By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.

Immunobiology: The Immune System in Health and Disease. 5th edition.
Show detailsAll T-cell effector functions involve the interaction of an armed effector T cell with a target cell displaying specific antigen. The effector proteins released by these T cells are focused on the appropriate target cell by mechanisms that are activated by recognition of antigen on the target cell. The focusing mechanism is common to all types of effector T cells, whereas their effector actions depend on the array of membrane and secreted proteins they express or release upon receptor ligation. The different types of effector T cell are specialized to deal with different types of pathogen, and the effector molecules they are programmed to produce cause distinct and appropriate effects on the target cell (Fig. 8.27).
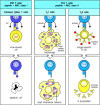
Figure 8.27
There are three classes of effector T cell, specialized to deal with three classes of pathogen. CD8 cytotoxic cells (left panels) kill target cells that display peptide fragments of cytosolic pathogens, most notably viruses, bound to MHC class I molecules (more...)
8-15. Effector T-cell interactions with target cells are initiated by antigen-nonspecific cell-adhesion molecules
Once an effector T cell has completed its differentiation in the lymphoid tissue it must find target cells that are displaying the MHC:peptide complex that it recognizes. Some TH2 cells encounter their B-cell targets without leaving the lymphoid tissue, as we discuss further in Chapter 9. However, most of the armed effector T cells emigrate from their site of activation in lymphoid tissues and enter the blood via the thoracic duct. Because of the cell-surface changes that have occurred during differentiation, they can now migrate into tissues, particularly at sites of infection. They are guided to these sites by changes in the adhesion molecules expressed on the endothelium of the local blood vessels as a result of infection, and by local chemotactic factors, as we will see in Chapter 10.
The initial binding of an effector T cell to its target, like that of a naive T cell to an antigen-presenting cell, is an antigen-nonspecific interaction mediated by LFA-1 and CD2. The level of LFA-1 and of CD2 is twofold to fourfold higher on armed effector T cells than on naive T cells, and so armed effector T cells can bind efficiently to target cells that have lower levels of ICAMs and LFA-3 on their surface than do the professional antigen-presenting cells. This interaction is normally transient unless recognition of antigen on the target cell through the T-cell receptor triggers an increase in the affinity of the T-cell's LFA-1 for its ligands on the target cell. The T cell binds more tightly to its target and remains bound for long enough to release its effector molecules. Armed CD4 effector T cells, which activate macrophages or induce B cells to secrete antibody, must maintain contact with their targets for relatively long periods. Cytotoxic T cells, by contrast, can be observed under the microscope attaching to and dissociating from successive targets relatively rapidly as they kill them (Fig. 8.28). Killing of the target, or some local change in the T cell, then allows the effector T cell to detach and address new targets. How armed CD4 effector T cells disengage from their antigen-negative targets is not known, although current evidence suggests that CD4 binding directly to MHC class II molecules on target cells that are not displaying specific antigen, signals the cell to detach.
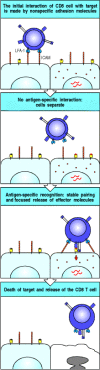
Figure 8.28
Interactions of T cells with their targets initially involve nonspecific adhesion molecules. The major initial interaction is between LFA-1 on the T cell, illustrated here as a cytotoxic CD8 T cell, and ICAM-1 or ICAM-2 on the target cell (top panel). (more...)
8-16. Binding of the T-cell receptor complex directs the release of effector molecules and focuses them on the target cell
When binding to peptide:MHC complexes, the T-cell receptor molecules and their cross-linked co-receptors cluster at the site of cell-cell contact. Clustering of the T-cell receptors then signals a reorientation of the cytoskeleton that polarizes the effector cell so as to focus the release of effector molecules at the site of contact with the target cell, as illustrated for a cytotoxic T cell in Fig. 8.29. Polarization of the cell starts with the local reorganization of the cortical actin cytoskeleton at the site of contact; this in turn leads to the reorientation of the microtubule-organizing center (MTOC), the center from which the microtubule cytoskeleton is produced, and of the Golgi apparatus (GA), through which most proteins destined for secretion travel. In the cytotoxic T cell, the cytoskeletal reorientation focuses exocytosis of the preformed lytic granules at the site of contact with its target cell.
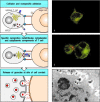
Figure 8.29
The polarization of T cells during specific antigen recognition allows effector molecules to be focused on the antigen-bearing target cell. The example illustrated here is a CD8 cytotoxic T cell. Cytotoxic CD8 cells contain specialized lysosomes called (more...)
The polarization of a T cell also focuses the secretion of soluble effector molecules whose synthesis is induced de novo by ligation of the T-cell receptor. For example, the secreted cytokine IL-4, which is the principal effector molecule of TH2 cells, is confined and concentrated at the site of contact with the target cell (see Fig. 9.6). It has been shown that the enhanced binding of LFA-1 to ICAM-1 creates a molecular seal surrounding the clustered T-cell receptors, CD4 co-receptors, and CD28 molecules (Fig. 8.30).
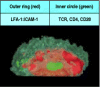
Figure 8.30
Tight junctions are formed between armed effector T cells and their targets. Confocal fluorescence micrograph of the area of contact between a T cell and a B cell (as viewed through one of the cells). The outer red ring is made up of LFA-1 on the T cell (more...)
Thus, the antigen-specific T-cell receptor controls the delivery of effector signals in three ways: it induces the stable binding of effector cells to their specific target cells to create a tightly held, narrow space in which effector molecules can be concentrated; it focuses their delivery at the site of contact by inducing a reorientation of the secretory apparatus of the effector cell; and it triggers their synthesis and/or release. All these receptor-coordinated mechanisms contribute to the selective action of effector molecules on the target cell bearing specific antigen. In this way, effector T-cell activity is highly selective for those target cells that display antigen, although the effector molecules themselves are not antigen-specific.
8-17. The effector functions of T cells are determined by the array of effector molecules they produce
The effector molecules produced by armed effector T cells fall into two broad classes: cytotoxins, which are stored in specialized lytic granules and released by cytotoxic CD8 T cells, and cytokines and related membrane-associated proteins, which are synthesized de novo by all effector T cells. The cytotoxins are the principal effector molecules of cytotoxic T cells and will be discussed further in Section 8-22. Their release, in particular, must be tightly regulated as they are not specific: they can penetrate the lipid bilayer and trigger an intrinsic death program in any cell. By contrast, cytokines and membrane-associated proteins act by binding to specific receptors on the target cell. Cytokines and membrane-associated proteins are the principal mediators of CD4 T-cell effector actions, and the main effector actions of CD4 cells are therefore directed at specialized cells that express receptors for these proteins.
The effector actions and main effector molecules of all three functional classes of effector T cell are summarized in Fig. 8.31. The cytokines are a diverse group of proteins and we will briefly review them before discussing the T-cell cytokines and their contributions to the effector actions of cytotoxic CD8 T cells, TH1 cells, and TH2 cells. As we will see, soluble cytokines and membrane-associated molecules often act in combination to mediate the effects of T cells on their target cells.
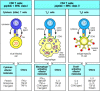
Figure 8.31
The three main types of armed effector T cell produce distinct sets of effector molecules. CD8 T cells are predominantly killer T cells that recognize pathogen-derived peptides bound to MHC class I molecules. They release perforin (which creates holes (more...)
The membrane-associated effector molecules, which we will discuss further in Section 8-20, are all structurally related to tumor necrosis factor (TNF), and their receptors on target cells are members of the TNF receptor (TNFR) family. All three classes of effector T cell express one or more members of the TNF family upon recognizing their specific antigen on the target cell. The membrane-bound TNF family member CD40 ligand is of particular importance for CD4 T-cell effector function; it is induced on TH1 and TH2 cells, and delivers activating signals to B cells and macrophages through the TNFR protein CD40. TNF-α is made by TH1 cells, some TH2 cells, and by cytotoxic T cells in soluble and membrane-associated forms, and can also deliver activating signals to macrophages. Some members of the TNF family can stimulate death by apoptosis. Thus Fas ligand (CD95L), the principal membraneassociated TNF-related molecule expressed by cytotoxic T cells, can trigger death by apoptosis in target cells bearing the receptor protein Fas (CD95); some TH1 cells also express Fas ligand and can kill Fas-bearing cells with which they interact. Death by this mechanism appears to be important for removing activated Fas-bearing lymphocytes; if it fails, a lymphoproliferative disease associated with severe autoimmunity results.
8-18. Cytokines can act locally or at a distance
Cytokines are small soluble proteins secreted by one cell that can alter the behavior or properties of the cell itself or of another cell. They are released by many cells in addition to those of the immune system. We have already discussed the cytokines released by phagocytic cells in Chapter 2, where we dealt with the inflammatory reactions that play an important part in innate immunity; here we are concerned mainly with the cytokines that mediate the effector functions of T cells. Cytokines produced by lymphocytes are often called lymphokines, but this nomenclature can be confusing because some lymphokines are also secreted by nonlymphoid cells; we will therefore use the generic term ‘cytokine’ for all of them. Most cytokines produced by T cells are given the name interleukin (IL) followed by a number: we have encountered several interleukins already in this chapter. Cytokines of immunological interest are listed in Appendix III.
Most cytokines have a multitude of different biological effects when tested at high concentration in biological assays in vitro but targeted disruption of genes for cytokines and cytokine receptors in knockout mice (see Appendix I, Section A-47) has helped to clarify their physiological roles. The major actions of the cytokines produced by effector T cells are given in Fig. 8.32. As the effect of a cytokine varies depending on the target cell, the actions are listed according to the major target cell types—B cells, T cells, macrophages, hematopoietic cells, and tissue cells.
The main cytokine released by CD8 effector T cells is IFN-γ, which can block viral replication or even lead to the elimination of virus from infected cells without killing them. TH1 cells and TH2 cells release different, but overlapping, sets of cytokines, which define their distinct actions in immunity. TH2 cells secrete IL-4 and IL-5, which activate B cells, and IL-10, which inhibits macrophage activation. TH1 cells secrete IFN-γ, which is the main macrophage-activating cytokine, and lymphotoxin (LT-α or TNF-β), which activates macrophages, inhibits B cells and is directly cytotoxic for some cells. The TH0 cells from which both of these functional classes derive (see Fig. 8.24) also secrete cytokines, including IL-2, IL-4, and IFN-γ, and may therefore have a distinctive effector function.
We have already discussed in Section 8-16 how the T-cell receptor can orchestrate the polarized release of these cytokines so that they are concentrated at the site of contact with the target cell. Furthermore, most of the soluble cytokines have local actions that synergize with those of the membrane-bound effector molecules. The effect of all these molecules is therefore combinatorial, and as the membrane-bound effectors can only bind to receptors on an interacting cell, this is another mechanism by which selective effects of cytokines are focused on the target cell. The effects of some cytokines are further confined to target cells by tight regulation of their synthesis: as we will see later, the synthesis of cytokines such as IL-2, IL-4, and IFN-γ is controlled, so that secretion from T cells does not continue after the interaction with a target cell ends.
Some cytokines, however, have more distant effects. IL-3 and GM-CSF (see Fig. 8.32), for example, which are released by both types of CD4 effector T cell, act on bone marrow cells to stimulate the production of macrophages and granulocytes, both of which are important nonspecific effector cells in both humoral and cell-mediated immunity. IL-3 and GM-CSF also stimulate the production of dendritic cells from bone marrow precursors. IL-5, produced by TH2 cells, can increase the production of eosinophils, which contribute to the late phase of allergic reactions, in which there is a predominant activation of TH2 cells (see Chapter 12). Whether a cytokine effect is local or more distant is likely to reflect the amounts released, the degree to which this release is focused on the target cell, and the stability of the cytokine in vivo but, for most of the cytokines, in particular those with more distant effects, these factors are not yet known.
8-19. Cytokines and their receptors fall into distinct families of structurally related proteins
Cytokines can be grouped by structure into families—the hematopoietins, the interferons, and the TNF family (Fig. 8.33)—and their receptors can likewise be grouped (Fig. 8.34). We have already encountered members of all of these families in Chapter 2, and have given an overview of the chemokine family there (see Section 2-20). We will focus here on the receptors for the hematopoietins, the TNF family, and IFN-γ on account of their role in T-cell effector function. Members of the TNF family act as trimers, most of which are membrane-bound and so are quite distinct in their properties from the other cytokines. Nevertheless, they share some important properties with the soluble T-cell cytokines, as they are also synthesized de novo upon antigen recognition by T cells, and affect the behavior of the target cell.

Figure 8.33
Cytokines and their receptors can be grouped into a small number of structural families. Representatives of the hematopoietin and TNF families are shown here, as most of the cytokines made by effector T cells belong to one or other of these families. (more...)
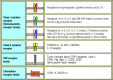
Figure 8.34
Cytokine receptors belong to families of receptor proteins, each with a distinctive structure. There is a large family of cytokine receptors, which are divided into two subsets on the basis of the presence or absence of particular sequence motifs. Many (more...)
Many of the soluble cytokines made by effector T cells are members of the hematopoietin family. These cytokines and their receptors can be further divided into subfamilies characterized by functional similarities and genetic linkage. For instance, IL-3, IL-4, IL-5, IL-13, and GM-CSF are related structurally, their genes are closely linked in the genome, and all are major cytokines produced by TH2 cells. In addition, they bind to closely related receptors, which form the family of class I cytokine receptors. The IL-3, IL-5, and GM-CSF receptors share a common β chain. Another subgroup of class I cytokine receptors is defined by their use of the γ chain of the IL-2 receptor; this is shared by receptors for the cytokines IL-2, IL-4, IL-7, IL-9, and IL-15 and is now called the common γ chain (γc). More distantly related, the receptor for IFN-γ is a member of a small family of cytokine receptors with some similarities to the hematopoietin receptor family. These so-called class II cytokine receptors include the receptor for IFN-α and IFN-β, and the IL-10 receptor. Overall, the structural, functional, and genetic relations between the cytokines and their receptors suggest that they may have diversified in parallel during the evolution of increasingly specialized effector functions.
These specific functional effects depend on intracellular signaling events that are triggered by the cytokines binding to their specific receptors. The hematopoietin and interferon receptors all signal through a similar pathway, which is described in Chapter 6. The key signaling molecules of this pathway are members of the Janus family of cytoplasmic tyrosine kinases (JAKs) and their targets the signal transducing activators of transcription (STATs), which enter the nucleus to activate specific genes. As the JAKs and STATs are present as families of related molecules, different members may be activated to achieve different effects.
8-20. The TNF family of cytokines are trimeric proteins that are often associated with the cell surface
TNF-α is made by T cells in soluble and membrane-associated forms, both of which are made up of three identical protein chains (a homotrimer, see Fig. 8.33). TNF-β (LT-α) can be produced as a secreted homotrimer, but is usually linked to the cell surface by forming heterotrimers with a third, membrane-associated, member of this family called LT-β. The receptors for these molecules, TNFR-I and TNFR-II, form homotrimers when bound to either TNF-α or LT. The trimeric structure is characteristic of all members of the TNF family, and the ligand-induced trimerization of their receptors seems to be the critical event in initiating signaling.
Most effector T cells express members of the TNF protein family as cell-surface molecules. The most important TNF-family proteins in T-cell effector function are TNF-α, TNF-β, Fas ligand, and CD40 ligand, the latter two always being cell-surface associated. These molecules all bind receptors that are members of the TNFR family; TNFR-I and II can each interact with either TNF-α or TNF-β, whereas Fas ligand and CD40 ligand bind respectively to the transmembrane protein Fas and to the transmembrane protein CD40 on target cells
Fas is expressed on many cells, especially on activated lymphocytes. Activation of Fas by the Fas ligand has profound consequences for the cell as Fas contains a ‘death domain’ in its cytoplasmic tail, which can initiate an activation cascade of cellular proteases called caspases that leads to apoptotic cell death (see Fig. 6.23). Fas is important in maintaining lymphocyte homeostasis, as can be seen from the effects of mutations in the Fas or Fas ligand genes. Mice and humans with a mutant form of Fas develop a lymphoproliferative disease associated with severe autoimmunity. A mutation in the gene encoding the Fas ligand in another mouse strain creates a nearly identical phenotype. These mutant phenotypes represent the best-characterized examples of generalized autoimmunity caused by single-gene defects. Other TNFR family members, including TNFR-I, are also associated with death domains and can also induce programmed cell death. Thus, TNF-α and TNF-β can induce programmed cell death by binding to TNFR-I.
The cytoplasmic tail of CD40 lacks a death domain; instead, it appears to be
linked to proteins called TRAFs
(TNF-receptor-associated factors), about which little is known. CD40 is involved
in macrophage and B-cell activation; the ligation of CD40 on B cells promotes
growth and isotype switching, whereas CD40 ligation on macrophages induces them
to secrete TNF-α and to become receptive to much lower concentrations of IFN-γ.
Deficiency in CD40 ligand expression is associated with immunodeficiency, as we
will learn in Chapters 9 and 11. ( Hyper IgM Immunodeficiency, in
Case Studies in Immunology, see Preface for details)
Hyper IgM Immunodeficiency, in
Case Studies in Immunology, see Preface for details)
Summary
Interactions between armed effector T cells and their targets are initiated by transient nonspecific adhesion between the cells. T-cell effector functions are elicited only when peptide:MHC complexes on the surface of the target cell are recognized by the receptor on an armed effector T cell. This recognition event triggers the armed effector T cell to adhere more strongly to the antigen-bearing target cell and to release its effector molecules directly at the target cell, leading to the activation or death of the target. The consequences of antigen recognition by an armed effector T cell are determined largely by the set of effector molecules it produces on binding a specific target cell. CD8 cytotoxic T cells store preformed cytotoxins in specialized lytic granules whose release can be tightly focused at the site of contact with the infected target cell. Cytokines, and one or more members of the TNF family of membrane-associated effector proteins, are synthesized de novo by all three types of effector T cell. TH2 cells express B-cell activating effector molecules, whereas TH1 cells express effector molecules that activate macrophages. CD8 T cells express membrane-associated Fas ligand that induces programmed cell death in cells bearing Fas; they also release IFN-γ. Membrane-associated effector molecules can deliver signals only to an interacting cell bearing the appropriate receptor, whereas soluble cytokines can act on cytokine receptors expressed locally on the target cell, or on hematopoietic cells at a distance. The actions of cytokines and membrane-associated effector molecules through their specific receptors, together with the effects of cytotoxins released by CD8 cells, account for most of the effector functions of T cells.
- Effector T-cell interactions with target cells are initiated by antigen-nonspecific cell-adhesion molecules
- Binding of the T-cell receptor complex directs the release of effector molecules and focuses them on the target cell
- The effector functions of T cells are determined by the array of effector molecules they produce
- Cytokines can act locally or at a distance
- Cytokines and their receptors fall into distinct families of structurally related proteins
- The TNF family of cytokines are trimeric proteins that are often associated with the cell surface
- Summary
- General properties of armed effector T cells - ImmunobiologyGeneral properties of armed effector T cells - Immunobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...