NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Nebivolol is a beta-blocker and antihypertensive medication that has additional vasodilatory activity mediated by nitric oxide release. Nebivolol has yet to be linked to instances of clinically apparent liver injury.
Background
Nebivolol (ne biv' oh lol) is an antihypertensive agent that has both beta-adrenergic receptor blocking activity and separate direct vasodilatory actions. The beta blockade is cardioselective, acting largely on beta-1 adrenergic receptors. Beta-1 adrenergic blockade reduces the heart rate and myocardial contractility by slowing the atrioventricular (AV) conduction and suppressing automaticity. Nebivolol also has vasodilatory activity that is not explained by typical beta blockade and appears to be mediated by release of nitric oxide from endothelial cells. Nebivolol was approved for use in the United States in 2007 and it is currently indicated for the management of hypertension either as monotherapy or in combination with other antihypertensive agents. Nebivolol is available in tablets of 2.5, 5, 10 and 20 mg under the trade name Bystolic. The typical initial oral dose in adults is 5 mg once daily, with subsequent dose modification based upon clinical response and tolerance; the total daily maintenance dose ranges from 5 to 40 mg. Common side effects of nebivolol include bradycardia, hypotension, fatigue, dizziness, depression, memory loss, impotence, cold limbs and, less commonly, severe hypotension, heart failure and bronchospasm. Sudden withdrawal can trigger rebound hypertension.
Hepatotoxicity
Mild-to-moderate elevations in serum aminotransferase levels occur in less than 2% of patients on beta-blockers and are usually transient and asymptomatic, resolving even with continuation of therapy. There is no information on the rates of ALT elevations during nebivolol therapy. Despite its use in several large clinical trials, nebivolol has not been linked to cases of clinically apparent liver injury.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Nebivolol undergoes extensive metabolism by the liver, is a substrate of CYP 2D6 and its excretion is largely biliary. The liver injury linked to other beta-blockers is likely to be idiosyncratic.
References to the safety and potential hepatotoxicity of nebivolol are provided in the overview on Beta-Adrenergic Receptor Antagonists, last updated in June 2019.
Drug Class: Beta-Adrenergic Receptor Antagonists
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nebivolol – Bystolic®
DRUG CLASS
Beta-Adrenergic Receptor Antagonists
Product labeling at DailyMed, National Library of Medicine, NIH
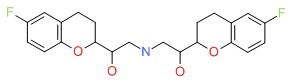
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Nebivolol | 42200-33-9 | C17-H27-N-O4 |
 |
- PubChem SubstanceRelated PubChem Substances
- Review Nebivolol: a highly selective beta1-adrenergic receptor blocker that causes vasodilation by increasing nitric oxide.[Cardiovasc Ther. 2008]Review Nebivolol: a highly selective beta1-adrenergic receptor blocker that causes vasodilation by increasing nitric oxide.Gupta S, Wright HM. Cardiovasc Ther. 2008 Fall; 26(3):189-202.
- Comparison of Nebivolol monotherapy versus Nebivolol in combination with other antihypertensive therapies for the treatment of hypertension.[Am J Cardiol. 2009]Comparison of Nebivolol monotherapy versus Nebivolol in combination with other antihypertensive therapies for the treatment of hypertension.Papademetriou V. Am J Cardiol. 2009 Jan 15; 103(2):273-8. Epub 2008 Nov 7.
- Nebivolol: a new antihypertensive agent.[Am J Health Syst Pharm. 2008]Nebivolol: a new antihypertensive agent.Gray CL, Ndefo UA. Am J Health Syst Pharm. 2008 Jun 15; 65(12):1125-33.
- Review Experimental evidences of nitric oxide-dependent vasodilatory activity of nebivolol, a third-generation beta-blocker.[Blood Press Suppl. 2004]Review Experimental evidences of nitric oxide-dependent vasodilatory activity of nebivolol, a third-generation beta-blocker.Ignarro LJ. Blood Press Suppl. 2004 Oct; 1:2-16.
- Effects of vasodilatory beta-adrenoceptor antagonists on endothelium-derived nitric oxide release in rat kidney.[Hypertension. 1999]Effects of vasodilatory beta-adrenoceptor antagonists on endothelium-derived nitric oxide release in rat kidney.Kakoki M, Hirata Y, Hayakawa H, Nishimatsu H, Suzuki Y, Nagata D, Suzuki E, Kikuchi K, Nagano T, Omata M. Hypertension. 1999 Jan; 33(1 Pt 2):467-71.
- Nebivolol - LiverToxNebivolol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...