NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
The fluoroquinolones are a family of broad spectrum, systemic antibacterial agents that have been used widely as therapy of respiratory and urinary tract infections. Fluoroquinolones are active against a wide range of aerobic gram-positive and gram-negative organisms. Gram-positive coverage includes penicillinase- and non-penicillinase producing Staphylococci, Streptococcus pneumoniae and viridans, Enterococcus faecalis, Listeria monocytogenes, and Nocardia species. Gram negative coverage includes Neisseria meningitides and gonorrhoeae, Haemophilus influenzae, and most clinically important Enterobacteriaceae species, Pseudomonas aeruginosa and Vibrio species. The fluoroquinolones are believed to act by inhibition of type II DNA topoisomerases (gyrases) that are required for synthesis of bacterial mRNAs (transcription) and DNA replication. They demonstrate little inhibition of human, host enzymes and have had an excellent safety record. The fluoroquinolones are indicated for treatment of several bacterial infections, including bacterial bronchitis, pneumonia, sinusitis, urinary tract infections, septicemia and intraabdominal infections, joint and bone infections, soft tissue and skin infections, typhoid fever, anthrax, bacterial gastroenteritis, urethral and gynecological infections, and pelvic inflammatory disease and several other infectious conditions.
The fluoroquinolones currently available in the United States include ciprofloxacin, gemifloxacin, levofloxacin, moxifloxacin, norfloxacin, and ofloxacin. These agents are well absorbed orally and well tolerated with a low rate of adverse effects. Several quinolones and fluoroquinolones were introduced but were subsequently withdrawn after spontaneous reports of severe adverse events including hepatotoxicity: temafloxacin (1992), gatifloxacin (2006), and trovafloxacin (1999). The currently available fluoroquinolones appear to cause idiosyncratic liver injury rarely, at an estimated rate of 1:100,000 persons exposed. Idiosyncratic liver injury due to fluoroquinolones may be a “class” effect; the pattern of injury is similar, marked by acute and often severe hepatocellular pattern of injury arising within 1 to 4 weeks of starting therapy. The fluoroquinolones most frequently linked to liver injury are ciprofloxacin and levofloxacin, but these two agents also have been most widely used. Minor elevations in liver enzymes occur in 1% to 3% of patients receiving ciprofloxacin, norfloxacin or ofloxacin. Rates with levofloxacin and moxifloxacin are less well defined, but probably similar. The common side effects of the fluoroquinolones are gastrointestinal disturbances, headaches, skin rash and allergic reactions. Less common but more severe side effects include QT prolongation, seizures, hallucinations, tendon rupture, angioedema and photosensitivity.
Ciprofloxacin, delafloxacin, gemifloxacin, levofloxacin, moxifloxacin, norfloxacin, and ofloxacin are discussed separately with individual clinical cases and references. General references and selected publications on fluoroquinolones no longer in use are given below.
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ciprofloxacin HCl | 86393-32-0 | C17-H18-F-N3-O3.Cl-H.H2-O |
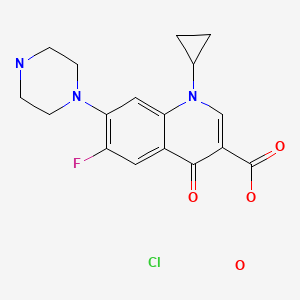
|
| Delafloxacin Meglumine | 352458-37-8 | C18-H12-Cl-F3-N4- O4.C7-H17-N-O5 |
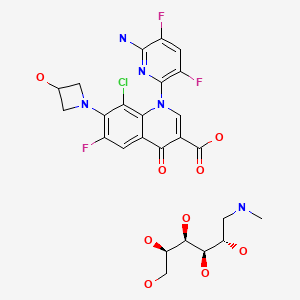
|
| Gemifloxacin | 175463-14-6 | C18-H20-F-N5-O4 |
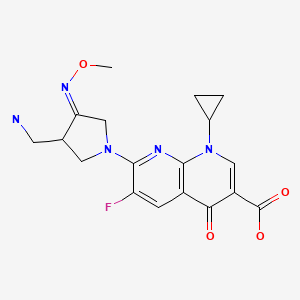
|
| Levofloxacin | 100986-85-4 | C18-H20-F-N3-O4 |
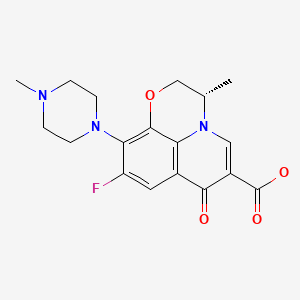
|
| Moxifloxacin HCl | 186826-86-8 | C21-H24-F-N3-O4.Cl-H |
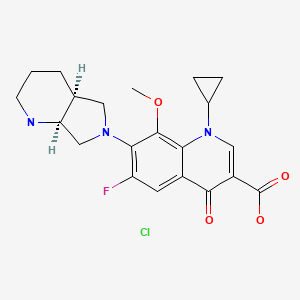
|
| Norfloxacin | 70458-96-7 | C16-H18-F-N3-O3 |
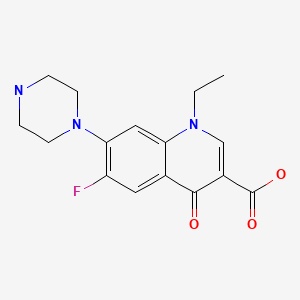
|
| Ofloxacin | 82419-36-1 | C18-H20-F-N3-O4 |
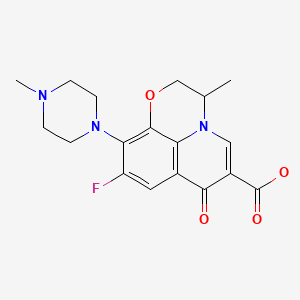
|
ANNOTATED BIBLIOGRAPHY
References updated: 10 March 2020
- Zimmerman HJ. Quinolones. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999. p. 603.(Expert review of hepatotoxicity published in 1999; mentions that cinoxacin, nalidixic acid, ciprofloxacin, norfloxacin, enoxacin, and ofloxacin are associated with minor serum enzyme elevations during therapy and with rare instances of clinically apparent liver injury).
- Moseley RH. Fluoroquinolones. Antibacterial and Antifungal Agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013. p. 468-9.(Review of hepatotoxicity of fluoroquinolones mentions that hepatocellular and cholestatic forms of injury have been reported due to the quinolones, including cases of ductopenia, acute liver failure and death).
- MacDougall C. The quinolones. Sulfonamides, trimethoprim-sulfamethoxazole, quinolones, and agents for urinary tract infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1015-8.(Textbook of pharmacology and therapeutics).
- Halkin H. Adverse effects of the fluoroquinolones. Rev Infect Dis. 1988;10 Suppl 1:S258–61. [PubMed: 3279499](Combined analysis of databases provided by manufacturers on adverse events of fluoroquinolones in approximately 30,000 persons receiving ciprofloxacin, ofloxacin, pefloxacin, norfloxacin and enoxacin found similar types and rates of adverse events among the agents, overall in 4-8%, elevated liver enzymes in 1.8-2.5%, but eosinophilia in 2.4% with ciprofloxacin and 5-19% with ofloxacin).
- Wolfson JS, Hooper DC. Overview of fluoroquinolone safety. Am J Med. 1991;91 Suppl 6A:153S–61S. [PubMed: 1767803](Review of side effects reported in 22 clinical trials of fluoroquinolones; elevations in ALT and/or Alk P levels occurred in 1.8-2.7% of patients on cipro-, nor-, or ofloxacin).
- Blum MD, Graham DJ, McCloskey CA. Temafloxacin syndrome: review of 95 cases. Clin Infect Dis. 1994;18:946–50. [PubMed: 8086558](FDA analysis of 95 cases showing hemolysis occurring within 1-2 weeks of starting temafloxacin, most with LDH elevations, half with AST elevations, many with multisystem disease).
- Ball P, Tillotson G. Tolerability of fluoroquinolone antibiotics. Past, present and future. Drug Saf. 1995;13:343–58. [PubMed: 8652079](Review of the nature and frequency of adverse reactions to the fluoroquinolones mentions that clinically apparent liver injury is rare, but that severe and occasional fatal cases have been reported with ciprofloxacin, levofloxacin, norfloxacin and ofloxacin and from postmarketing surveillance has estimated the frequency of clinically apparent liver injury to be 0.8 per 100,000 recipients).
- FDA. http://www
.fda.gov/cder /news/trovan/default.htm. (FDA advisory concerning trovafloxacin; despite no hepatic adverse event reports during prelicensure studies, spontaneous reports after licensure were common and included 140 cases of hepatotoxicity which led to its withdrawal in 1999). - Bertino J Jr, Fish D. The safety profile of the fluoroquinolones. Clin Ther. 2000;22:798–817. [PubMed: 10945507](Systematic review of side effects of fluoroquinolones; common side effects being gastrointestinal intolerance [1-5%], headaches and dizziness [1-2%], and skin rashes [~2%]; trovafloxacin was associated with hepatotoxicity, which was not seen in clinical trials in 7000 patients before approval in 1997, but since then 150 cases of clinically apparent liver toxicity and at least 14 fatalities have been reported).
- Chen HJ, Bloch KJ, Maclean JA. Acute eosinophilic hepatitis from trovafloxacin. N Engl J Med. 2000;342:359–60. [PubMed: 10660405](66 year old man developed fever and eosinophilia with ALT elevations [841 U/L] without jaundice [bilirubin 1.6 mg/dL] after 4 weeks of trovafloxacin therapy, that rapidly resolved with steroid therapy).
- Lucena MI, Andrade RJ, Rodrigo L, Salmeron J, Alvarez A, Lopez-Garrdio MJ, Camargo R, et al. Trovafloxacin-induced acute hepatitis. Clin Infect Dis. 2000;30:400–1. [PubMed: 10671353](3 men, ages 33 to 68 years, developed liver injury 7-14 days after starting trovafloxacin [bilirubin 1.2, 5.1 and 29.5 mg/dL, ALT 1990, 716 and 731 U/L, Alk P 1-2.5 times ULN, eosinophils 6-14%], self-limited in all, resolving within 6-8 weeks of stopping).
- Henann NE, Zambie MF. Gatifloxacin-associated acute hepatitis. Pharmacotherapy. 2001;21:1579–82. [PubMed: 11765309](44 year old woman developed jaundice after 5 days of gatifloxacin therapy [bilirubin 4.1 rising to 9.4 mg/dL, ALT 669 U/L, Alk P 243 U/L], resolving within 6 weeks of stopping).
- Lazarczyk DA, Goldstein NS, Gordon SC. Trovafloxacin hepatotoxicity. Dig Dis Sci. 2001;46:925–6. [PubMed: 11330435](19 year old woman developed rash and fever 5 days after starting trovafloxacin followed one week later by ascites and jaundice [bilirubin 1.3 mg/dL, ALT 901 U/L, Alk P 233 U/L], liver biopsy showing centrolobular necrosis, treated with corticosteroids and recovered).
- Rubinstein E. History of quinolones and their side effects. Chemotherapy. 2001;47 Suppl 3:3–8. [PubMed: 11549783](Between 1985 and 1999, 11 fluoroquinolones in clinical development or already in clinical use were withdrawn because of serious side effects, including trovafloxacin which was implicated in at least 140 cases of clinically apparent liver injury).
- Coleman CI, Spencer JV, Chung JO, Reddy P. Possible gatifloxacin-induced fulminant hepatic failure. Ann Pharmacother. 2002;36:1162–7. [PubMed: 12086547](76 year old man developed jaundice at the end 10 day course of gatifloxacin [bilirubin 5.4 rising to 34.2 mg/dL, ALT 545 U/L, Alk P 110 U/L, INR 1.3 rising to 7], with progressive liver failure and death 25 days later).
- Stahlmann R. Clinical toxicological aspects of fluoroquinolones. Toxicol Lett. 2002;127:269–77. [PubMed: 12052667](Review of the serious toxicities of the fluoroquinolones, their frequency and possible mechanisms; the hepatotoxicity of trovafloxacin became evident only after approval and approximately 2.5 million prescriptions worldwide, with 140 reported cases of "severe hepatic reactions" despite no serious hepatotoxicity in clinical trials in several thousand patients).
- Leone R, Venegoni M, Motola D, Moretti U, Piazzetta V, Cocci A, Resi D, et al. Adverse drug reactions related to the use of fluoroquinolone antimicrobials: an analysis of spontaneous reports and fluoroquinolone consumption data from three Italian regions. Drug Saf. 2003;26:109–20. [PubMed: 12534327](Analysis of 432 spontaneous adverse event reports on fluoroquinolones from Italy, 1/3 were serious none of which were liver related).
- Cheung O, Chopra K, Yu T, Nalesnik MA, Amin S, Shakil AO. Gatifloxacin-induced hepatotoxicity and acute pancreatitis. Ann Intern Med. 2004;140:73–4. [PubMed: 14706991](Two cases of liver injury and mild pancreatitis due to gatifloxacin; 41 year old woman and 19 year old man developed jaundice and abdominal within 1 week of starting therapy [bilirubin 5.1 and 5.5 mg/dL, ALT 145 and 520 U/L, Alk P 482 and 268 U/L], with elevations in amylase and lipase, resolving slowly and incompletely in the first, and rapidly and completely in the second).
- Galan MV, Potts JA, Silverman AL, Gordon SC. The burden of acute nonfulminant drug-induced hepatitis in a United States tertiary referral center. J Clin Gastroenterol. 2005;39:64–7. [corrected] [PubMed: 15599214](Ten year experience of 96 patients with acute liver injury, 64 due to viral hepatitis and 32 to drugs; major agents identified being amiodarone, augmentin, minocycline and nitrofurantoin; trovafloxacin accounted for 1 case which was symptomatic but anicteric, resolving within 6 weeks of stopping).
- Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41 Suppl 2:S144–57. [PubMed: 15942881](Review of common and rare complications of fluoroquinolones focusing upon QT prolongation, tendon rupture, diabetes, renal injury, and seizures; liver injury discussed in concept of immune related idiosyncratic reactions such as hemolytic anemia, thrombocytopenia, acute interstitial nephritis, Stevens Johnson syndrome, pancreatitis, serum sickness-like syndrome, and eosinophilic meningitis).
- Iannini PB. The safety profile of moxifloxacin and other fluoroquinolones in special patient populations. Curr Med Res Opin. 2007;23:1403–13. Erratum in: Curr Med Res Opin 2007; 23: 2303. Erratum in: Curr Med Res Opin 2007; 23(9): 2303; dosage error in article text. [PubMed: 17559736](Review of safety of moxifloxacin and fluoroquinolones in the elderly and patients with liver, kidney, endocrine and cardiovascular disease; no discussion of hepatotoxicity).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which only 1 was attributed to a fluoroquinolone, ciprofloxacin).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, but no fluoroquinolone was among the top 41 specific causes).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India, none of which were attributed to a fluoroquinolone).
- Orman ES, Conjeevaram HS, Vuppalanchi R, Freston JW, Rochon J, Kleiner DE, Hayashi PH., DILIN Research Group. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol. 2011;9:517–23.e3. [PMC free article: PMC3718017] [PubMed: 21356330](Among 679 cases of drug induced liver injury presenting between 2004 and 2010 at 8 US medical centers, 12 [1.8%] were attributed to fluoroquinolones including 6 cipro-, 4 moxi-, 1 levo-, and 1 gatifloxacin; average time to onset 4 days [range 1-39], with both hepatocellular and cholestatic enzyme patterns, seven with rash or fever, mortality limited to those with hepatocellular injury and jaundice; hepatic injury appeared to be class specific).
- Paterson JM, Mamdani MM, Manno M, Juurlink DN., Canadian Drug Safety and Effectiveness Research Network. Fluoroquinolone therapy and idiosyncratic acute liver injury: a population-based study. CMAJ. 2012;184:1565–70. [PMC free article: PMC3470619] [PubMed: 22891208](In a population based study using Canadian health care databases, the risk of admission to hospital for acute liver injury was increased for persons who received a prescription for moxifloxacin or levofloxacin relative to clarithromycin, but not for ciprofloxacin).
- Hayashi PH, Chalasani NP. Liver injury in the elderly due to fluoroquinolones: should these drugs be avoided? CMAJ. 2012;184:1555–6. [PMC free article: PMC3470615] [PubMed: 22891207](Editorial in response to Paterson [2013] stressing the low absolute risk of liver injury from the fluoroquinolones [4-9 per 100,000 exposures]).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to a fluoroquinolone).
- Kwon H, Lee SH, Kim SE, Lee JH, Jee YK, Kang HR, Park BJ, et al. Spontaneously reported hepatic adverse drug events in Korea: multicenter study. J Korean Med Sci. 2012;27:268–73. [PMC free article: PMC3286773] [PubMed: 22379337](Summary of 2 years of adverse event reporting in Korea; of 9360 reports, 567 were liver related, including 29 [5.1%] attributed to quinolones).
- Harr T, French LE. Stevens-Johnson syndrome and toxic epidermal necrolysis. Chem Immunol Allergy. 2012;97:149–66. [PubMed: 22613860](Review of the clinical features, epidemiology, genetics and pathogenesis of SJS and TEN).
- Patel TK, Barvaliya MJ, Sharma D, Tripathi C. A systematic review of the drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Indian population. Indian J Dermatol Venereol Leprol. 2013;79:389–98. [PubMed: 23619444](Systematic review of 10 case series of SJS/TEN from India identified 352 cases, among which 342 implicated a medication with most common being antimicrobials [37%], anticonvulsants [16%] and NSAIDs [16%]; fluoroquinolones accounted for 33 cases [10%], 4 of which were due to ciprofloxacin).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, one due to trovafloxacin [acute liver failure], but none attributed to ciprofloxacin or other fluoroquinolones).
- Alshammari TM, Larrat EP, Morrill HJ, Caffrey AR, Quilliam BJ, LaPlante KL. Risk of hepatotoxicity associated with fluoroquinolones: a national case-control safety study. Am J Health Syst Pharm. 2014;71:37–43. [PubMed: 24352180](Retrospective analysis of Veterans Affairs patients receiving a fluoroquinolone [n=7862] found a higher relative risk of developing acute liver injury after receipt of ciprofloxacin compared to matched controls [adjusted odds ratio: OR=1.29], but not after receipt of levofloxacin [OR=1.16) or moxifloxacin [OR=0.98]).
- Levine C, Trivedi A, Thung SN, Perumalswami PV. Severe ductopenia and cholestasis from levofloxacin drug-induced liver injury: a case report and review. Semin Liver Dis. 2014;34:246–51. [PubMed: 24879988](67 year old woman with streptococcal infection developed jaundice 8 weeks after starting levofloxacin [bilirubin 6.3 mg/dL, ALT 614 U/L, Alk P 483 U/L], with prolonged jaundice and biopsy showing bile duct loss).
- Kaye JA, Castellsague J, Bui CL, Calingaert B, McQuay LJ, Riera-Guardia N, Saltus CW, et al. Risk of acute liver injury associated with the use of moxifloxacin and other oral antimicrobials: a retrospective, population-based cohort study. Pharmacotherapy. 2014;34:336–49. [PMC free article: PMC4260122] [PubMed: 24865821](In a nested case control analysis of a health care network database of persons between 2001 and 2009, 8 selected antibiotics were assessed for association with risk of hospitalization for liver injury, adjusted relative risks being significantly elevated for levofloxacin [3.2], moxifloxacin [2.3], doxycycline [2.5], amoxicillin/clavulanate [2.5] and amoxicillin [2.3], but not for clarithromycin [1.8], telithromycin [1.7] or cefuroxime [0.9]).
- Goldberg DS, Forde KA, Carbonari DM, Lewis JD, Leidl KB, Reddy KR, Haynes K, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology 2015; 148: 1353-61. e3. [PMC free article: PMC4446162] [PubMed: 25733099](Analysis of Kaiser Permanente health care database from 2004 to 2011 identified 62 patients with suspected acute liver failure, 32 [52%] of whom had a presumed drug etiology, the most common being acetaminophen [18: 56%] and various herbal products [5: 16%], with single instances attributed to imatinib, simvastatin, leflunomide, isoniazid and valproate, but none to fluoroquinolones).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 38 cases [4%] were attributed to fluoroquinolones, including 16 due to ciprofloxacin [the 8th most common prescription drug cause], 13 due to levofloxacin and 8 to moxifloxacin).
- Xu P, Chen H, Xu J, Wu M, Zhu X, Wang F, Chen S, Xu J. Moxifloxacin is an effective and safe candidate agent for tuberculosis treatment: a meta-analysis. Int J Infect Dis. 2017;60:35–41. [PubMed: 28495364](Metaanalysis of nine controlled studies of the addition of moxifloxacin to antituberculosis drug regimens concludes that addition of moxifloxacin may increase the rate of culture conversion and decrease the rate of relapse but does not increase the rate of adverse events; no specific mention or discussion of rates of ALT elevations or hepatotoxicity).
- Marin AC, Nyssen OP, McNicholl AG, Gisbert JP. Efficacy and safety of quinolone-containing rescue therapies after the failure of non-bismuth quadruple treatments for Helicobacter pylori eradication: systematic review and meta-analysis. Drugs. 2017;77:765–76. [PubMed: 28361211](Systematic review of efficacy of fluoroquinolone-containing therapies of H. pylori infection after failure of standard treatments found eradication in 80% with triple and 90% with quadruple therapy; rates of specific adverse events were not discussed).
- Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, Fontana RJ, Ghabril MS, et al. U.S. Drug Induced Liver Injury Network Investigators. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65:1267–77. [PMC free article: PMC5360519] [PubMed: 27981596](Among 363 patients with drug induced liver injury who underwent liver biopsy, 26 [7%] had bile duct loss of whom 94% developed evidence of chronic liver injury suggestive of vanishing bile duct syndrome, 2 of which were due to fluoroquinolones, 1 to moxifloxacin and 1 levofloxacin).
- Markham A. Delafloxacin: first global approval. Drugs. 2017;77:1481–6. [PMC free article: PMC6208769] [PubMed: 28748399](Review of the mechanism of action, pharmacology, clinical efficacy and safety of delafloxacin; mentions that aminotransferase elevations occur in 3-4% of treated patients, but cases of clinically apparent liver injury with jaundice were not observed in the prelicensure studies).
- Yim HJ, Suh SJ, Jung YK, Yim SY, Seo YS, Lee YR, Park SY, Jang JY, Kim YS, Kim HS, Kim BI, Um SH. Daily norfloxacin vs. weekly ciprofloxacin to prevent spontaneous bacterial peritonitis: a randomized controlled trial. Am J Gastroenterol. 2018;113:1167–76. [PubMed: 29946179](Among 124 patients with cirrhosis and ascites given prophylaxis with daily norfloxacin [400 mg] or weekly oral ciprofloxacin [750 mg], subsequent rates of bacterial peritonitis were similar [7% vs 5%] as were rates of liver transplantation and death).
- Moreau R, Elkrief L, Bureau C, Perarnau JM, Thévenot T, Saliba F, Louvet A, et al. NORFLOCIR Trial Investigators. Effects of long-term norfloxacin therapy in patients with advanced cirrhosis. Gastroenterology. 2018;155:1816–27.e9. [PubMed: 30144431](Among 291 patients with advanced cirrhosis treated for 6-12 months with daily oral norfloxacin or placebo, 6-month mortality rate was less with norfloxacin [15% vs 19%] as were gram-negative bacterial infections and no norfloxacin related severe adverse events were identified).
- Dixit A, Karandikar MV, Jones S, Nakamura MM. Safety and tolerability of moxifloxacin in children. J Pediatric Infect Dis Soc. 2018;7:e92–e101. [PubMed: 29939314](Among 221 children who received 300 course of moxifloxacin, drug related aminotransferase elevations occurred in 8 [2.7%], with ALT ranging from 114-805 U/L and AST from 96-807 U/L]; no mention of jaundice or need for discontinuation).
- Tweed CD, Wills GH, Crook AM, Dawson R, Diacon AH, Louw CE, McHugh TD, et al. Liver toxicity associated with tuberculosis chemotherapy in the REMoxTB study. BMC Med. 2018;16:46. [PMC free article: PMC5875008] [PubMed: 29592805](Analysis of liver test abnormalities occurring in a trial of 1928 patients comparing standard 6 month isoniazid based regimens with shorter 4 month regimens using moxifloxacin and ethambutol found ALT elevations above 3 times ULN in 5.2% overall, 9.4% with standard therapy, 8% with 4 month course and 6% with 4 month course using ethambutol instead of isoniazid; two patients died of liver failure, one with rash, hemodynamic instability and rising bilirubin that was considered “hepatitis of unknown cause”).
- Comparison table: some systemic fluoroquinolones. Med Lett Drugs Ther. 2018;60:e57–e58. [PubMed: 29635268](Table comparing 4 fluoroquinolones [cipro-, levo-, dela- and moxifloxacin] mentions that ALT and AST elevations are a class adverse event).
- Delafloxacin (Baxdela)--a new fluoroquinolone antibiotic. Med Lett Drugs Ther. 2018;60(1543):49–51. [PubMed: 29635263](Concise review of the mechanism of action, clinical efficacy, safety and costs of delafloxacin shortly after its approval for use in the US; mentions adverse side effects of aminotransferase elevations [3%] and that it, like other fluoroquinolones, has a boxed warning about tendinitis, tendon rupture, peripheral neuropathy and CNS effects, and that it can also lead to C. difficile diarrhea).
- Mavros MN, Theochari NA, Kyriakidou M, Economopoulos KP, Sava JA, Falagas ME. Fluoroquinolone-based versus β-lactam-based regimens for complicated intra-abdominal infections: a meta-analysis of randomised controlled trials. Int J Antimicrob Agents. 2019;53:746–54. [PubMed: 30639629](Systematic review of controlled trials of fluoroquinolones versus β-lactam based antibiotic regimens found similar rates of efficacy and adverse events; no discussion of ALT elevations or liver related toxicities).
- Kuula LSM, Viljemaa KM, Backman JT, Blom M. Fluoroquinolone-related adverse events resulting in health service use and costs: A systematic review. PLoS One. 2019;14:e0216029. [PMC free article: PMC6485715] [PubMed: 31026286](Systematic review of observational studies on safety of fluoroquinolones concluded that due to lack of published literature, health service and costs could not be evaluated).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review A critical review of the fluoroquinolones: focus on respiratory infections.[Drugs. 2002]Review A critical review of the fluoroquinolones: focus on respiratory infections.Zhanel GG, Ennis K, Vercaigne L, Walkty A, Gin AS, Embil J, Smith H, Hoban DJ. Drugs. 2002; 62(1):13-59.
- In vitro activity of gatifloxacin compared with gemifloxacin, moxifloxacin, trovafloxacin, ciprofloxacin and ofloxacin against uropathogens cultured from patients with complicated urinary tract infections.[Int J Antimicrob Agents. 2000]In vitro activity of gatifloxacin compared with gemifloxacin, moxifloxacin, trovafloxacin, ciprofloxacin and ofloxacin against uropathogens cultured from patients with complicated urinary tract infections.Naber KG, Hollauer K, Kirchbauer D, Witte W. Int J Antimicrob Agents. 2000 Nov; 16(3):239-43.
- Review Levofloxacin and sparfloxacin: new quinolone antibiotics.[Ann Pharmacother. 1998]Review Levofloxacin and sparfloxacin: new quinolone antibiotics.Martin SJ, Meyer JM, Chuck SK, Jung R, Messick CR, Pendland SL. Ann Pharmacother. 1998 Mar; 32(3):320-36.
- Comparative in vitro activity of gemifloxacin, ciprofloxacin, levofloxacin and ofloxacin in a North American surveillance study.[Diagn Microbiol Infect Dis. 2001]Comparative in vitro activity of gemifloxacin, ciprofloxacin, levofloxacin and ofloxacin in a North American surveillance study.Hoban DJ, Bouchillon SK, Johnson JL, Zhanel GG, Butler DL, Miller LA, Poupard JA, Gemifloxacin Surveillance Study Research Group. Diagn Microbiol Infect Dis. 2001 May-Jun; 40(1-2):51-7.
- Gatifloxacin, gemifloxacin, and moxifloxacin: the role of 3 newer fluoroquinolones.[Clin Infect Dis. 2003]Gatifloxacin, gemifloxacin, and moxifloxacin: the role of 3 newer fluoroquinolones.Saravolatz LD, Leggett J. Clin Infect Dis. 2003 Nov 1; 37(9):1210-5. Epub 2003 Oct 2.
- Fluoroquinolones - LiverToxFluoroquinolones - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...