes for Clostridioides difficile. Front Public Health. 2019;7:309. doi: 10.3389/fpubh.2019.00309. PMID: 31709221. [PMC free article: PMC6821651] [PubMed: 31709221] [CrossRef]
- 33.
- Rowlands J, Dufort E, Chaturvedi S, et al. Candida auris admission screening pilot in select units of New York City health care facilities, 2017-2019. Am J Infect Control. 2023;51:866–870. doi: 10.1016/j.ajic.2023.01.012. PMID: 36736380. [PMC free article: PMC10902794] [PubMed: 36736380] [CrossRef]
- 34.
- Currie K, King C, McAloney-Kocaman K, et al. Barriers and enablers to meticillin-resistant Staphylococcus aureus admission screening in hospitals: a mixed-methods study. J Hosp Infect. 2019;101(1):100–8. doi: 10.1016/j.jhin.2018.08.006. PMID: 30098382. [PubMed: 30098382] [CrossRef]
- 35.
- King C, Grandison T, Cawthorne J, et al. Patient experience of hospital screening for carbapenemase-producing Enterobacteriaceae: A qualitative study. J Clin Nurs. 2019;28(21–22):3890–900. doi: 10.1111/jocn.14982. PMID: 31240778. [PubMed: 31240778] [CrossRef]
- 36.
- Garcia-Jeldes F, Mitchell R, Bharat A, McGeer A. Preparedness for Candida auris in Candadian Nosocomial Infection Surveillance Program (CNISP) hospitals, 2018. Infect Control Hosp Epidemiol. 2020;41:361–364. doi: 10.1017/ice.2019.369. PMID: 31928546. [PubMed: 31928546] [CrossRef]
- 37.
- Lin G, Tseng KK, Gatalo O, et al. Cost-effectiveness of carbapenem-resistant Enterobacteriaceae (CRE) surveillance in Maryland. Infect Control Hosp Epidemiol. 2022;43(9):1162–70. doi: 10.1017/ice.2021.361. PMID: 34674791. [PMC free article: PMC9023597] [PubMed: 34674791] [CrossRef]
- 38.
- Datta S, Dexter F, Ledolter J, et al. Sample times for surveillance of S. aureus transmission to monitor effectiveness and provide feedback on intraoperative infection control. Perioper Care Oper Room Manag. 2020;21:100137. doi: 10.1016/j.pcorm.2020.100137. PMID: 33072894. [PMC free article: PMC7547614] [PubMed: 33072894] [CrossRef]
- 39.
- Guidance for detection of colonization of Candida auris. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Foodborne, Waterborne, and Environmental Diseases (DFWED); 2022. https://www
.cdc.gov/fungal /candida-auris/c-auris-guidance .html. Accessed on July 28, 2023. - 40.
- Popovich KJ, Green SJ, Okamoto K, et al. MRSA transmission in intensive care units: genomic analysis of patients, their environments, and healthcare workers. Clinical infectious diseases. 2021;72(11):1879–87. doi: 10.1093/cid/ciaa731. PMID: 32505135. [PMC free article: PMC8315036] [PubMed: 32505135] [CrossRef]
Acknowledgments
The authors gratefully acknowledge the following individuals for their contributions to this project: Leyi Lin, M.D., and Melissa A. Miller, M.D., M.S., of AHRQ’s Center for Quality Improvement and Patient Safety; and Jonathan Treadwell, Ph.D., Laura Koepfler, M.L.S., Helen Dunn, and Kitty Donohue of ECRI, Plymouth Meeting, PA.
Afterword
Recognized for excellence in conducting comprehensive systematic reviews, the Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Center (EPC) Program is developing a range of rapid evidence products to assist end-users in making specific decisions in a limited timeframe. AHRQ recognizes that people are struggling with urgent questions on how to make healthcare safer. AHRQ is using this rapid format for the fourth edition of its Making Healthcare Safer series of reports, produced by the EPC Program and the General Patient Safety Program. To shorten timelines, reviewers make strategic choices about which processes to abridge. However, the adaptations made for expediency may limit the certainty and generalizability of the findings from the review, particularly in areas with a large literature base. Transparent reporting of the methods used and the resulting limitations of the evidence synthesis are extremely important.
AHRQ expects that these rapid evidence products will be helpful to health plans, providers, purchasers, government programs, and the healthcare system as a whole. Transparency and stakeholder input are essential to AHRQ. If you have comments related to this report, they may be sent by mail to the Task Order Officer named below at: Agency for Healthcare Research and Quality, 5600 Fishers Lane, Rockville, MD 20857, or by email to vog.shh.qrha@SHM.
- Robert Otto Valdez, Ph.D., M.H.S.A.DirectorAgency for Healthcare Research and Quality
- Therese Miller, D.P.H.DirectorCenter for Evidence and Practice ImprovementAgency for Healthcare Research and Quality
- Christine Chang, M.D., M.P.H.Acting DirectorEvidence-based Practice Center DivisionCenter for Evidence and Practice ImprovementAgency for Healthcare Research and Quality
- David W. Niebuhr, M.D., M.P.H., M.Sc.Evidence-based Practice Center Division LiaisonCenter for Evidence and Practice ImprovementAgency for Healthcare Research and Quality
- Craig A. Umscheid, M.D., M.S.DirectorCenter for Quality Improvement and Patient SafetyAgency for Healthcare Research and Quality
- Margie Shofer, B.S.N., M.B.A.Director, General Patient Safety ProgramCenter for Quality Improvement and Patient SafetyAgency for Healthcare Research and Quality
- Jennifer EskandariTask Order OfficerCenter for Quality Improvement and Patient SafetyAgency for Healthcare Research and Quality
- Farzana Samad, Pharm.D., FISMP, CPPSHealth Scientist AdministratorCenter for Quality Improvement and Patient SafetyAgency for Healthcare Research and Quality
Appendixes
Appendix A. Methods: Search Strategy for Published Literature
Appendix B. List of Excluded Studies Upon Full-Text Review
- 1.
- Almond J, Leal J, Bush K et al. Hospital-acquired Clostridioides difficile infections in Alberta: The validity of laboratory-identified event surveillance versus clinical infection surveillance. Am J Infect Control. 2020 48(6):633–637. – No outcome of interest [PubMed: 31733811]
- 2.
- Al Musawi S, Alkhaleefa Q, Alnassri S, Alamri A and Alnimr A. Predictive role of targeted, active surveillance cultures for detection of methicillin-resistant Staphylococcus aureus. Infect Drug Resist. 2021 14:4757–4764. – No comparator [PMC free article: PMC8594744] [PubMed: 34795491]
- 3.
- Ambretti S, Bassetti M, Clerici P et al. Screening for carriage of carbapenem-resistant Enterobacteriaceae in settings of high endemicity: a position paper from an Italian working group on CRE infections. Antimicrob Resist Infect Control. 2019 8:136. – Study design – review [PMC free article: PMC6693230] [PubMed: 31423299]
- 4.
- Amick M, O’Marr JM and Schuster KM. Evaluation of MRSA surveillance nasal swabs for predicting MRSA infection in surgical intensive care unit patients. J Surg Res. 2021 268:712–719. – No comparator [PubMed: 34487964]
- 5.
- Berenguer EY, Morales JC, Revuelto PS et al. Results of a preoperative screening and decolonization programme for Staphylococcus aureus in primary hip and knee arthroplasty. Rev Esp Cir Ortop Traumatol. 2023. – No pathogen of interest [PubMed: 36863522]
- 6.
- Bagal UR, Phan J, Welsh RM et al. MycoSNP: A portable workflow for performing whole-genome sequencing analysis of candida auris. Methods Mol Biol. 2022 2517:215–228. – Study design - review [PubMed: 35674957]
- 7.
- Baghdadi J, Ganz DA, Chumpia M, Chang ET and de Peralta SS. Holding firm: Use of clinical correlation to improve Clostridioides difficile testing. Am J Infect Control. 2020 48(9):1104–1107. – No comparator [PubMed: 31862165]
- 8.
- Barker AK, Scaria E, Safdar N and Alagoz O. Evaluation of the cost-effectiveness of infection control strategies to reduce hospital-onset Clostridioides difficile infection. JAMA Netw Open. 2020 3(8):e2012522. – Effect of surveillance not evaluated separately [PMC free article: PMC7426752] [PubMed: 32789514]
- 9.
- Bartels MD, Holm MKA, Worning P et al. Whole genome sequencing reveals two genetically distinct MRSA outbreaks among people who inject drugs and homeless people in Copenhagen. Apmis. 2023 131(6):294–302. – No intervention of interest [PubMed: 37026991]
- 10.
- Ben Natan O, Stein M and Reisfeld S. Audit and feedback as a tool to increase compliance with carbapenemase-producing Enterobacteriaceae (CPE) screening and decrease CPE transmission in the hospital. Infect Control Hosp Epidemiol. 2022 doi:10.1017/ice.2022.224. – No intervention of interest [PMC free article: PMC10665877] [PubMed: 36081188] [CrossRef]
- 11.
- Benulič K, Pirš M, Couto N et al. Whole genome sequencing characterization of Slovenian carbapenem-resistant Klebsiella pneumoniae, including OXA-48 and NDM-1 producing outbreak isolates. PLoS One. 2020 15(4):e0231503. – No intervention of interest [PMC free article: PMC7153892] [PubMed: 32282829]
- 12.
- Blanco N, Robinson GL, Heil EL et al. Impact of a C. difficile infection (CDI) reduction bundle and its components on CDI diagnosis and prevention. Am J Infect Control. 2021 49(3):319–326. – No intervention of interest [PubMed: 33640109]
- 13.
- Borg MA, Suda D, Scicluna E, Brincat A and Zarb P. Universal admission screening: a potential game-changer in hospitals with high prevalence of MRSA. J Hosp Infect. 2021 113:77–84. – No outcome of interest [PubMed: 33811962]
- 14.
- Büchler AC, Wicki M, Frei R et al. Matching Clostridioides difficile strains obtained from shoe soles of healthcare workers epidemiologically linked to patients and confirmed by whole-genome sequencing. J Hosp Infect. 2022 126:10–15. – No intervention of interest [PubMed: 35562075]
- 15.
- Buckley MS, Kobic E, Yerondopoulos M et al. Comparison of methicillin-resistant Staphylococcus aureus nasal screening predictive value in the intensive care unit and general ward. Ann Pharmacother. 2022 DOI:10.1177/10600280221145152. – No outcome of interest [PubMed: 36575978] [CrossRef]
- 16.
- Burgoon R, Weeda E, Mediwala KN and Raux BR. Clinical utility of negative methicillin-resistant Staphylococcus aureus (MRSA) nasal surveillance swabs in skin and skin structure infections. Am J Infect Control. 2022 50(8):941–946. – No intervention of interest [PubMed: 34958856]
- 17.
- Cai Y, Hoo GSR, Lee W et al. Estimating the economic cost of carbapenem resistant Enterobacterales healthcare associated infections in Singapore acute-care hospitals. PLOS Glob Public Health. 2022 2(12): e0001311. – No intervention of interest [PMC free article: PMC10021918] [PubMed: 36962882]
- 18.
- Chang E, Chang HE, Shin IS et al. Investigation on the transmission rate of carbapenemase-producing carbapenem-resistant Enterobacterales among exposed persons in a tertiary hospital using whole-genome sequencing. J Hosp Infect. 2022 124:1–8. – No intervention of interest [PubMed: 35307505]
- 19.
- Collison M, Murillo C, Marrs R et al. Universal screening for Clostridioides difficile at an urban academic medical center. Infect Control Hosp Epidemiol. 2021 42(3):351–352. – No comparator [PubMed: 32959739]
- 20.
- Contreras DA and Morgan MA. Surveillance diagnostic algorithm using real-time PCR assay and strain typing method development to assist with the control of C. auris amid COVID-19 pandemic. Front Cell Infect Microbiol. 2022 12:887754. – No comparator [PMC free article: PMC9471137] [PubMed: 36118039]
- 21.
- Crobach MJT, Hornung BVH, Verduin C et al. Screening for Clostridioides difficile colonization at admission to the hospital: a multi-centre study. Clin Microbiol Infect. 2023 29(7):891–896. – No comparator [PubMed: 36871826]
- 22.
- Currie K, King C, McAloney-Kocaman K et al. Barriers and enablers to meticillin-resistant Staphylococcus aureus admission screening in hospitals: a mixed-methods study. J Hosp Infect. 2019 101(1);100–108. – No outcome of interest [PubMed: 30098382]
- 23.
- Dancer SJ, Adams CE, Smith J et al. Tracking Staphylococcus aureus in the intensive care unit using whole-genome sequencing. J Hosp Infect. 2019 103(1):13–20. – No intervention of interest [PubMed: 31039382]
- 24.
- Datta S, Dexter F, Ledolter J, Wall RW and Loftus RW. Sample times for surveillance of S. aureus transmission to monitor effectiveness and provide feedback on intraoperative infection control. Perioper Care Oper Room Manag. 2020 21:100137. – No comparator [PMC free article: PMC7547614] [PubMed: 33072894]
- 25.
- Dexter F, Ledolter J, Wall RT, Datta S and Loftus RW. Sample sizes for surveillance of S. aureus transmission to monitor effectiveness and provide feedback on intraoperative infection control including for COVID-19. Perioper Care Oper Room Manag. 2020 20:100115. – No comparator [PMC free article: PMC7240254] [PubMed: 32501426]
- 26.
- Diallo OO, Baron SA, Abat C et al. Antibiotic resistance surveillance systems: A review. J Glob Antimicrob Resist. 2020 23:430–438. – Study design - review [PubMed: 33176216]
- 27.
- Dymond A, Davies H, Mealing S et al. Genomic surveillance of methicillin-resistant Staphylococcus aureus: A mathematical early modeling study of cost-effectiveness. Clin Infect Dis. 2020 70(8):1613–1619. – No outcome of interest [PMC free article: PMC7145999] [PubMed: 31219153]
- 28.
- Elliott TM, Hare N, Hajkowicz K et al. Evaluating the economic effects of genomic sequencing of pathogens to prioritise hospital patients competing for isolation beds. Aust Health Rev. 2021 45(1):59–65. – Effect of surveillance not evaluated separately [PubMed: 33049199]
- 29.
- Emery A, Jeanvoine A, Bailly P et al. Management of carbapenemase-producing Enterobacteriaceae in a low incidence area: A six-year experience in a university hospital. Infect Control Hosp Epidemiol. 2019 40(8):936–938. – No comparator [PubMed: 31203818]
- 30.
- Evans ME, Simbartl LA, Kralovic SM et al. Healthcare-associated infections in Veterans Affairs acute-care and long-term healthcare facilities during the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2023 44(3):420–426. – No intervention of interest [PMC free article: PMC9043628] [PubMed: 35379366]
- 31.
- Forde BM, Bergh H, Cuddihy T et al. Clinical implementation of routine whole-genome sequencing for hospital infection control of multi-drug resistant pathogens. Clin Infect Dis. 2023 76(3):e1277–e1284. – No intervention of interest [PubMed: 36056896]
- 32.
- Foschi C, Gaibani P, Lombardo D, Re MC and Ambretti S. Rectal screening for carbapenemase-producing Enterobacteriaceae: a proposed workflow. J Glob Antimicrob Resist. 2020 21:86–90. – No comparator [PubMed: 31639545]
- 33.
- Freire MP, de Oliveira Garcia D, Lima SG et al. Performance of two methods of carbapenem-resistant Enterobacterales surveillance on a kidney transplant ward: selective culture of and real-time PCR directly from rectal swabs. Infection. 50(6):1525–1533. – No outcome of interest [PubMed: 35534755]
- 34.
- Garcia-Jeldes F, Mitchell R, Bharat A and McGeer A. Preparedness for Candida auris in Canadian Nosocomial Infection Surveillance Program (CNISP) hospitals, 2018. Infect Control Hosp Epidemiol. 2020 41(3):361–364. – No outcome of interest [PubMed: 31928546]
- 35.
- Gomides MDA, Fontes AMS, Silveira A et al. The importance of active surveillance of carbapenem-resistant Enterobacterales (CRE) in colonization rates in critically ill patients. PLoS One. 2022 17(1): e0262554. – No intervention of interest [PMC free article: PMC8775193] [PubMed: 35051212]
- 36.
- Gonzales-Luna AJ, Dureja C, Eubank TA et al. Surveillance of Clostridioides difficile antimicrobial resistance in the United States. Clin Infect Dis. 2023 76:2038–2039. – Study design - correspondence [PMC free article: PMC10249984] [PubMed: 36883582]
- 37.
- Harrison C, Zent R, Schneck E, Flynn CE and Drees M. Infection prevention versus antimicrobial stewardship: Does nasal povidone-iodine interfere with methicillin-resistant Staphylococcus aureus (MRSA) screening? Infect Control Hosp Epidemiol. 2022 43(7):945–947. – No intervention of interest [PubMed: 34933698]
- 38.
- Heindel J, Zweigner J, Fuchs F and Hamprecht A. Usefulness of screening for Candida auris colonisation in international patients admitted to a large university hospital. Mycoses 2023 66(2):138–143. – No comparator [PubMed: 36135346]
- 39.
- Hong F, Salmon S, Ong XY et al. Routine antiseptic baths and MRSA decolonization: diverse approaches across Singapore’s acute-care hospitals. J Hosp Infect. 2021 112:87–91. – No intervention of interest [PubMed: 33812940]
- 40.
- Hyun IK, Park PJ, Park D et al. Methicillin-resistant Staphylococcus aureus screening is important for surgeons. Ann Hepatobiliary Pancreat Surg. 2019 23(3):265–273. – No intervention of interest [PMC free article: PMC6728247] [PubMed: 31501816]
- 41.
- Jakharia KK, Ilaiwy G, Moose SS et al. Use of whole-genome sequencing to guide a Clostridioides difficile diagnostic stewardship program. Infect Control Hosp Epidemiol. 2019 40(7):804–806. – No intervention of interest [PubMed: 31088580]
- 42.
- Janezic S and Rupnik M. Development and implementation of whole genome sequencing-based typing schemes for Clostridioides difficile. Front Public Health. 2019 7:309. – Study design - review [PMC free article: PMC6821651] [PubMed: 31709221]
- 43.
- Kamboj M, McMillen T, Syed M et al. Evaluation of a combined multilocus sequence typing and whole-genome sequencing two-step algorithm for routine typing of Clostridioides difficile. J Clin Microbiol. 2021 59(2). – No outcome of interest [PMC free article: PMC8111118] [PubMed: 33177119]
- 44.
- Karanfilovska D, Cheng AC, Spelman D and Worth LJ. Development and piloting of a prevention assessment and response tool for healthcare-associated Staphylococcus aureus bloodstream infection (the SAB-PART Study) using a Delphi method. J Hosp Infect. 2021 115:17–26. – No intervention of interest [PubMed: 34126103]
- 45.
- Kardaś-Słoma L, Fournier S, Dupont JC et al. Cost-effectiveness of strategies to control the spread of carbapenemase-producing Enterobacterales in hospitals: a modelling study. Antimicrob Resist Infect Control. 2022 11(1):117. - Effect of surveillance not evaluated separately [PMC free article: PMC9484055] [PubMed: 36117231]
- 46.
- Kelly BJ, Bekele S, Loughrey S et al. Healthcare microenvironments define multidrug-resistant organism persistence. Infect Control Hosp Epidemiol. 2022 43(9):1135–1141. – No intervention of interest [PMC free article: PMC8866524] [PubMed: 34425925]
- 47.
- King C, Grandison T, Cawthorne J and Currie K. Patient experience of hospital screening for carbapenemase-producing Enterobacteriaceae: A qualitative study. J Clin Nurs. 2019 28(21–22):3890–3900. – No outcome of interest [PubMed: 31240778]
- 48.
- Kinnevey PM, Kearney A, Shore AC et al. Meticillin-susceptible Staphylococcus aureus transmission among healthcare workers, patients and the environment in a large acute hospital under non-outbreak conditions investigated using whole-genome sequencing. J Hosp Infect. 2022 127:15–25. – No pathogen of interest [PubMed: 35594983]
- 49.
- Kinnevey PM, Kearney A, Shore AC et al. Meticillin-resistant Staphylococcus aureus transmission among healthcare workers, patients and the environment in a large acute hospital under non-outbreak conditions investigated using whole-genome sequencing. J Hosp Infect. 2021 118:99–107. – No outcome of interest [PubMed: 34428508]
- 50.
- Kossow A, Kampmeier S, Schaumburg F et al. Whole genome sequencing reveals a prolonged and spatially spread nosocomial outbreak of Panton-Valentine leucocidin-positive meticillin-resistant Staphylococcus aureus (USA300). J Hosp Infect. 2019 101(3):327–332. – No intervention of interest [PubMed: 30240815]
- 51.
- Kumar P, Sundermann AJ, Martin EM et al. Method for economic evaluation of bacterial whole genome sequencing surveillance compared to standard of care in detecting hospital outbreaks. Clin Infect Dis. 2021 73(1):e9–e18. – No pathogen of interest [PMC free article: PMC8246822] [PubMed: 32367125]
- 52.
- Lal AK, Sprawka N, Darji H et al. MRSA screening: incidence and maternal postpartum outcomes in an obstetric population at a tertiary care center. Arch Gynecol Obstet. 2023 307(4):1203–1208. – No comparator [PubMed: 35396975]
- 53.
- Lane CR, Brett J, Schultz M et al. Search and contain: Impact of an integrated genomic and epidemiological surveillance and response program for control of carbapenemase-producing Enterobacterales. Clin Infect Dis. 2021 73(11):e3912–e3920. – No comparator [PMC free article: PMC8662772] [PubMed: 32663248]
- 54.
- Ledda A, Cummins M, Shaw LP et al. Hospital outbreak of carbapenem-resistant Enterobacterales associated with a bla(OXA-48) plasmid carried mostly by Escherichia coli ST399. Microb Genom. 2022 8(4). – No intervention of interest [PMC free article: PMC9453065] [PubMed: 35442183]
- 55.
- Lee BY, Bartsch SM, Hayden MK et al. How to choose target facilities in a region to implement carbapenem-resistant Enterobacteriaceae control measures. Clin Infect Dis. 2021 72(3):438–447. – No intervention of interest [PMC free article: PMC7850552] [PubMed: 31970389]
- 56.
- Lee BY, Bartsch SM, Hayden MK et al. How introducing a registry with automated alerts for carbapenem-resistant Enterobacteriaceae (CRE) may help control CRE spread in a region. Clin Infect Dis. 2020 70(5):843–849. – No intervention of interest [PMC free article: PMC7931833] [PubMed: 31070719]
- 57.
- Lin G, Tseng KK, Gatalo O et al. Cost-effectiveness of carbapenem-resistant Enterobacteriaceae (CRE) surveillance in Maryland. Infect Control Hosp Epidemiol. 2022 43(9):1162–1170. – No outcome of interest [PMC free article: PMC9023597] [PubMed: 34674791]
- 58.
- Lin L, Ke ZY, Wang Y et al. Efficacy of preoperative screening and decolonization for staphylococcus aureus in total joint arthroplasty: A meta-analysis. Asian J Surg. 2021 44(6):807–818. – Study design - review [PubMed: 33468375]
- 59.
- Lin R, Akgun E, Erenay FS et al. Effectiveness of Methicillin-resistant Staphylococcus aureus surveillance among exposed roommates in community hospitals: Conventional culture vs direct PCR. Am J Infect Control. 2023 doi:10.1016/j.ajic.2023.04.009. – Study design - simulation [PubMed: 37059122] [CrossRef]
- 60.
- Manoukian S, Stewart S, Dancer SJ et al. Probabilistic microsimulation to examine the cost-effectiveness of hospital admission screening strategies for carbapenemase-producing enterobacteriaceae (CPE) in the United Kingdom. Eur J Health Econ. 2022 23(7):1173–1185. – No outcome of interest [PMC free article: PMC8689289] [PubMed: 34932169]
- 61.
- Marimuthu K, Venkatachalam I, Koh V et al. Whole genome sequencing reveals hidden transmission of carbapenemase-producing Enterobacterales. Nat Commun. 2022 13(1):3052. – No intervention of interest [PMC free article: PMC9160272] [PubMed: 35650193]
- 62.
- Maurer NR, Hogan TH and Walker DM. Hospital- and system-wide interventions for health care-associated infections: A systematic review. Med Care Res Rev. 2021 78(6):643–659. – Study design - review [PubMed: 32842879]
- 63.
- McKew G, Ramsperger M, Cheong E et al. Hospital MRSA outbreaks: Multiplex PCR-reverse line blot binary typing as a screening method for WGS, and the role of the environment in transmission. Infect Dis Health. 2020 25(4):268–276. – No comparator [PubMed: 32616448]
- 64.
- Migliara G, Di Paolo C, Barbato D et al. Multimodal surveillance of healthcare associated infections in an intensive care unit of a large teaching hospital. Ann Ig. 2019 31(5):399–413. – No comparator [PubMed: 31304521]
- 65.
- Moreno C, Seaman R and Noles K. Quality improvement project to decrease the rates of clostridium difficile infections in a tertiary care center. J Nurs Care Qual. 2023 38(1):10. – No intervention of interest [PubMed: 36409655]
- 66.
- Mulet Bayona JV, Salvador García C, Tormo Palop N and Gimeno Cardona C. Validation and implementation of a commercial real-time PCR assay for direct detection of Candida auris from surveillance samples. Mycoses. 2021 64(6):612–615. – No outcome of interest [PubMed: 33529398]
- 67.
- Nelson RE, Goto M, Samore MH et al. Expanding an economic evaluation of the Veterans Affairs (VA) methicillin-resistant Staphylococcus aureus (MRSA) prevention initiative to include prevention of infections from other pathogens. Clin Infect Dis. 2021 72:S50–S58. No comparator [PubMed: 33512526]
- 68.
- Ong KM, Phillips MS and Peskin CS. A mathematical model and inference method for bacterial colonization in hospital units applied to active surveillance data for carbapenem-resistant enterobacteriaceae. PLoS One. 2020 15(11): e0231754. – No intervention of interest [PMC free article: PMC7660488] [PubMed: 33180781]
- 69.
- Ötleş E, Balczewski EA, Keidan M. et al. Clostridioides difficile infection surveillance in intensive care units and oncology wards using machine learning. Infect Control Hosp Epidemiol. 2023 1–6. doi:10.1017/ice.2023.54. – No outcome of interest [PMC free article: PMC10665879] [PubMed: 37088695] [CrossRef]
- 70.
- Otter JA, Mookerjee S, Davies F et al. Detecting carbapenemase-producing Enterobacterales (CPE): an evaluation of an enhanced CPE infection control and screening programme in acute care. J Antimicrob Chemother. 2020 75(9):2670–2676. – No outcome of interest [PubMed: 32479615]
- 71.
- Page B, Klompas M, Chan C et al. Surveillance for healthcare-associated infections: hospital-onset adult sepsis events versus current reportable conditions. Clin Infect Dis. 2021 73(6):1013–1019. – No intervention of interest [PubMed: 33780544]
- 72.
- Pei S, Liljeros F and Shaman J. Identifying asymptomatic spreaders of antimicrobial-resistant pathogens in hospital settings. Proc Natl Acad Sci. 2021 118(37). – No intervention of interest [PMC free article: PMC8449327] [PubMed: 34493678]
- 73.
- Perreault SK, Binks B, McManus DS and Topal JE. Evaluation of the negative predictive value of methicillin-resistant Staphylococcus aureus nasal swab screening in patients with acute myeloid leukemia. Infect Control Hosp Epidemiol. 2021 42(7):853–856. – No outcome of interest [PubMed: 33228818]
- 74.
- Popovich KJ, Davila S, Chopra V et al. A tiered approach for preventing methicillin-resistant Staphylococcus aureus infection. Ann Intern Med. 2019 171:S59–S65. – Study design - review [PubMed: 31569224]
- 75.
- Renzoni AJ, Peksa GD and DeMott JM. Emergency department methicillin-resistant Staphylococcus aureus nare screen effect on pneumonia treatment duration. Am J Emerg Med 2021 44:68–71. – No outcome of interest [PubMed: 33581603]
- 76.
- Rohde JM, Jones K, Padron N et al. A tiered approach for preventing Clostridioides difficile infection. Ann Intern Med. 2019 171:S45–S51. – Study design -review [PubMed: 31569223]
- 77.
- Rose R, Nolan DJ, Moot S et al. Molecular surveillance of methicillin-resistant Staphylococcus aureus genomes in hospital unexpectedly reveals discordance between temporal and genetic clustering. Am J Infect Control. 2021 49(1):59–64. – No intervention of interest [PubMed: 32565273]
- 78.
- Scholten R, Hannink G, Willemsen K et al. Preoperative Staphylococcus aureus screening and eradication. Bone Joint J. 2020 102B(10):1341–1348. – No pathogen of interest [PubMed: 32993339]
- 79.
- Sonnevend A, Abdulrazzaq N, Ghazawi A et al. The first nationwide surveillance of carbapenem-resistant Enterobacterales in the United Arab Emirates - increased association of Klebsiella pneumoniae CC14 clone with Emirati patients. Int J Infect Dis. 2022 120:103–112. - No intervention of interest [PubMed: 35470020]
- 80.
- Sova C, Lewis SS, Smith BA and Reynolds S. Multi-faceted strategies improve collection compliance and sample acceptance rate for carbapenem-resistant Enterobacteriaceae (CRE) active surveillance testing. Am J Infect Control. 2021 49(8):1043–1047. – No outcome of interest [PubMed: 33556392]
- 81.
- Stuart RL, Marshall C, Harrington G et al. ASID/ACIPC position statement - Infection control for patients with Clostridium difficile infection in healthcare facilities. Infect Dis Health. 2019 24(1):32–43. Study design - review [PubMed: 30691583]
- 82.
- Sumon ZE, Lesse AJ, Sellick JA, Tetewsky S and Mergenhagen KA. Temporal trends of inpatient C. difficile infections within the Veterans Health Administration hospitals: An analysis of the effect of molecular testing, time to testing, and mandatory reporting. Infect Control Hosp Epidemiol. 2020 41(1):44–51. No intervention of interest [PubMed: 31708000]
- 83.
- Sundermann AJ, Chen J, Kumar P et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin Infect Dis. 2022 75(3):476–482. – No intervention of interest [PMC free article: PMC9427134] [PubMed: 34791136]
- 84.
- Tai CH, Liu WL, Pan SC et al. Evaluation of the negative predictive value of methicillin-resistant Staphylococcus aureus nasal swab screening in the medical intensive care units and its effect on antibiotic duration. Infect Drug Resist. 2022 15:1259–1266. – No outcome of interest [PMC free article: PMC8959872] [PubMed: 35355623]
- 85.
- Takaya S, Hayakawa K, Matsunaga N et al. Surveillance systems for healthcare-associated infection in high and upper-middle income countries: A scoping review. J Infect Chemother. 2020 26(5):429–437. – Study design - review [PubMed: 32081645]
- 86.
- Taori SK, Rhodes J, Khonyongwa K et al. First experience of implementing Candida auris real-time PCR for surveillance in the UK: detection of multiple introductions with two international clades and improved patient outcomes. J Hosp Infect. 2022 127:111–120. – No comparator [PubMed: 35753522]
- 87.
- Tchouaket Nguemeleu E, Beogo I, Sia D et al. Economic analysis of healthcare-associated infection prevention and control interventions in medical and surgical units: systematic review using a discounting approach. J Hosp Infect. 2020 106(1):134–154. – Study design - review [PMC free article: PMC7341040] [PubMed: 32652215]
- 88.
- Tucker A, George R, Welfare W et al. Screening for carbapenemase-producing Enterobacteriaceae in previous carriers readmitted to hospital: evaluation of a change in screening policy. J Hosp Infect. 2019 103(2):156–159. – No comparator [PubMed: 31039383]
- 89.
- van Hout D, Bruijning-Verhagen PCJ, Blok HEM, Troelstra A and Bonten MJM. Universal risk assessment upon hospital admission for screening of carriage with multidrug-resistant micro-organisms in a Dutch tertiary care centre. J Hosp Infect. 2021 109:32–39. – No comparator [PubMed: 33347938]
- 90.
- Vellinga A, Brennan W, Humphreys H and Burns K. Initial impact of a national programme to contain the spread of carbapenemase-producing Enterobacterales in Ireland. J Hosp Infect. 2021 109:107–114. – No comparator [PubMed: 33429000]
- 91.
- Vigário A, Gonçalves JA, Costa AR et al. Implementation of an infection control program with emphasis on cohorting to patients with carbapenemase-producing Enterobacteriaceae. The experience of 2 years in a tertiary teaching hospital in northern Portugal. Porto Biomed J. 2020 5(3):e68. – No comparator [PMC free article: PMC7722402] [PubMed: 33299948]
- 92.
- Vihta KD, Gordon NC, Stoesser N et al. Antimicrobial resistance in commensal opportunistic pathogens isolated from non-sterile sites can be an effective proxy for surveillance in bloodstream infections. Sci Rep. 2021 11(1):23359. – No intervention of interest [PMC free article: PMC8642463] [PubMed: 34862445]
- 93.
- Wangchinda W, Thamlikitkul V, Watcharasuwanseree S and Tangkoskul T. Active surveillance for carbapenem-resistant Enterobacterales (CRE) colonization and clinical course of CRE colonization among hospitalized patients at a university hospital in Thailand. Antibiotics. 2022 11(10). – No intervention of interest [PMC free article: PMC9598097] [PubMed: 36290059]
- 94.
- Ward DV, Hoss AG, Kolde R et al. Integration of genomic and clinical data augments surveillance of healthcare-acquired infections. Infect Control Hosp Epidemiol. 2019 40(6):649–655. – No comparator [PubMed: 31012399]
- 95.
- Worley JN, Crothers JW, Wolfgang WJ et al. Prospective genomic surveillance reveals cryptic MRSA outbreaks with local to international origins among NICU patients. J Clin Microbiol. 2023 e0001423. – No comparator [PMC free article: PMC10204624] [PubMed: 37022157]
- 96.
- Yang S, He L, Li K et al. Efficacy of active rapid molecular screening and IPC interventions on carbapenem-resistant enterobacterales infections in emergency intensive care units without enough single-room isolation. Infect Drug Resist. 2023 16:1039–1048. – Effect of surveillance not evaluated separately [PMC free article: PMC9951601] [PubMed: 36845019]
- 97.
- Yang M, Huang Y, Li Q et al. A matrix management of prevention and control for carbapenem-resistant Enterobacteriaceae in an urban compact medical union. Indian J Med Microbiol. 2023 40:30–35. – No intervention of interest [PubMed: 36357265]
- 98.
- Yavor A, Ben-Zvi H, Freeman S, Geffen Y and Adler A. Institutional burden of carbapenemase-producing Enterobacterales: The effect of changes in surveillance culture methodology. Microb Drug Resist. 2020 26(11):1350–1356. – No intervention of interest [PubMed: 32380896]
- 99.
- Zhu X, Sun X, Zeng Y et al. Can nasal Staphylococcus aureus screening and decolonization prior to elective total joint arthroplasty reduce surgical site and prosthesis-related infections? A systematic review and meta-analysis. J Orthop Surg Res. 2020 15(1):60. – Study design - review [PMC free article: PMC7031963] [PubMed: 32075670]
Appendix C. Data Tables
Table C-1. Overview of the studies of patient safety practices (PSPs) focused on infection surveillance for MRSA (MS Word, 357K)
Table C-2. Overview of the studies of patient safety practices (PSPs) focused on infection surveillance for C. difficile (MS Word, 359K)
Table C-3. Overview of the studies of patient safety practices (PSPs) focused on infection surveillance for CRE (MS Word, 359K)
Table C-4. Risk of bias assessment for non-randomized studies (MS Word, 361K)
- Disclaimers: This report is based on research conducted by the Johns Hopkins University under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 75Q80120D00003). The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.None of the investigators have any affiliations or financial involvement that conflicts with the material presented in this report.The information in this report is intended to help healthcare decision makers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of healthcare services. This report is not intended to be a substitute for the application of clinical judgment. Anyone who makes decisions concerning the provision of clinical care should consider this report in the same way as any medical reference and in conjunction with all other pertinent information, i.e., in the context of available resources and circumstances presented by individual patients.This report is made available to the public under the terms of a licensing agreement between the author and the Agency for Healthcare Research and Quality. Most AHRQ documents are publicly available to use for noncommercial purposes (research, clinical or patient education, quality improvement projects) in the United States and do not need specific permission to be reprinted and used unless they contain material that is copyrighted by others. Specific written permission is needed for commercial use (reprinting for sale, incorporation into software, incorporation into for-profit training courses) or for use outside of the United States. If organizational policies require permission to adapt or use these materials, AHRQ will provide such permission in writing.AHRQ or U.S. Department of Health and Human Services endorsement of any derivative products that may be developed from this report, such as clinical practice guidelines, other quality enhancement tools, or reimbursement or coverage policies, may not be stated or implied.A representative from AHRQ served as a Contracting Officer’s Representative and reviewed the contract deliverables for adherence to contract requirements and quality. AHRQ did not directly participate in the literature search, determination of study eligibility criteria, data analysis, interpretation of data, or preparation or drafting of this report.AHRQ appreciates appropriate acknowledgment and citation of its work. Suggested language for acknowledgment: This work was based on an evidence report, Active Surveillance Culturing of Clostridioides difficile and Multi-Drug Resistant Organisms: Methicillin-Resistant Staphylococcus aureus, Carbapenem-Resistant Enterobacterales, and Candida auris, by the Evidence-based Practice Center Program at the Agency for Healthcare Research and Quality (AHRQ).
- Leas BF, Pegues DA, Mull NK. Active Surveillance Culturing of Clostridioides difficile and Multi-Drug Resistant Organisms: Methicillin-Resistant Staphylococcus aureus (MRSA), Carbapenem-Resistant Enterobacterales (CRE), and Candida auris. (Prepared by the ECRI-Penn Evidence-based Practice Center under Contract No. 75Q80120D00003). AHRQ Publication No. 23(24)-EHC019-14. Rockville, MD: Agency for Healthcare Research and Quality. May 2024. DOI: https://doi.org/10.23970/AHRQEPC_MHS4CULTURING. Posted final reports are located on the Effective Health Care Program search page.
- Review Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities Around the World: A Review.[Antibiotics (Basel). 2021]Review Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities Around the World: A Review.Rodríguez-Villodres Á, Martín-Gandul C, Peñalva G, Guisado-Gil AB, Crespo-Rivas JC, Pachón-Ibáñez ME, Lepe JA, Cisneros JM. Antibiotics (Basel). 2021 Jun 7; 10(6). Epub 2021 Jun 7.
- Presence of Clostridioides difficile and multidrug-resistant healthcare-associated pathogens in stool specimens from hospitalized patients in the USA.[J Hosp Infect. 2020]Presence of Clostridioides difficile and multidrug-resistant healthcare-associated pathogens in stool specimens from hospitalized patients in the USA.Tickler IA, Dela Cruz CM, Obradovich AE, Goering RV, Dewell S, Le VM, Tenover FC, Healthcare Associated Infections Consortium. J Hosp Infect. 2020 Sep; 106(1):179-185. Epub 2020 Jul 7.
- Predominance of methicillin-resistant Staphylococcus aureus in the residents and environments of long-term care facilities in Taiwan.[J Microbiol Immunol Infect. 2019]Predominance of methicillin-resistant Staphylococcus aureus in the residents and environments of long-term care facilities in Taiwan.Liu CY, Lai CC, Chiang HT, Lu MC, Wang LF, Tsai TL, Kang MY, Jan YN, Lo YT, Ko WC, et al. J Microbiol Immunol Infect. 2019 Feb; 52(1):62-74. Epub 2018 Feb 21.
- Presence of multidrug-resistant organisms in the residents and environments of long-term care facilities in Taiwan.[J Microbiol Immunol Infect. 2017]Presence of multidrug-resistant organisms in the residents and environments of long-term care facilities in Taiwan.Lee CM, Lai CC, Chiang HT, Lu MC, Wang LF, Tsai TL, Kang MY, Jan YN, Lo YT, Ko WC, et al. J Microbiol Immunol Infect. 2017 Apr; 50(2):133-144. Epub 2017 Feb 20.
- Review Barrier Precautions in the Era of Multidrug Pathogens.[Curr Treat Options Infect Dis....]Review Barrier Precautions in the Era of Multidrug Pathogens.Pryor R, Viola-Luqa C, Hess O, Bearman G. Curr Treat Options Infect Dis. 2020; 12(3):321-331. Epub 2020 Jun 29.
- Active Surveillance Culturing of Clostridioides difficile and Multidrug-Resistan...Active Surveillance Culturing of Clostridioides difficile and Multidrug-Resistant Organisms: Methicillin-Resistant Staphylococcus aureus, Carbapenem-Resistant Enterobacterales, and Candida auris - Making Healthcare Safer IV
- hypothetical protein SDJN02_08340 [Cucurbita argyrosperma subsp. argyrosperma]hypothetical protein SDJN02_08340 [Cucurbita argyrosperma subsp. argyrosperma]gi|2053612007|gb|KAG7029996.1||gnl| DJN|SDJN02_08340Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...