NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Adefovir dipivoxil is an acyclic nucleotide analogue of adenosine used either alone or in combination with other agents as therapy of chronic hepatitis B. Adefovir does not appear to be a significant cause of drug induced liver injury, but initiation of therapy and sudden withdrawal of therapy can induce a transient exacerbation of the underlying hepatitis B.
Background
Adefovir dipivoxil (bis-pom PMEA) is an acyclic nucleotide analog and prodrug of adefovir (a def' oh vir). The dipivoxil moiety is hydrolyzed after absorption, and adefovir is phosphorylated intracellularly to its active form, adefovir triphosphate, which competes with deoxyadenosine triphosphate for incorporation into the growing HBV DNA strand, causing inhibition of the viral DNA polymerase and chain termination. Adefovir is indicated for the treatment of chronic hepatitis B as a single agent and with lamivudine for lamivudine-resistant HBV infection. Adefovir was approved by the FDA in 2002 as therapy for hepatitis B. Adefovir is available generically and under the brand name Hepsera in 10 mg tablets. The recommended dose of adefovir is 10 mg orally once daily in adults and children age 12 years and older. The dose should be adjusted for renal impairment. Adefovir has minimal activity against human immunodeficiency virus (HIV) infection and is considered contraindicated in patients with HBV-HIV coinfection. Recently, adefovir has been largely replaced by tenofovir which has a similar mechanism and spectrum of activity, but is approximately 30 times more potent against HBV. Side effects of adefovir include asthenia and renal injury which is marked by increases in serum creatinine levels, hypophosphatemia, hypouricemia and renal tubular acidosis.
Hepatotoxicity
Serum aminotransferase elevations are common during or after therapy of hepatitis B, but appear to be due to exacerbations of the underlying HBV infection rather than hepatotoxicity. Sudden withdrawal of adefovir therapy can lead to an acute flare of hepatitis as viral levels suddenly rise. These withdrawal flares are usually transient and self-limited, but in rare instances are symptomatic and severe and can lead to death or need for liver transplantation. Instances of moderate serum aminotransferase elevations early during treatment have been described in clinical trials, but these elevations are usually transient and asymptomatic and are found in up to 25% of persons who start nucleoside analogue therapy of hepatitis B. Finally, development of antiviral resistance can be followed by a flare of the underlying hepatitis B as HBV DNA levels rise. Antiviral resistance to adefovir is rare during the first 1 to 2 years of therapy, but increasing rates are found with long-term therapy.
Adefovir has not been associated with cases of lactic acidosis with hepatic steatosis and liver failure. Tenofovir, a nucleotide analogue similar to adefovir, has been associated with isolated cases of lactic acidosis, but only in combination with other antiretroviral agents that are more closely linked to this syndrome. Because adefovir is considered contraindicated in HIV infection (it has weak anti-HIV activity), it is not used in combination with typical antiretroviral drugs. There have been no convincing cases of lactic acidosis or of clinically apparent liver injury with symptoms or jaundice due to adefovir.
Likehood score: E (unlikely cause of clinically apparent, idiosyncratic liver injury).
Mechanism of Injury
The apparent absence of significant hepatotoxicity from adefovir may be due to its minimal hepatic metabolism and rapid urinary excretion. In vitro, adefovir demonstrates little inhibitory activity against mitochondrial polymerase gamma.
Outcome and Management
The mild-to-moderate ALT elevations associated with initiating adefovir use are usually asymptomatic and transient. Due to the high percentage of patients who have flares of hepatitis B after discontinuation of adefovir, serum aminotransferase testing should be monitored for several months and antiviral therapy resumed if symptoms or jaundice arise or serum aminotransferases remain significantly above baseline (pretreatment) levels. Patients who develop antiviral resistance to adefovir can have significant flares of hepatitis B and should be switched to or have added another agent with a different profile of resistance.
[Agents used in therapy of HBV infection include adefovir, emtricitabine, entecavir, lamivudine, telbivudine, tenofovir, interferon alfa and peginterferon.]
Drug Class: Antiviral Agents, Antiretroviral Agents, Hepatitis B Agents
Other Drugs in the Subclass, Nucleoside Analogues: Abacavir, Didanosine, Emtricitabine, Entecavir, Lamivudine, Stavudine, Telbivudine, Tenofovir, Zidovudine
CASE REPORT
Case 1. Flare of hepatitis B accompanying development of adefovir resistance.
[NIH Case: Adefovir-Lamivudine #6]
A 56 year old man with chronic hepatitis B and previous therapy with interferon and lamivudine was treated with adefovir (10 mg daily). He took no other medications and did not drink alcohol. He had no symptoms of hepatitis, but appeared to have Gilbert’s syndrome with intermittently elevated total bilirubin levels, but normal direct fractions. He was positive for HBsAg and HBeAg in serum and HBV DNA levels were high. Molecular testing revealed evidence of lamivudine-resistant virus (rtM204V/rtL180M). ALT levels were only modestly elevated (Table). A liver biopsy showed moderate histological activity and bridging hepatic fibrosis. With adefovir therapy, serum HBV DNA levels decreased by only 3 to 4 log10 copies per mL and ALT levels improved minimally. Liver histology did not change. He remained asymptomatic and had no other medical problems. During the third year of therapy, HBV DNA levels began to rise towards pretreatment values and serum ALT levels increased five-fold. Molecular testing showed presence of the typical adefovir-resistant mutation in the HBV polymerase gene: rtN236T. Switching therapy to tenofovir led to prompt decrease in HBV DNA levels and improvements in ALT values.
Key Points
| Medication: | Adefovir (10 mg daily) |
|---|---|
| Pattern: | Hepatocellular |
| Severity: | 1+ (aminotransferase elevations without jaundice) |
| Latency: | 3 years |
| Recovery: | After switching to tenofovir therapy |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | AST (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | HBV DNA (copies/mL) | Other |
|---|---|---|---|---|---|---|
| 0 | 55 | 95 | 1.7 | 456,000,000 | Liver biopsy | |
| 6 months | 46 | 105 | 1.6 | 892,000 | ||
| 1 year | 48 | 90 | 2.7 | 489,000 | Liver biopsy | |
| 2 years | 45 | 108 | 1.7 | 120,000 | ||
| 3 years | 33 | 114 | 2.2 | 121,506 | ||
| 3.7 years | 43 | 94 | 1.2 | 132,026,000 | rtN236T mutation | |
| 4 years | 205 | 98 | 1.9 | 31,349,600 | ||
| Switched to tenofovir therapy (300 mg daily) | ||||||
| 2 months* | 2 months | 61 | 108 | 2.5 | 1,715 | |
| 6months* | 6 months | 27 | 129 | 2.5 | <58 | |
| 5 years* | 5 years | 25 | 130 | 1.5 | <10 | |
| Normal Values | <42 | <115 | <1.2 | |||
*Time after switching to tenofovir
Comment
Adefovir therapy of chronic hepatitis B is associated with a low rate of antiviral resistance during the first 1 to 2 years of treatment, but a somewhat high rate of poor initial response (as in this patient). Antiviral resistant rates increase to 17% to 28% after 3 to 4 years of treatment. Development of virological breakthrough is commonly followed by a flare in the underlying hepatitis. In this patient, the flare was mild and the adefovir-resistant virus was successfully suppressed by tenofovir therapy. These features make monotherapy with adefovir problematic in chronic hepatitis B and it is now rarely used.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Adefovir – Hepsera®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Adefovir | 106941-25-7 | C8-H12-N5-O4-P |
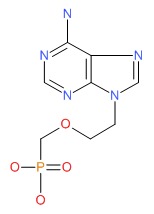 |
ANNOTATED BIBLIOGRAPHY
References updated: 15 February 2016
- Núñez M. Hepatitis treatments. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 511-2.(Review of hepatotoxicity of antiviral agents; mentions the potential of severe flares of disease upon withdrawal of therapy in chronic hepatitis B).
- Acosta EP, Flexner C. Antiviral agents (nonretroviral). In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1593-1622.(Textbook of pharmacology and therapeutics).
- Gilson RJ, Chopra KB, Newell AM, Murray-Lyon IM, Nelson MR, Rice SJ, Tedder RS, et al. A placebo-controlled phase I/II study of Adefovir dipivoxil in patients with chronic hepatitis B virus infection. J Viral Hepat 1999; 6: 387-95. [PubMed: 10607255](Controlled trial of high doses of adefovir [125 mg/day] vs placebo in 20 patients with chronic hepatitis B; ALT elevations of 724 U/L and 980 U/L occurred in 2 patients early during therapy and elevations above 300 U/L in 4 after withdrawal; none developed jaundice).
- Marcellin P, Change TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, et al.; Adefovir Dipivoxil 437 Study Group. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med 2003; 348: 808-16. [PubMed: 12606735](Controlled trial of adefovir vs placebo for 48 weeks in 515 patients with HBeAg-positive chronic hepatitis B: ALT elevations >10 times ULN occurred in 9% of adefovir vs 19% placebo recipients; in the adefovir group, no patient had jaundice or needed to stop therapy for ALT elevations).
- Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, et al.; Adefovir Dipivoxil 438 Study Group. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med 2003; 348: 800-7. Erratum in: N Engl J Med 2003; 348: 1192. [PubMed: 12606734](Controlled trial of adefovir vs placebo for 48 weeks in 185 patients with HBeAg-negative chronic hepatitis B; one placebo- but no adefovir-treated patient had severe flare of hepatitis).
- Rivas P, Polo J, de Górgolas M, Fernández-Guerrero ML. Drug Points: Fatal lactic acidosis associated with tenofovir. BMJ 2003, 327: 711. [PMC free article: PMC200801] [PubMed: 14512477](45 year old woman with HIV-HCV coinfection developed jaundice and lactic acidosis on tenofovir [8 weeks], didanosine and stavudine [long term] [bilirubin 12.6 mg/dL, ALT 157 U/L], with progressive liver failure and death; role of tenofovir vs didanosine and stavudine uncertain).
- Murphy MD, O.Hearn M, Chou S. Fatal lactic acidosis and acute renal failure after addition of tenofovir to an antiretroviral regimen containing didanosine. CID 2003; 36: 1982-6. [PubMed: 12684925](49 year old man with HIV and renal insufficiency on long term didanosine developed progressive, fatal lactic acidosis 6 weeks after starting tenofovir; lactate of 5.5 rising to 16.7 mM; no mention of liver injury).
- Guo Y, Fung HB. Fatal lactic acidosis associated with coadministration of didanosine and tenofovir disoproxil fumarate. Pharmacotherapy 2004; 24: 1089-94. [PubMed: 15338857](63 year old man with HIV and HCV developed fatal lactic acidosis 1.5 years after starting didanosine-tenofovir-lopinavir-ritonavir regimen, with pancreatitis, multiorgan failure and death; liver injury not mentioned).
- Abrescia N, D'Abbraccio M, Figoni M, Busto A, Maddaloni A, De Marco M. Hepatotoxicity of antiretroviral drugs. Curr Pharm Des 2005; 11: 3697-710. [PubMed: 16305505](Review of risk factors, epidemiology and pathogenic mechanisms of hepatotoxicity caused by antiretroviral drugs).
- Masiá M, Gutiérrez F, Padilla S, Ramos JM, Pascual J. Severe toxicity associated with the combination of tenofovir and didanosine: case report and review. Int J STD AIDS 2005; 16: 646-8. [PubMed: 16176639](45 year old with HIV-HCV coinfection developed lactic acidosis and "mild cholestasis" 3 months after adding tenofovir to didanosine, resolving slowly after stopping therapy).
- Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, et al.; Adefovir Dipivoxil 438 Study Group. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006; 131: 1743-51. [PubMed: 17087951](Results after 2 years in 185 HBeAg-negative chronic hepatitis B; 13 of 38 [32%] patients who stopped adefovir after 1 year had rise in ALT >10 times ULN, one with jaundice).
- Schiff E, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, Tillmann H, et al.; Adefovir Dipivoxil Study 45 International Investigators Group. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl 2007; 13: 349-60. [PubMed: 17326221](Among 226 pre- and 241 post-transplant patients with chronic hepatitis B treated with adefovir for up to 2 years, ALT elevations occurred in 2-4%; none considered serious or requiring drug discontinuation).
- Chan HL, Heathcote EJ, Marcellin P, Lai CL, Cho M, Moon YM, Chao YC, et al.; 018 Study Group. Treatment of hepatitis B e antigen positive chronic hepatitis with telbivudine or adefovir: a randomized trial. Ann Intern Med 2007; 147: 745-54. [PubMed: 17909201](Controlled trial of telbivudine vs adefovir in 135 patients with HBeAg-positive chronic hepatitis B, mild transient elevations in ALT occurred in both groups).
- Marcellin P, Chang TT, Lim SG, Sievert W, Tong M, Arterburn S, Borroto-Esoda K, et al. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2008; 48: 750-8. [PubMed: 18752330](Among 65 patients with HBeAg-positive chronic hepatitis B treated for up to 5 years, 9% had ALT elevation above 5 times ULN, all resolving without change in therapy).
- Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359: 2442-55. [PubMed: 19052126](Two controlled trials of tenofovir vs adefovir in 641 patients with chronic hepatitis B; ALT levels above 5 times ULN occurred in 6% of tenofovir and 3% of adefovir recipients, usually within 8 weeks of starting; all were self-limited even with continuing drug).
- Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology 2009; 49 (5 Suppl): S185-95. [PubMed: 19399802](Review of side effects of nucleoside analogues used to treat chronic hepatitis B).
- Gara N, Zhao X, Collins MT, Chong WH, Kleiner DE, Liang JT, Ghany MG, et al. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther 2012; 35: 1317-25. [PMC free article: PMC3443969] [PubMed: 22506503](Among 51 patients with chronic hepatitis B treated with adefovir or tenofovir for 1 to 10 years, 7 [14%] developed renal tubular dysfunction).
- Ghany MG, Feld JJ, Zhao X, Heller T, Doo E, Rotman Y, Nagabhyru P, et al. Randomised clinical trial: the benefit of combination therapy with adefovir and lamivudine for chronic hepatitis B. Aliment Pharmacol Ther 2012 Mar 26. [Epub ahead of print] [PMC free article: PMC7448290] [PubMed: 22449251](Controlled trial of adefovir alone vs its combination with lamivudine in 41 patients with chronic hepatitis B treated for up to 4 years demonstated a better long term response rate with the combination; no hepatotoxicity mentioned).
- Antiviral drugs. Treat Guidel Med Lett 2013; 11 (127): 19-30. [PubMed: 23459414](Review of safety and efficacy of antivirals used to treated hepatitis B mentions that acute flares of hepatitis B occur in up to 25% of patients who discontinue adefovir).
- Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016; 63: 261-83. [PMC free article: PMC5987259] [PubMed: 26566064](Guidelines for management and treatment of hepatitis B from the American Association for the Study of Liver Diseases lists adefovir as an approved oral therapy of chronic hepatitis B).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Severe exacerbation of chronic hepatitis B after emergence of lamivudine resistance in a cirrhotic patient: immediate switch to adefovir dipivoxil appears to be indicated.[Z Gastroenterol. 2004]Severe exacerbation of chronic hepatitis B after emergence of lamivudine resistance in a cirrhotic patient: immediate switch to adefovir dipivoxil appears to be indicated.Wiegand J, Tischendorf JJ, Nashan B, Klempnauer J, Flemming P, Niemann P, Rohde P, Manns MP, Trautwein C, Tillmann HL. Z Gastroenterol. 2004 Jan; 42(1):15-8.
- Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B.[Gastroenterology. 2004]Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B.Peters MG, Hann Hw Hw, Martin P, Heathcote EJ, Buggisch P, Rubin R, Bourliere M, Kowdley K, Trepo C, Gray Df Df, et al. Gastroenterology. 2004 Jan; 126(1):91-101.
- Review Adefovir dipivoxil in chronic hepatitis B infection.[Expert Opin Pharmacother. 2004]Review Adefovir dipivoxil in chronic hepatitis B infection.Yuen MF, Lai CL. Expert Opin Pharmacother. 2004 Nov; 5(11):2361-7.
- Review Adefovir dipivoxil: a review of its use in chronic hepatitis B.[Drugs. 2003]Review Adefovir dipivoxil: a review of its use in chronic hepatitis B.Dando T, Plosker G. Drugs. 2003; 63(20):2215-34.
- Review [Application and efficacy of adefovir dipivoxil in hepatitis B virus-associated chronic liver diseases].[Korean J Gastroenterol. 2003]Review [Application and efficacy of adefovir dipivoxil in hepatitis B virus-associated chronic liver diseases].Cho YK, Kim BI. Korean J Gastroenterol. 2003 Nov; 42(5):357-62.
- Adefovir - LiverToxAdefovir - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...