NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Nateglinide is an oral hypoglycemic agent and amino acid derivative that stimulates insulin secretion from the pancreas and is used in the therapy of type 2 diabetes. Nateglinide has been linked to rare instances of clinically apparent acute liver injury.
Background
Nateglinide (na teg' li nide) is an insulin secretagogue that is similar in action but different in structure from the sulfonylureas. It is a derivative of phenylalanine and stimulates insulin secretion by blocking ATP sensitive potassium channels in pancreatic beta-cells, causing cell membrane depolarization which results in calcium influx and insulin secretion. Nateglinide has been shown to reduce the postprandial increase in glucose in patients with type 2 diabetes and improve glycemic control. Nateglinide was approved for use in the United States in 2000. The current indications are for management of type 2 diabetes used in combination with diet and exercise, with or without other oral hypoglycemic agents. Nateglinide is available generically and under the brand name Starlix in tablets of 60 and 120 mg. The typical initial dose in adults is 120 mg three times daily before meals. Side effects of nateglinide include diarrhea, nausea, gastrointestinal upset, hypoglycemia, dizziness and rash.
Hepatotoxicity
In several large clinical trials, serum aminotransferase elevations were no more common with nateglinide than with placebo. The enzyme elevations that occurred were asymptomatic and resolved rapidly with stopping therapy. Since its approval and with wide scale use, the FDA and the sponsor have received reports of clinically apparent liver injury attributed to nateglinide. However, none of these cases has been published and the clinical features, time to onset, course and outcome of the injury have not been described. The metiglinide analogue repaglinide has been impicated in rare cases of cholestatic or mixed hepatitis, but whether nateglinide liver injury is similar is not known. Thus, nateglinide is likely to be a very rare cause of clinically apparent liver disease.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of nateglinide induced liver injury is not known, but nateglinide is extensively metabolized by the liver via the P450 system (CYP 2C9 and 3A4), and liver injury may be the result of production of a toxic or immunoreactive intermediate.
Outcome and Management
There is no information on results of rechallenge on cross sensitivity to hepatic damage among the various metiglinides. Reexposure and use of other metiglinides after clinically apparent liver injury related to nateglinide should be done with caution.
References regarding the safety and hepatotoxicity of nateglinide and repaglinide are given with the Overview section on the Metiglinide Analogues (updated June 2018).
Drug Class: Antidiabetic Agents
Other Drugs in the Subclass Metiglinide Analogues: Repaglinide
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nateglinide – Generic, Starlix®
DRUG CLASS
Antidiabetic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
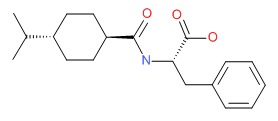
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Nateglinide | 105816-04-4 | C19-H27-N-O3 |
 |
- PubChem SubstanceRelated PubChem Substances
- Review Nateglinide.[Drugs. 2000]Review Nateglinide.Dunn CJ, Faulds D. Drugs. 2000 Sep; 60(3):607-615; discussion 616-7.
- Nateglinide: A structurally novel, short-acting, hypoglycemic agent.[Drugs Today (Barc). 2001]Nateglinide: A structurally novel, short-acting, hypoglycemic agent.Norman P, Rabasseda X. Drugs Today (Barc). 2001 Jun; 37(6):411-426.
- Review Nateglinide.[Am J Health Syst Pharm. 2001]Review Nateglinide.Halas CJ. Am J Health Syst Pharm. 2001 Jul 1; 58(13):1200-5.
- Effects of the new oral hypoglycaemic agent nateglinide on insulin secretion in Type 2 diabetes mellitus.[Diabet Med. 2000]Effects of the new oral hypoglycaemic agent nateglinide on insulin secretion in Type 2 diabetes mellitus.Whitelaw DC, Clark PM, Smith JM, Nattrass M. Diabet Med. 2000 Mar; 17(3):225-9.
- Review Clinical pharmacokinetics of nateglinide: a rapidly-absorbed, short-acting insulinotropic agent.[Clin Pharmacokinet. 2004]Review Clinical pharmacokinetics of nateglinide: a rapidly-absorbed, short-acting insulinotropic agent.McLeod JF. Clin Pharmacokinet. 2004; 43(2):97-120.
- Nateglinide - LiverToxNateglinide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...