By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.

Immunobiology: The Immune System in Health and Disease. 5th edition.
Show detailsAll viruses, and some bacteria, multiply in the cytoplasm of infected cells; indeed, the virus is a highly sophisticated parasite that has no biosynthetic or metabolic apparatus of its own and, in consequence, can replicate only inside cells. Once inside cells, these pathogens are not accessible to antibodies and can be eliminated only by the destruction or modification of the infected cells on which they depend. This role in host defense is fulfilled by cytotoxic CD8 T cells. The critical role of cytotoxic T cells in limiting such infections is seen in the increased susceptibility of animals artificially depleted of these T cells, or of mice or humans that lack the MHC class I molecules that present antigen to CD8 T cells. As well as controlling infection by viruses and cytoplasmic bacteria, CD8 T cells are important in controlling some protozoan infections and are crucial, for example, in host defense against the protozoan Toxoplasma gondii, a vesicular parasite that exports peptides from the infected vesicles to the cytosol, from which they enter the MHC class I processing pathway. The elimination of infected cells without the destruction of healthy tissue requires the cytotoxic mechanisms of CD8 T cells to be both powerful and accurately targeted.
8-21. Cytotoxic T cells can induce target cells to undergo programmed cell death
Cells can die in either of two ways. Physical or chemical injury, such as the deprivation of oxygen that occurs in heart muscle during a heart attack, or membrane damage with antibody and complement, leads to cell disintegration or necrosis. The dead or necrotic tissue is taken up and degraded by phagocytic cells, which eventually clear the damaged tissue and heal the wound. The other form of cell death is known as programmed cell death or apoptosis. Apoptosis is a normal cellular response that is crucial in the tissue remodeling that occurs during development and metamorphosis in all multicellular animals. As we saw in Chapter 7, most thymocytes die an apoptotic death when they fail positive selection or, much less often, are negatively selected as a result of recognizing self antigens. Early changes seen in apoptosis are nuclear blebbing, alteration in cell morphology, and, eventually, fragmentation of the DNA. The cell then destroys itself from within, shrinking by shedding membrane-bound vesicles, and degrading itself until little is left. A hallmark of this type of cell death is the fragmentation of nuclear DNA into 200-base-pair (bp) pieces through the activation of endogenous nucleases that cleave the DNA between nucleosomes, each of which contains about 200 bp of DNA.
Cytotoxic T cells kill their targets by programming them to undergo apoptosis (Fig. 8.35). When cytotoxic T cells are mixed with target cells and rapidly brought into contact by centrifugation, they can program antigen-specific target cells to die within 5 minutes, although death may take hours to become fully evident. The short period required by cytotoxic T cells to program their targets to die reflects the release of preformed effector molecules, which activate an endogenous apoptotic pathway within the target cell.

Figure 8.35
Cytotoxic CD8 T cells can induce apoptosis in target cells. Specific recognition of peptide:MHC complexes on a target cell (top panels) by a cytotoxic CD8 T cell (CTL) leads to the death of the target cell by apoptosis. Cytotoxic T cells can recycle to (more...)
As well as killing the host cell, the apoptotic mechanism may also act directly on cytosolic pathogens. For example, the nucleases that are activated in apoptosis to destroy cellular DNA can also degrade viral DNA. This prevents the assembly of virions and thus the release of infectious virus, which could otherwise infect nearby cells. Other enzymes activated in the course of apoptosis may destroy nonviral cytosolic pathogens. Apoptosis is therefore preferable to necrosis as a means of killing infected cells; in necrosis, intact pathogens are released from the dead cell and these can continue to infect healthy cells, or can parasitize the macrophages that ingest them.
8-22. Cytotoxic effector proteins that trigger apoptosis are contained in the granules of CD8 cytotoxic T cells
The principal mechanism through which cytotoxic T cells act is by the calcium-dependent release of specialized lytic granules upon recognition of antigen on the surface of a target cell. These granules are modified lysosomes that contain at least two distinct classes of cytotoxic effector protein that are expressed selectively in cytotoxic T cells (Fig. 8.36). Such proteins are stored in the lytic granules in an active form, but conditions within the granules prevent them from functioning until after their release. One of these cytotoxic proteins, known as perforin, polymerizes to form transmembrane pores in target cell membranes. The other class of cytotoxic proteins comprises at least three proteases called granzymes, which belong to the same family of enzymes—the serine proteases—as the digestive enzymes trypsin and chymotrypsin. Granules that store perforin and granzymes can be seen in armed CD8 cytotoxic effector cells in tissue lesions.

Figure 8.36
Cytotoxic effector proteins released by cytotoxic T cells.
When purified granules from cytotoxic T cells are added to target cells in vitro, they lyse the cells by creating pores in the lipid bilayer. The pores consist of polymers of perforin, which is a major constituent of these granules. On release from the granule, perforin forms a cylindrical structure that is lipophilic on the outside and hydrophilic down a hollow center with an inner diameter of 16 nm (Fig. 8.37). It is not known whether this structure is first formed and then inserted into the lipid bilayer of the target cell membrane, or whether it is formed in the bilayer itself. The pore that is formed allows water and salts to pass rapidly into the cell. With the integrity of the cell membrane destroyed, the cells die rapidly. Large numbers of purified granules can kill target cells in vitro without inducing fragmentation of cellular DNA, but this lytic mechanism of cell killing probably occurs only at artificially high levels of perforin that do not reflect the physiological activity of cytotoxic T cells.
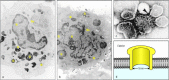
Figure 8.37
Perforin released from the lytic granules of cytotoxic T cells can insert into the target cell membrane to form pores. Perforin molecules, as well as several other effector molecules, are contained in the granules of cytotoxic T cells (panel a). When (more...)
Both perforin and granzymes are required for effective cell killing. The separate roles of perforin and granzymes have been investigated in a cell system that relies upon similarities between the lytic granules of T cells and the granules of mast cells. Release of mast cell granules occurs on cross-linking of the Fcε receptor (see Chapter 9), just as release of lytic granules from CD8 T cells occurs on cross-linking of the T-cell receptor. The mechanism of signaling for granule release is thought to be the same or similar in both cases, as both the Fcε receptor and the T-cell receptor have ITAM motifs in their cytoplasmic domains, and cross-linking leads to tyrosine phosphorylation of the ITAMs (see Chapter 6).
When a mast-cell line is transfected with the gene for perforin or for granzyme, the gene products are stored in mast cell granules, and when the cell is activated through its Fcε receptor, these granules are released. When transfected with the gene for perforin alone, mast cells can kill other cells, but large numbers of the transfected cells are needed as the killing is not very efficient. By contrast, mast cells transfected with the gene for granzyme B alone are unable to kill other cells. However, when perforin-transfected mast cells are also transfected with the gene encoding granzyme B, the cells or their purified granules become as effective at killing targets as granules from cytotoxic cells, and granules from both types of cell induce DNA fragmentation. This suggests that perforin makes pores through which the granzymes can move into the target cell.
The granzymes are proteases, so although they have a role in triggering apoptosis in the target cell, they cannot act directly to fragment the DNA. Rather, they must activate an enzyme, or more probably an enzyme cascade, in the target cell. Granzyme B can cleave the ubiquitous cellular enzyme CPP-32, which is believed to have a key role in programmed cell death in all cells. CPP-32 is a caspase and activates a nuclease, called caspase-activated deoxyribonuclease or CAD, by cleaving an inhibitory protein (ICAD) that binds to and inactivates CAD. This enzyme is believed to be the final effector of DNA degradation in apoptosis.
Cells undergoing programmed cell death are rapidly ingested by nearby phagocytic cells. The phagocytes recognize some change in the cell membrane, most probably the exposure of phosphatidylserine, which is normally found only in the inner leaflet of the membrane. The ingested cell is then completely broken down and digested by the phagocyte without the induction of co-stimulatory proteins. Thus, apoptosis is normally an immunologically ‘quiet’ process; that is, apoptotic cells do not normally contribute to or stimulate immune responses.
The importance of perforin in this process is well illustrated in mice that have had their perforin gene knocked out. Such mice are severely defective in their ability to mount a cytotoxic T-cell response to many but not all viruses, whereas mice that are defective in the granzyme B gene have a less profound defect, probably because there are several genes coding for granzymes.
8-23. Activated CD8 T cells and some CD4 effector T cells express Fas ligand, which can also activate apoptosis
The release of granule contents accounts for most of the cytotoxic activity of CD8 effector T cells, as shown by the loss of most killing activity in perforin knockout mice. This granule-mediated killing is strictly calcium-dependent, yet some cytotoxic actions of CD8 T cells survive calcium depletion. Moreover, some CD4 T cells can also kill other cells, yet do not contain granules and make neither perforin nor granzymes. These observations imply that there must be a second perforin-independent mechanism of cytotoxicity. This mechanism involves the binding of Fas in the target cell membrane by the Fas ligand, which is present in the membranes of activated cytotoxic T cells and TH1 cells. Ligation of Fas leads to activation of caspases, which induce apoptosis in the target cell (see Fig. 6.23). As discussed in Section 8-20, the lymphoproliferative and autoimmune disorders seen in mice and humans with mutations in genes for either Fas or Fas ligand imply that this pathway of killing is very important in regulating peripheral immune responses. Fas is expressed on activated lymphocytes and Fas-Fas ligand interactions are important in terminating lymphocyte growth after the removal of the initiating pathogen.
8-24. Cytotoxic T cells are selective and serial killers of targets expressing specific antigen
When cytotoxic T cells are offered a mixture of equal amounts of two target cells, one bearing specific antigen and the other not, they kill only the target cell bearing the specific antigen. The ‘innocent bystander’ cells and the cytotoxic T cells themselves are not killed, despite the fact that cloned cytotoxic T cells can be recognized and killed by other cytotoxic T cells just like any tissue cell. At first sight this may seem surprising, because the effector molecules released by cytotoxic T cells lack any specificity for antigen. The explanation probably lies in the highly polar release of the effector molecules. As we saw in Fig. 8.29, cytotoxic T cells orient their Golgi apparatus and microtubule-organizing center to focus secretion on the point of contact with a target cell. Granule movement toward the point of contact is shown in Fig. 8.38. Cytotoxic T cells attached to several different target cells reorient their secretory apparatus toward each cell in turn and kill them one by one, strongly suggesting that the mechanism whereby cytotoxic mediators are released allows attack at only one point of contact at any one time. The narrowly focused action of cytotoxic CD8 T cells allows them to kill single infected cells in a tissue without creating widespread tissue damage (Fig. 8.39) and is of critical importance in tissues where cell regeneration does not occur, as in neurons of the central nervous system, or is very limited, as in the pancreatic islets.
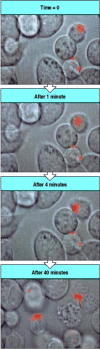
Figure 8.38
Effector molecules are released from T-cell granules in a highly polar fashion. The granules of cytotoxic T cells can be labeled with fluorescent dyes, allowing them to be seen under the microscope, and their movements followed by time-lapse photography. (more...)

Figure 8.39
Cytotoxic T cells kill target cells bearing specific antigen while sparing neighboring uninfected cells. All the cells in a tissue are susceptible to lysis by the cytotoxic proteins of armed effector CD8 T cells, but only infected cells are killed. Specific (more...)
Cytotoxic T cells can kill their targets rapidly because they store preformed cytotoxic proteins in forms that are inactive in the environment of the lytic granule. Cytotoxic proteins are synthesized and loaded into the lytic granules during the first encounter of a naive cytotoxic precursor T cell with its specific antigen. Ligation of the T-cell receptor similarly induces de novo synthesis of perforin and granzymes in armed effector CD8 T cells, so that the supply of lytic granules is replenished. This makes it possible for a single CD8 T cell to kill many targets in succession.
8-25. Cytotoxic T cells also act by releasing cytokines
Although the secretion of perforin and granzymes is the main way by which cytotoxic CD8 T cells eliminate infection, with the expression of Fas ligand playing a lesser role, most cytotoxic CD8 T cells also release the cytokines IFN-γ, TNF-α, and TNF-β, which contribute to host defense in several ways. IFN-γ directly inhibits viral replication, and also induces the increased expression of MHC class I and other molecules involved in peptide loading of the newly synthesized MHC class I proteins in infected cells. This increases the chance that infected cells will be recognized as target cells for cytotoxic attack. IFN-γ also activates macrophages, recruiting them to sites of infection both as effector cells and as antigen-presenting cells. The activation of macrophages by IFN-γ is a critical component of the host immune response to intracellular protozoan pathogens such as Toxoplasma gondii. IFN-γ also has a secondary role in decreasing the tryptophan concentration within responsive cells and thus can kill intracellular parasites, effectively by starvation. TNF-α or TNF-β can synergize with IFN-γ in macrophage activation, and in killing some target cells through their interaction with TNFR-I. Thus, armed effector cytotoxic CD8 T cells act in a variety of ways to limit the spread of cytosolic pathogens. The relative importance of each of these mechanisms remains to be determined.
Summary
Armed effector cytotoxic CD8 T cells are essential in host defense against pathogens that live in the cytosol, the commonest of which are viruses. These cytotoxic T cells can kill any cell harboring such pathogens by recognizing foreign peptides that are transported to the cell surface bound to MHC class I molecules. Cytotoxic CD8 T cells carry out their killing function by releasing two types of preformed cytotoxic protein: the granzymes, which seem able to induce apoptosis in any type of target cell, and the pore-forming protein perforin, which punches holes in the target-cell membrane through which the granzymes can enter. These properties allow the cytotoxic T cell to attack and destroy virtually any cell that is infected with a cytosolic pathogen. A membrane-bound molecule, the Fas ligand, expressed by CD8 and some CD4 T cells, is also capable of inducing apoptosis by binding to Fas expressed by some target cells. Cytotoxic CD8 T cells also produce IFN-γ, which is an inhibitor of viral replication and is an important inducer of MHC class I expression and macrophage activation. Cytotoxic T cells kill infected targets with great precision, sparing adjacent normal cells. This precision is critical in minimizing tissue damage while allowing the eradication of infected cells.
- Cytotoxic T cells can induce target cells to undergo programmed cell death
- Cytotoxic effector proteins that trigger apoptosis are contained in the granules of CD8 cytotoxic T cells
- Activated CD8 T cells and some CD4 effector T cells express Fas ligand, which can also activate apoptosis
- Cytotoxic T cells are selective and serial killers of targets expressing specific antigen
- Cytotoxic T cells also act by releasing cytokines
- Summary
- T cell-mediated cytotoxicity - ImmunobiologyT cell-mediated cytotoxicity - Immunobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...