NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Occupational Exposures in Insecticide Application, and Some Pesticides. Lyon (FR): International Agency for Research on Cancer; 1991. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 53.)
1. Exposure Data
1.1. Chemical and physical data
1.1.1. Synonyms, structural and molecular data
- Chem. Abstr. Serv. Reg. No.: 116-06-3
- Chem. Abstr. Name: 2-Methyl-2-(methylthio)propanal, O-[(methylamino)carbonylloxime
- IUPAC Systematic Name: 2-Methyl-2-(methylthio)propionaldehyde O-methylcarbamoyloxime
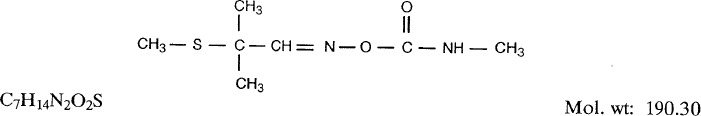
1.1.2. Chemical and physical properties
- Description: Colourless crystals (Worthing & Walker, 1987)
- Boiling-point: Decomposes (Rhone-Poulenc Ag Co., 1987)
- Melting-point: 98–100°C (Worthing & Walker, 1987)
- Spectroscopy data: Infrared spectroscopy data have been reported (US Environmental Protection Agency, 1976).
- Solubility: Slightly soluble in water (6 g/l at 20°C); soluble in most organic solvents: at 25 °C, acetone, 350 g/kg; dichloromethane, 300 g/kg; benzene, 150 g/kg; xylene, 50 g/kg; practically insoluble in heptane (Worthing & Walker, 1987)
- Volatility: Vapour pressure, 9.75 × 10−5 mm Hg [1.3 × 10−5 Pal at 25°C (Worthing & Walker, 1987)
- Stability: Stable in neutral, acidic and weakly alkaline media; hydrolysed by concentrated alkalis; decomposes above 100°C; rapidly converted by oxidizing agents to the sulfoxide, which is more slowly oxidized to the sulfone (Worthing & Walker, 1987; Royal Society of Chemistry, 1989)
- Conversion factor for airborne concentrations1: mg/m3 = 7.78 × ppm
1.1.3. Trade names, technical products and impurities
Some common trade names are AI3-27 093, Aldicarb, ENT 27 093, OMS 771, Temik and UC 21149.
Aldicarb is available in the USA as a technical grade product with a purity of 98% (minimum) (Rhone-Poulenc Ag Co., 1987).
It is formulated in the USA and Europe as a granular product with the concentration of active ingredient ranging from 5 to 15%; some formulated products also contain gypsum and dichloromethane (Royal Society of Chemistry, 1986; Rhone-Poulenc Ag Co., 1990a,b; see IARC, 1987a). Aldicarb is also formulated as mixtures with pentachloronitrobenzene (see IARC, 1974,1987b), 5-ethoxy-3-(trichloromethyl)-l,2,4-thiadiazole and lindane (see IARC, 1987c) (Royal Society of Chemistry, 1986; Rhone-Poulenc Ag Co., 1990c). The granular carrier material is impregnated with aldicarb and a bonding agent which helps prevent dustiness that may be caused by abrasion during shipping; dust is also removed during the manufacturing process to minimize inhalation exposure and hazards of direct handling (Baron & Merriam, 1988).
1.1.4. Analysis
Selected methods for the analysis of aldicarb in various matrices are given in Table 1. Several more methods and the environmental fate and transport of aldicarb and its metabolites have been reviewed (Moye & Miles, 1988).
Table 1.
Methods for the analysis of aldicarb.
1.2. Production and use
1.2.1. Production
Aldicarb was first prepared by Payne and Weiden (1965) and was first made available as a commercial product in 1970, following its registration in the USA for use on cotton (Romine, 1974).
Aldicarb is synthesized by reacting nitrosyl chloride with isobutylene to obtain 2-chloro-2-methyl-1-nitrosopropane dimer, which is further reacted with methyl mercaptan and sodium hydroxide to obtain 2-methyl-2-(methylthio)propionaldoxime. Aldicarb is obtained by reaction of the oxime with methyl isocyanate (Romine, 1974).
Aldicarb is currently produced in the USA and in France (Meister, 1990). Production in the USA in 1979–81 was estimated at 2000 tonnes per annum (US Environmental Protection Agency, 1987).
1.2.2. Use
Aldicarb acts as a systemic insecticide, acaricide and nematicide and is applied to soil under cotton, potatoes, sugar beets, peanuts, soya beans, ornamental plants, sweet potatoes, pecans, citrus (grapefruit, lemons, limes and oranges only), dry beans, sorghum and sugar-cane (Rhone-Poulenc Ag Co., 1989; Meister, 1990). In the USA during 1979–81, annual usage of aldicarb (active ingredient) was estimated as follows (tonnes;%): cotton, 520 (29%); potatoes, 430 (25%); peanuts, 250 (14%); soya beans, 205 (12%); pecans, 180 (10%); ornamental plants (lilies, roses, holly), 45 (3%); sugar beets, 45 (3%); citrus, 34 (2%); sweet potatoes, 22 (< 1%); and tobacco, 6 (< 1%) (Holtorf, 1982). In Finland, 132 kg aldicarb (active ingredient) were sold in 1988 (Hynninen & Blomqvist, 1989). In the USA in 1989, about 1000–1500 tonnes aldicarb are believed to have been used. Aldicarb use is restricted in certain areas in various parts of the world, usually because of its potential to leach to groundwater (IRPTC/UNEP, 1990).
1.3. Occurrence
1.3.1. Air
Few studies are available on the stability or migration of aldicarb in air over or near treated fields. Laboratory studies with 14C-labelled aldicarb in various soil types resulted in its loss, which could be explained only on the grounds that aldicarb or its decomposition products had been transferred to the vapour phase. Subsequent experiments showed that transfer of radioactivity to the atmosphere was inversely proportional to the depth of application in the soil (Coppedge et al., 1977). Little aldicarb was released from a clay soil treated with this pesticide and placed in a volatilizer (Supak et al., 1977).
1.3.2. Water
Aldicarb has been detected in ground- and drinking-water in 15 states in the USA at levels ranging from traces to 500 µg/kg (WHO, 1991).
It was detected in water in Suffolk County, NY, in August 1979: a monitoring programme for aldicarb in water indicated that 1121 (13.5%) of 8404 wells examined exceeded the State recommended guideline of 7 µg/1. Of the contaminated wells, 52% contained between 8 and 30 µg/l, 32% between 31 and 75 µg/l and 16% contained more than 75 µg/l (Zaki et al., 1982). Following the banning of aldicarb in Suffolk County in 1979, approximately 74% of all wells sampled in the County in 1981 contained no detectable level of aldicarb. Of the 27% of wells (2054 samples) that did, residue levels were 1–10 µg/l in 56%, 11–100 µg/l in 40% and > 100 µg/l in 4% (US Environmental Protection Agency, 1988). Data derived from monitoring of all drinking-water wells in Suffolk County in which contamination had occurred are shown in Table 2.
Table 2.
Numbers of wells containing aldicarb residues in Suffolk County, NY, 1980–85.
Extensive monitoring studies in the USA have mostly been related to potato and citrus production. Relatively high percentages (5-> 50%) of positive findings occurred in Wisconsin and north-eastern states (New York, Massachusetts, Rhode Island, Connecticut, Maine). There was substantial evidence for leaching to shallow groundwater associated with citrus production in Florida (US Environmental Protection Agency, 1988; WHO, 1991).
1.3.3. Soil
Numerous studies have been carried out with aldicarb under field and laboratory conditions to study its translocation, persistence and degradation (WHO 1991) It has a half-time in soil of approximately 30 days; this can vary depending on microbial populations, soil composition, moisture, temperature and farming practices (Meister, 1990) Its half-time in the root zone varied from one week to over two months. The primary mode of degradation in this zone is oxidative metabolism by microorganisms, although some hydrolysis may occur. Warm soil temperatures, high moisture content and high organic contents may result in more rapid degradation (US Environmental Protection Agency, 1988; WHO, 1991).
Aldicarb is mobile in most types of soil, with adsorption coefficients typically of < 1.0 and often 0.1. Incidents of groundwater contamination have primarily been associated with sandy soils, to which aldicarb residues are poorly bound (US Environmental Protection Agency, 1988).
Aldicarb has been used extensively since 1979–80 to control cotton whitefly in the Sudan, reaching a maximum of nearly 84 000 ha in 1984–85. When uptake and distribution were studied and the maximum uptake recorded two weeks after application of aqueous treatments and four weeks after application of granular formulations, no aldicarb or its sulfoxide or sulfone metabolites were detected in plant tissues or in soil at harvest (El-Zorgani et al., 1988).
1.3.4. Food
In a market-basket survey carried out in the USA in 1983–85 on 491 samples of raw agricultural commodities, 76 (72 of white potatoes, two of sweet potatoes, one peach and one collard green) contained aldicarb residues. The mean residue level in potato samples taken in 1984 and 1985 was 200 µg/kg, while that taken in 1983 was 720 µg/kg (US Environmental Protection Agency, 1988).
In 1984–85 to 1988–89 in Canada, aldicarb residues were found in 13 of 30 samples of potatoes, at 0.02–0.78 mg/kg (mean, 0.13 mg/kg). No residue was detected in samples of bananas, oranges, cucumbers or wine (Government of Canada, 1990).
In 1978, national monitoring of potatoes treated at 3 kg/ha active ingredient in the Netherlands found total residues (expressed as the sulfone) in 23 samples, ranging from < 0.03 to 0.38 mg/kg, 100–205 days after broadcast application (mean, 0.11 mg/kg). Monitoring in 1982 of potatoes treated similarly showed residues of < 0.03–0.25 mg/kg (mean, 0.06 mg/kg) (FAO/WHO, 1986).
In a total diet study in 1986–88, 3737 domestic and imported food samples were analysed in the USA. Of these, 3656 (98%) contained no detectable residue. Potatoes (312) were included and residues found in 18% of samples, the highest level being 0.71 mg/kg No residue was found in 98% of bananas sampled (55); one sample contained 0.12 mg/kg (US Food and Drug Administration, 1989b).
1.4. Regulations and guidelines
Limits for residues of aldicarb in foods in various countries or regions are given in Table 3.
Table 3.
National or regional residue limits for aldicarb in foods.
The FAO/WHO Joint Meeting on Pesticide Residues evaluated aldicarb at meetings in 1979, 1982, 1985 and 1988 (FAO/WHO, 1980, 1983a, 1986, 1988). In 1982, an acceptable daily intake in food of 0.005 mg/kg bw was established (FAO/WHO, 1983b).
Maximum residue levels have been established by the Codex Alimentarius Commission for aldicarb (sum of aldicarb, its sulfoxide and its sulfone, expressed as aldicarb) in or on the following commodities (in mg/kg): maize forage, 5; sugar beets (leaves or tops), 1; bananas, dry sorghum (straw and fodder), pecans, potatoes, 0.5; citrus fruit, sorghum, 0.2; coffee beans, dry beans, cottonseed, sweet potatoes, 0.1; maize, onion (bulb), peanuts, sugar beets, 0.05; dry soya beans, 0.02; meat, milk, 0.01 (Codex Commission on Pesticide Residues, 1990).
The Office of Drinking Water of the US Environmental Protection Agency established a Health Advisory Level of 10 ppb (µg/l) for residues of aldicarb in drinking-water (US Environmental Protection Agency, 1984), with a proposed revision of 3 ppb (µg/l) (US Environmental Protection Agency, 1991). Aldicarb was included in the 1987 Canadian guidelines for drinking-water quality, with a maximum acceptable concentration of 9 µg/l (Minister of National Health and Welfare, 1987).
The technical product aldicarb has been classified as 'extremely hazardous' by WHO (1990).
2. Studies of Cancer in Humans
No data were available to the Working Group.
3. Studies of Cancer in Experimental Animals
Oral administration
Mouse
Groups of 50 male and 50 female B6C3F1 mice, six weeks old, were fed aldicarb (approximately 99% pure) at 2 or 6 mg/kg of diet for 103 weeks. A control group of 25 males and 25 females was available. Survival at 90 weeks was 21/25 control, 48/50 low-dose and 45/50 high-dose males and 19/25 control, 45/50 low-dose and 44/50 high-dose females. No reduction in body weight was observed in treated animals, and there was no treatment-related increase in tumour incidence at any site. The authors stated that the dietary concentrations of aldicarb used were too low to be considered a maximum tolerated dose (US National Cancer Institute, 1979).
Rat
Groups of 50 male and 50 female Fischer 344/N rats, eight weeks of age, were fed aldicarb (approximately 99% pure) at 2 or 6 mg/kg of diet for 103 weeks. A control group of 25 males and 25 females was available. Survival at 90 weeks was 18/25 control, 44/50 low-dose and 39/50 high-dose males and 24/25 control, 44/50 low-dose and 46/50 high-dose females. No reduction in body weight was observed in treated animals, and there was no treatment-related increase in tumour incidence at any site. The authors stated that the dietary concentration of aldicarb used were too low to be considered a maximum tolerated dose (US National Cancer Institute, 1979).
4. Other Relevant Data
The toxicityof aldicarb has been reviewed (FAO/WHO, 1980, 1983a; Risher et al., 1987; Baron & Merriam, 1988; WHO, 1991).
4.1. Absorption, distribution, metabolism and excretion
The metabolism of aldicarb is shown in Figure 1 (Risher et al., 1987).

Fig. 1.
Metabolic pathways of aldicarb in rats (from Risher et al., 1987)
4.1.1. Humans
Most carbamate insecticides are readily absorbed from the gastrointestinal tract They may also be absorbed to varying degrees through skin (Feldman & Maibach, 1970; Sterling, 1983).
Reports on the toxicity of aldicarb in humans (section 4.2.1) suggest that it enters the human body following skin contact, inhalation and ingestion. It is metabolized to aldicarb sulfoxide and aldicarb sulfone. Aldicarb derivatives (aldicarb, aldicarb sulfoxide and aldicarb sulfone) were found in the tissues of a 20-year-old man run over by a tractor following overexposure for about 2 h without adequate protection. The levels found were 482 µg/l in blood, 187 µg/kg in liver, 683 µg/kg in kidney and 823 µg/kg in skin from the hand Aldicarb itself was not detected in blood, but aldicarb sulfoxide was present at 108 ppb [µg/l] and aldicarb sulfone at 374 ppb [µg/l], indicating an almost complete two-step oxidation process. The same trend was seen in the liver and kidney, while aldicarb occurred at the highest level in the skin. The total body burden of aldicarb was estimated at 18.2 mg (equivalent to 0.275 mg/kg bw) (Lee & Ransdell, 1984).
4.1.2. Experimental systems
Aldicarb is readily absorbed through the gut in rats and cows and through the skin in rats and rabbits. It is rapidly metabolized and excreted within 24 h of exposure, almost all of the toxic and nontoxic metabolites being excreted in urine (Risher et al., 1987) Signs of poisoning were reported to occur only a few minutes after administration of higher doses in rats given aldicarb at up to 0.1 mg/kg bw by intubation (Cambon et al., 1979) In rats administered radiolabelled aldicarb orally, 80% of the label was excreted in the urine within 24 h; less than 0.4% consisted of unchanged aldicarb (Andrawes et al., 1967) Almost complete absorption via the gut was observed in cows (Dorough & Ivie, 1968; Dorough et al.,1970).
Analysis of tissue samples taken from rats one to four days following oral administration of radiolabelled aldicarb indicated general distribution and elimination (Andrawes et al., 1967)
The metabolism of aldicarb in rats involves both hydrolysis of the carbamate ester and oxidation of the sulfur to the sulfoxide and sulfone derivatives (Andrawes et al., 1967). While the hydrolysis results m compounds with little or no insecticidal activity or toxicity to other organisms, the sulfoxide and sulfone metabolites are active cholinesterase inhibitors (Bull et al., 1967; Risher et al., 1987). In rats, the sulfoxide and the oxime sulfoxide constitute the major urinary metabolites, at 40% and 30%, respectively (Knaak et al., 1966).
4.2. Toxic effects
4.2.1. Humans
Aldicarb is one of the most toxic pesticides known (Marshall, 1985). Its toxicity is based on a transient inhibition of acetylcholinesterase. Carbamates form unstable complexes with chlolinesterases by carbamoylation of the active sites of the enzymes (Done 1979; Mortensen, 1986). Unlike the relatively irreversible anticholinesterase activity of the organophosphorus insecticides, the carbamoylation process which produces the esterase inhibition is quickly reversible.
Several reports on the acute and chronic toxicity of aldicarb are summarized in Table 4 In most exposure situations, the clinical symptoms observed were consistent with the known mechanism of action (inhibition of acetylcholinesterase). In some of the studies, the dose has been estimated. chlolinesterases measurements have been of additional use in characterizing dose or extent of exposure.
Table 4.
Acute and chronic toxicity of aldicarb in human populations.
4.2.2. Experimental systems
The acute toxicity of alidcarb is high, with oral LD50s in rats and mice generally below 1 mg/kg bw. Depression of cholinesterase activity has been reported in rats administered aldicarb, its sulfoxide or its sulfone. The relative order of inhibition of cholinesterase was plasma > red blood cells > brain (DePass et al., 1985; studies reported by Risher et al., 1987).
After a review of several studies, Risher et al. (1987) concluded there was no effect of subchronic and chronic oral feeding to rats of aldicarb at 0.3 mg/kg bw/day; of aldicarb sulfoxide at 0.3 mg/kg bw/day; of aldicarb sulfone at 2.4 mg/kg bw/day; or of 1:1 aldicarb sulfoxide:sulfone mixture at 0.6 mg/kg bw/day. The latter was tested owing to the observation that residues found in drinking-water are generally present as this mixture.
Suppressed humoral immune response (splenic plaque-forming cell assay) has been reported after exposure of mice to aldicarb in drinking-water for up to 34 days. Suppression on day 34 was significant, however, only at 1 ppb [µg/l] water and was less pronounced and not significant at 10, 100 or 1000 µg/l water (Olson et al., 1987). In a similar study in mice exposed to aldicarb in drinking-water at levels of 0.1–1000 ppb [µg/l] for 34 days using a variety of immunological endpoints including that used by Olson et al., no significant effect on the immunological system was noted (Thomas et al., 1987). Shirazi et al. (1990) however performed a follow-up experiment using the same assay and aldicarb at 0.01–1000 µg/l in drinking-water but prolonging the study time up to 180 days. After extensive statistical analysis, the authors concluded that there was a stimulatory effect at 30 and 60 days and an inhibitory effect at 90 and 180 days. Dean et al. (1990) reported that aldicarb given mtrapentoneally to mice at doses of 0.01–100 ng per mouse (0.1 ml of a 0.1, 1, 10, 100 or 1000 ppb solution) decreased the stimulatory function of macrophages without affecting T-lymphocyte function. There was no clear dose-response relationship.
4.3. Reproductive and developmental effects
4.3.1. Humans
No data were available to the Working Group.
4.3.2. Experimental systems
Few data are available on the reproductive and developmental effects of aldicarb. In two studies described in a review by Risher et al. (1987), there was reported to be no evidence of toxicity in the offspring of rats treated with aldicarb in the feed at doses as high as 1 mg/kg bw [near the LD50] throughout pregnancy and lactation, and offspring of rabbits treated with aldicarb by gavage at doses of up to 0.5 mg/kg bw on days 7–27 of gestation were reported to exhibit no developmental toxicity. Rats investigated in a three-generation study in which aldicarb was incorporated into the diet were also reported to exhibit no significant difference in any parameter assessed compared to control animals.
Cambon et al. (1979, 1980) evaluated the effects of administering a single dose of aldicarb (0–0.1 mg/kg bw) on gestation day 18 on acetylcholinesterase activity in maternal and fetal tissue of Sprague-Dawley rats. Maternal blood acetylcholinesterase activity was reduced at doses greater than 0.001 mg/kg, and fetal blood acetylcholinesterase activity was reduced at doses of 0.001 mg/kg and above in samples taken > 1 h after dosing. The effect persisted for up to 24 h at doses of 0.01 mg/kg and above. Tissues were stored overnight at 4°C before analysis. Acetylcholinesterase was assayed in maternal and fetal brain homogenates 1 h after administration of 0.1 mg/kg bw aldicarb, and isoenzymes were analysed using polyacrylamide electrophoresis. Both maternal and fetal brain acetylcholinesterase levels were decreased by aldicarb; the levels of two of three cholinesterase isozymes were decreased in fetal brain and that of only one of the cholinesterase isozymes was decreased in maternal brain.
4.4. Genetic and related effects (see Table 5 and Appendices 1 and 2)
4.4.1. Humans
No data were available to the Working Group.
4.4.2. Experimental systems
Aldicarb induced differential toxicity in Salmonella typhimurium but not in strains of Escherichia coli. Aldicarb was not mutagenic to bacteria, whereas mutations were induced in cultured mammalian cells. Gene mutation, sister chromatid exchange and chromosomal aberrations were induced by aldicarb in cultured human cells. In a single study, no DNA strand breakage was observed in human cells.
In vivo, aldicarb induced chromosomal aberrations in cells of rat bone marrow.
5. Summary of Data Reported and Evaluation
5.1. Exposure data
Aldicarb is a moderately persistent systemic insecticide, acaricide and nematicide formulated as granules. It was first used in 1970 and is applied mainly on cotton and potatoes.
Exposure to aldicarb may occur during its production and application and, at lower levels, via contamination of groundwater and consumption of food containing residues.
Table 5.
Genetic and related effects of aldicarb.
5.2. Carcinogenicity in humans
No data were available to the Working Group.
5.3. Carcinogenicity in experimental animals
Aldicarb has not been tested adequately for carcinogenicity in experimental animals.
5.4. Other relevant data
Aldicarb is highly acutely toxic: it is one of the most potent cholinesterase-inhibiting carbamate insecticides.
No data were available on the genetic and related effects of aldicarb in humans.
Aldicarb induced chromosomal aberrations in rat bone-marrow cells in vivo. It induced various kinds of chromosomal damage and gene mutation in cultured human cells and induced gene mutation in rodent cells. It did not cause mutation in bacteria.
5.5. Evaluation1
No data were available from studies in humans.
There is inadequate evidence for the carcinogenicity of aldicarb in experimental animals.
Overall evaluation
Aldicarb is not classifiable as to its carcinogenicity to humans (Group 3).
6. References
- Andrawes N.R., Dorough H.W., Lindquist D.A. Degradation and elimination of Temik in rats. J. Econ. Entomol. 1967;60:979–987. [PubMed: 6073199]
- Association of Official Analytical Chemists, author. Changes in methods. N-Methylcarbamate insecticide and metabolite residues. Liquid chromatographic method. First action. J. Assoc. off. anal. Chem. 1985;68:386–388.
- Baron R.L., Merriam T.L. Toxicology of aldicarb. Rev. environ. Contam. Toxicol. 1988;105:2–70. [PubMed: 3062705]
- Blevins R.D., Lijinsky W., Regan J.D. Nitrosated methylcarbamate insecticides: effect on the DNA of human cells. Mutat. Res. 1977;44:1–7. [PubMed: 895750]
- Bull D.L., Lindquist D.A., Coppedge J.R. Metabolism of 2-methyl-2-(methylthio)-propionaldehyde O-(methylcarbamoyt)oxirne (Temik, UC-21149)in insects. J. agric. Food Chem. 1967;15:610–616.
- Cambon C., Declume C., Derache R. Effect of the insecticidal carbamate derivatives (carbofuran, pirimicarb, aldicarb) on the activity of acetylcholinesterase in tissues from pregnant rats and fetuses. Toxicol. appl. Pharmacol. 1979;49:203–208. [PubMed: 494273]
- Cambon C., Declume C., Derache R. Foetal and maternal rat brain acetylcholinesterase: isoenzymes changes following insecticidal carbamate derivatives poisoning. Arch. Toxicol. 1980;45:257–262. [PubMed: 7447699]
- Caspary W.J., Langenbach R., Penman B.W., Crespi C., Myhr B.C., Mitchell A.D. The mutagenic activity of selected compounds at the TK locus: rodent vs. human cells. Mutat. Res. 1988;196:61–81. [PubMed: 3292900]
- Cid M.G., Matos E. Induction of sister-chromatid exchanges in cultured human lymphocytes by aldicarb, a carbamate pesticide. Mutat. Res. 1984;138:175–179. [PubMed: 6513973]
- Cid M.G., Matos E. Chromosomal aberrations in cultured human lymphocytes treated with aldicarb, a carbamate pesticide. Mutat. Res. 1987;191:99–103. [PubMed: 3600695]
- Codex Committee on pesticide Residues (1990) Guide to Codex Maximum Limits for Pesticide Residues, Part 2 (CAC/PR 2—1990; CCPR Pesticide Classification No. 117) The Hague.
- Coppedge J.R., Bull D.L., Ridgway R.L. Movement and persistence of aldicarb in certain soils. Arch. environ. Contam. Toxicol. 1977;5:129–141. [PubMed: 596936]
- Dean T.N., Selvan R.S., Misra H.P., Nagarkatti M., Nagarkatti P.S. Aldicarb treatment inhibits the stimulatory activity of macrophages without affecting the T-cell responses in the syngeneic mixed lymphocyte reaction. Int. J. Immunopharmacol. 1990;12:337–348. [PubMed: 2139433]
- Debuyst, B. & Van Larebeke, N. (1983) Induction of sister-chromatid exchanges in human lymphocytes by aldicarb, thiofanox and methomyl (Abstract No. 35) Mutat. Res., 113 242–243.
- DePass L.R., Weaver E.V, Mirro E.J. Aldicarb sulfoxide/aldicarb sulfone mixture in drinking water of rats: effects on growth and acetylcholinesterase activity. J. Toxicol. environ. Health. 1985;16:163–172. [PubMed: 4078930]
- Done A.K. The toxic emergency. The great equalizers? II. Anticholinesterases. Emerg. Med. 1979;11:167–175.
- Dorough H. W, Ivie G.W. Temik-S35 metabolism in a lactating cow. J. agric. Food Chem. 1968;16:460–464.
- Dorough H. W., Davis R.B., Ivie G.W. Fate of Temik-carbon-14 lactating cows during a 14-day feeding period. J. agric. Food Chem. 1970;18:135–142. [PubMed: 5535666]
- Dunkel V.C., Zeiger E., Brusick D., McCoy E., McGregor D., Mortelmans K., Rosenkranz H.S., Simmon V.F. Reproducibility of microbial mutagenicity assays. II. Testing of carcinogens and noncarcinogens in Salmonella typhimurium and Escherichia coli. Environ. Mutagenesis. 1985;7 (Suppl. 5):1–248. [PubMed: 3905369]
- El-Zorgani G.A., Bakhiet T.N., Eldin N.S. Distribution of residues of 14C-aldicarb applied to cotton plants in Gezira, Sudan. Int. atomic Energy Agency. 1988;297:149–156.
- FAO/WHO (1980) Pesticide Residues in Food: 1979 Evaluations. The Monographs (FAO Plant Production and Protection Paper 20 Sup.), Rome.
- FAO/WHO (1983a) Pesticide Residues in Food—1982 Evaluations. The Monographs (FAO Plant Production and Protection Paper 49), Rome.
- FAO/WHO (1983b) Pesticide Residues in Food—1982 (FAO Plant Production and Protection Paper 46), Rome.
- FAO/WHO (1986) Pesticide Residues in Food—1985 (FAO Plant Production and Protection Paper 72/1), Rome.
- FAO/WHO (1988) Pesticide Residues in Food—1988. Evaluations. Part I. Residues (FAO Plant Production and Protection Paper 93/1), Rome.
- Feldman R.J., Maibach H.I. Pesticide percutaneous penetration in man (Abstract No. 5). J. invest. Dermatol. 1970;54:435–436.
- Fiore M.C., Anderson H.A., Hong R., Golubjatnikov R., Seiser J.E., Nordstrom D., Hanrahan L., Belluck D. Chronic exposure to aldicarb-contaminated groundwater and human immune function. Environ. Res. 1986;41:633–645. [PubMed: 3490967]
- Goes E.A., Savage E.P., Gibbons G., Aaronson M., Ford S.A., Wheeler H.W. Suspected foodborne carbamate pesticide intoxications associated with ingestion of hydroponic cucumbers. Am. J. Epidemiol. 1980;111:254–260. [PubMed: 7355886]
- Government of Canada, (1990) Report on National Surveillance Data from 1984/85 to 1988/89, Ottawa.
- Green M.A., Heumann M.A., Wehr H.M., Foster L.R., Williams L.P. Jr, Polder J.A., Morgan C.L., Wagner S.L., Wanke L.A., Witt J.M. An outbreak of watermelon-borne pesticide toxicity. Am. J. public Health. 1987;77:1431–1434. [PMC free article: PMC1647114] [PubMed: 3661796]
- Griffith J., Duncan R.C. Grower reported pesticide poisoning among Florida citrus fieldworkers. J. environ. Sci. Health. 1985;B20:61–72. [PubMed: 3989222]
- Health and Welfare Canada (1990) National Pesticide Residue Limits in Foods, Ottawa, Bureau of Chemical Safety, Food Directorate, Health Protection Branch.
- Hirsch G.H., Mori B.T., Morgan G.B., Bennett P.R., Williams B.C. Report of illnesses caused by aldicarb-contaminated cucumbers. Food Addit. Contam. 1987;5:155–160. [PubMed: 3360204]
- Holtorf, R.C. (1982) Preliminary Quantitative Usage Analysis of Aldicarb as a Pesticide, Washington DC, US Environmental Protection Agency, Office of Pesticide Programs.
- Hynninen E.-L., Blomqvist H. Sales of pesticides in Finland in 1988 (Fin.). Kemia Kemi. 1989;16:614–617.
- IARC (1974) IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man, Vol. 5, Some Organochlorinic Pesticides, Lyon, pp. 211–218.
- IARC (1987a) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, Lyon, pp. 194–195. [PubMed: 3482203]
- IARC (1987b) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, Lyon, p. 71. [PubMed: 3482203]
- IARC (1987c) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, Lyon, pp. 220–222. [PubMed: 3482203]
- IRPTC/UNEP (1990) Data Profiles, Geneva.
- Jackson R.J., Goldman L. Aldicarb poisoning. Reply to a Letter. J. Am. med. Assoc. 1986;256:3218.
- Jackson R.J., Stratton J.W., Goldman L.R., Smith D.F., Pond E.M., Epstein D., Neutra R.R., Kelter A., Kizer K.W. Aldicarb food poisoning from contaminated melons-California. J. Am. med. Assoc. 1986;256:175–176.
- Knaak J.B., Tallant M.J., Sullivan L.J. The metabolism of 2-methyl-2-(methylthio)-propionaldehyde O-(methylcarbamoyl)oxime in the rat. J. agric. Food Chem. 1966;14:573–578.
- Lee M.H., Ransdell J.F. A farmworker death due to pesticide toxicity: a case report. J. Toxicol. environ. Health. 1984;14:239–246. [PubMed: 6502735]
- Marshall E. The rise and decline of Temik. Science. 1985;229:1369–1371. [PubMed: 17798373]
- Meister, R.T., ed. (1990) Farm Chemical Handbook '90, Willoughby, OH, Meister Publishing Co., pp. E-10, C-402, C-279-C-280.
- Minister of National Health and Welfare (1987) Guidelines for Canadian Drinking Water Quality, Ottawa.
- Mitchell A.D., Rudd C.J., Caspary W.J. Evaluation of the L5178Y mouse lymphoma cell mutagenesis assay: intralaboratory results for sixty-three coded chemicals tested at SRI International. Environ. mol. Mutagenesis. 1988;12 (Suppl. 13):37–101. [PubMed: 3416841]
- Mortensen M.L. Management of acute childhood poisoning caused by selected insecticides and herbicides. Pediatr. Clin. North Am. 1986;33:421–445. [PubMed: 3515303]
- Moye H.A., Miles C.J. Aldicarb contamination of groundwater. Rev. environ. Contam Toxicol. 1988;105:99–146. [PubMed: 3062707]
- Myhr B.C., Caspary W.J. Evaluation of the L5178Y mouse lymphoma cell mutagenesis assay: intralaboratory results for sixty-three coded chemicals tested at Litton Bionetics, Inc. Environ. mol. Mutagenesis. 1988;12 (Suppl. 13):103–194. [PubMed: 3416838]
- Narang A.S., Eadon G. Use of XAD-2 macroreticular resin for the recovery of aldicarb and its metabolites in drinking water. Int. J. environ. anal. Chem. 1982;11:167–174.
- Olson L.J., Erickson B.J., Hinsdil R.D., Wyman J.A., Porter W.P., Binning L.K., Bidgood R.C., Nordheim E.V. Aldicarb immunomodulation in mice: an inverse dose-response to parts per billion levels in drinking water. Arch. environ. Contam. Toxicol. 1987;16:433–439. [PubMed: 3300571]
- Payne, L.K., Jr & Weiden, M.H.J. (1965) 2-Hydrocarbylthiosulfinyl and sulfonylalkanal carbamoyloximes. US Patent 3,217,037 (to Union Carbide Co., NY)
- Peoples S.A., Maddy K.T., Smith C.R. Occupational exposure to Temic (aldicarb) as reported by California physicians for 1974–1976. Vet. hum. Toxicol. 1978;20:321–324.
- Rashid K.A., Mumma R.O. Screening pesticides for their ability to damage bacterial DNA. J. environ. Sci. Health. 1986;B21:319–334. [PubMed: 3531299]
- Rhone-Poulenc Ag Co. (1987) Material Safety Data Sheet: Aldicarb, Technical Grade, Research Triangle Park, NC.
- Rhone-Poulenc Ag Co. (1989) Product Label Guide, Research Triangle Park, NC, pp. 324–350.
- Rhone-Poulenc Ag Co. (1990a) Material Safety Data Sheet: Temik® Brand 10G Aldicarb Pesticide for Agricultural Use (Gypsum), Research Triangle Park, NC.
- Rhone-Poulenc Ag Co. (1990b) Material Safety Data Sheet: Temik® Brand 15G Aldicarb Pesticide (Gypsum), Research Triangle Park, NC.
- Rhone-Poulenc Ag Co. (1990c) Material Safety Data Sheet: Temik® Brand TSX Granular Pesticide, Research Triangle Park, NC.
- Risher J.F., Mink F.L., Stara J.F. The toxicologic effects of the carbamate insecticide aldicarb in mammals: a review. Environ. Health Perspect. 1987;72:267–281. [PMC free article: PMC1474664] [PubMed: 3304999]
- Romine, R.R. (1974) Aldicarb. In: Zweig, G., ed., Analytical Methods for Pesticides and Plant Growth Regulators, Vol. VII, New York, Academic Press, pp. 147–162.
- Royal Society of Chemistry (1986) European Directory of Agrochemical Products, Vol. 3, Insecticides, Acaricides, Nematicides, Cambridge, pp. 4–10.
- Royal Society of Chemistry (1989) The Agrochemicals Handbook [Dialog Information Services (File 306)], Cambridge.
- Sharaf A.A., Temtamy S.A., de Hondt H.A., Belai M.H., Kassam E.A. Effect of aldicarb (Temik) a carbamate insecticide on chromosomes of the laboratory rat. Egypt. J. genet. Cytol. 1982;11:135–144.
- Shirazi M.A., Erickson B.J., Hinsdill R.D., Wyman J.A. An analysis of risk from exposure to aldicarb using immune response of nonuniform populations of mice. Arch. environ. Contam. Toxicol. 1990;19:447–456. [PubMed: 2353843]
- Sterling G.H. Poisoning by cholinesterase-inhibiting insecticides. Am. Fam. Physician. 1983;27:159–162. [PubMed: 6858816]
- Sterman A.B., Varma A. Evaluating human neurotoxicity of the pesticide aldicarb: when man becomes the experimental animal. Neurobehav. Toxicol. Teratol. 1983;5:493–495. [PubMed: 6320022]
- Supak J.R., Swoboda A.R., Dixon J.B. Volatilization and degradation losses of aldicarb from soils. J. environ. Qual. 1977;6:413–417.
- Thomas P.T., Ratajczak H.V, Eisenberg W.C., Furedi-Machacek M., Ketels K.V, Barbera P.W. Evaluation of host resistance and immunity in mice exposed to the carbamate pesticide aldicarb. Fundam. appl. Toxicol. 1987;9:82–89. [PubMed: 3040502]
- US Environmental Protection Agency (1975) Substitute Chemical Program: Initial Scientific and Minieconomic Review of Aldicarb (EPA 540/1-75-013; NTIS PB-243-743), Washington DC.
- US Environmental Protection Agency (1976) Infrared spectra of pesticides. In: Manual of Chemical Methods for Pesticides and Devices, Arlington, VA, Association of Official Analytical Chemists.
- US Environmental Protection Agency (1984) Pesticide Fact Sheet Number 19: Aldicarb (PB 87–10881), Washington DC, Office of Pesticide Programs.
- US Environmental Protection Agency (1987) Aldicarb, Washington DC, Health Advisory Office of Drinking Water.
- US Environmental Protection Agency (1988) Aldicarb (Special Review, Technical Support Document), Washington DC, Office of Pesticides and Toxic Substances.
- US Environmental Protection Agency (1989a) Method 531.1 Measurement of N-methylcarbamoyloximes and N-methylcarbamates in water by direct aqueous injection HPLC with post column derivatization. In: Methods for the Determination of Organic Compounds in Drinking Water (EPA-600/4-88-039; US NTIS PB89-220461), Cincinnati, OH, Environmental Monitoring Systems Laboratory, pp. 357–378.
- US Environmental Protection Agency. US Code fed. Regul., Title 40, Part 180. Vol. 269. 1989b. Aldicarb; tolerances for residues; pp. 324–325.
- US Environmental Protection Agency. US Code fed. Regul., Title 40, Part 185. Vol. 150. 1989c. Aldicarb. Tolerances for pesticides in animal feed; p. 440.
- US Environmental Protection Agency. National primary drinking water regulations—monitoring for synthetic organic chemicals; MCLGs and MCLs for aldicarb, aldicarb sulfoxide, aldicarb sulfone, pentachlorophenol and barium. Fed. Reg. 1991;56:3600–3614.
- US Food and Drug Administration (1989a) Pesticide Analytical Manual, Vol. II, Washington DC, US Department of Health and Human Services.
- US Food and Drug Administration. J. Assoc. Off. Anal. Chem. Vol. 72. 1989b. Residues in foods—1988; pp. 133A–142A. [PubMed: 2681132]
- US National Cancer Institute (1979) Bioassay of Aldicarb for Possible Carcinogenicity (Carcinogenesis Technical Report Series No. 136; DHEW Publ. No. (NIH) 79-1391), Washington DC, US Government Printing Office. [PubMed: 12799656]
- WHO (1990) The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 1990–91, Geneva.
- WHO (1991) Aldicarb (Environmental Health Criteria No. 121), Geneva.
- Williams, S., ed. (1984) Official Methods of Analysis of the Association of Official Analytical Chemists, 14th ed., Washington DC, Association of Official Analytical Chemists, p. 137.
- Worthing, C.R. & Walker, S.B., eds (1987) The Pesticide Manual—A World Compendium, 8th ed., Thornton Heath, British Crop Protection Council, pp. 7–8.
- Zaki M.H., Moran D., Harris D. Pesticides in groundwater: the aldicarb story in Suffolk County, NY. Am. J. public Health. 1982;72:1391–1395. [PMC free article: PMC1650561] [PubMed: 7137437]
- Zeiger E., Anderson B., Haworth S., Lawlor T., Mortelmans K. Salmonella mutagenicity tests: IV. Results from the testing of 300 chemicals. Environ. mol. Mutagenesis. 1988;11 (Suppl. 12):1–158. [PubMed: 3277844]
Footnotes
- 1
Calculated from: mg/m3 = (molecular weight/24.45) × ppm, assuming standard temperature (25 °C) and pressure (760 mm Hg [101.3 kPa])
- 1
For definition of the italicized terms, see Preamble, pp. 26–28.
- Review Occupational exposures in spraying and application of insecticides.[IARC Monogr Eval Carcinog Risk...]Review Occupational exposures in spraying and application of insecticides.. IARC Monogr Eval Carcinog Risks Hum. 1991; 53:45-92.
- Review Picloram.[IARC Monogr Eval Carcinog Risk...]Review Picloram.. IARC Monogr Eval Carcinog Risks Hum. 1991; 53:481-93.
- Review Monuron.[IARC Monogr Eval Carcinog Risk...]Review Monuron.. IARC Monogr Eval Carcinog Risks Hum. 1991; 53:467-80.
- Review Simazine.[IARC Monogr Eval Carcinog Risk...]Review Simazine.. IARC Monogr Eval Carcinog Risks Hum. 1991; 53:495-513.
- Review Thiram.[IARC Monogr Eval Carcinog Risk...]Review Thiram.. IARC Monogr Eval Carcinog Risks Hum. 1991; 53:403-22.
- Aldicarb - Occupational Exposures in Insecticide Application, and Some Pesticide...Aldicarb - Occupational Exposures in Insecticide Application, and Some Pesticides
- NOTE TO THE READER - Wood Dust and FormaldehydeNOTE TO THE READER - Wood Dust and Formaldehyde
- Homo sapiens collagen type IV alpha 2 chain (COL4A2), RefSeqGene on chromosome 1...Homo sapiens collagen type IV alpha 2 chain (COL4A2), RefSeqGene on chromosome 13gi|2302519849|ref|NG_032137.2|Nucleotide
- Mus musculus VGF nerve growth factor inducible, mRNA (cDNA clone MGC:170179 IMAG...Mus musculus VGF nerve growth factor inducible, mRNA (cDNA clone MGC:170179 IMAGE:8861574), complete cdsgi|187951978|gb|BC138554.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...
