NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Committee on Nutrient Requirements of Dairy Cattle. Nutrient Requirements of Dairy Cattle: Eighth Revised Edition. Washington (DC): National Academies Press (US); 2021 Aug 30.

Nutrient Requirements of Dairy Cattle: Eighth Revised Edition.
Show detailsNot all minerals will be discussed in this chapter. For information on minerals that are primarily a toxicity concern (e.g., aluminum and fluoride) rather than a nutritional concern, see NRC (2005). Mineral requirements specific to preweaned calves are discussed in Chapter 10.
MACROMINERALS
Calcium
Functions
Extracellular calcium (Ca) is essential for formation of skeletal tissues, transmission of nervous tissue impulses, excitation of skeletal and cardiac muscle contraction, blood clotting, and as a component of milk. Intracellular Ca, while 1/10,000th of the concentration of extracellular Ca, is involved in the activity of a wide array of enzymes and serves as an important second messenger conveying information from the surface of the cell to the interior of the cell. About 98 percent of the Ca in the body is located within the skeleton, where Ca, along with phosphate anion, serves to provide structural strength and hardness to bone. The other 2 percent is found primarily in the extracellular fluids. Normally, the concentration of Ca in plasma is 2.2 to 2.5 mM (9 to 10 mg/dL) in the adult cow, with slightly higher values in calves. Between 40 and 45 percent of total Ca in plasma is bound to plasma proteins, primarily albumin, and another 5 percent is bound to organic components of the blood such as citrate or inorganic elements. From 45 percent (at higher blood pH) to 50 percent (at lower pH) of total Ca in plasma exists in the ionized, soluble form. The ionized Ca concentration of plasma must be maintained between 1 and 1.25 mM to ensure normal nerve membrane and muscle end-plate electric potential and conductivity, which has forced vertebrates to evolve an elaborate system to maintain Ca homeostasis. This system attempts to maintain a constant concentration of extracellular Ca by increasing Ca entry into the extracellular fluids whenever there is a loss of Ca from the extracellular compartment. When the loss of Ca exceeds entry, hypocalcemia can occur, resulting in loss of nerve and muscle function, which can lead to recumbency and the clinical condition referred to as milk fever.
Calcium Homeostasis
Ca leaves extracellular fluids during bone formation, in digestive secretions, sweat, and, in specific situations, urine. In lactating cows, secretion of Ca in milk is by far the greatest loss, accounting for 50 to 75 percent of Ca losses. Ca lost via these routes can be replaced from dietary Ca, from resorption of Ca stored in bone, or by resorbing the Ca filtered across the renal glomerulus (i.e., reducing urinary Ca loss). Under most circumstances urinary Ca losses are no more than 1 to 2 percent of absorbed Ca intake (Knowlton and Herbein, 2002; Taylor et al., 2009). When the loss of Ca from extracellular fluids exceeds the amount of Ca entering the extracellular fluids, plasma concentrations decrease. The parathyroid glands monitor the concentration of Ca in carotid arterial blood and secrete parathyroid hormone (PTH) when they sense a decrease in blood Ca. Release of PTH immediately increases renal reabsorption mechanisms for Ca and will stimulate processes to enhance intestinal absorption of Ca and resorption of Ca from bone.
Absorption of dietary Ca can occur by passive (paracellular) transport between epithelial cells across any portion of the digestive tract whenever ionized Ca in the digesta directly over the mucosa exceeds 6 mM (Bronner, 1987). These concentrations are reached when calves are fed all-milk diets and when cows are given oral Ca drenches for prevention or treatment of hypocalcemia (Goff and Horst, 1993). Following drenching, elevated Ca concentrations are short-lived as a result of dilution with digesta and the formation of complexes and chelates that reduce ionized Ca concentrations.
In nonruminants, as much as 50 percent of dietary Ca absorption may be passive (Nellans, 1988), but the amount of passive absorption of Ca that occurs in dairy cattle is unknown. The diluting effect of the rumen would likely reduce the degree to which passive Ca absorption occurs. When chelates and less-soluble salts such as calcium carbonate (CaCO3) and calcium sulfate (CaSO4) move out of the rumen and interact with hydrochloric acid secreted in the abomasum, ionized Ca increases (Goff, 2018). Ca absorption is tightly regulated and is one of the primary means by which Ca homeostasis is maintained. This suggests that active transport of Ca is the major route for Ca absorption in mature ruminants, and this process is controlled by 1,25-dihydroxyvitamin D, the hormone derived from vitamin D. By carefully regulating the amount of 1,25-dihydroxyvitamin D produced, the amount of dietary Ca absorbed can be adjusted to maintain a constant concentration of extracellular Ca (DeLuca, 1979; Wasserman, 1981; Bronner, 1987). Regulation of Ca absorption in the intestine occurs as 1,25-dihydroxyvitamin D in blood binds vitamin D receptors in the intestinal enterocyte that initiate transcription and translation of several key proteins needed for active transport of Ca (Goff, 2018). These include an apical membrane channel protein that facilitates movement of ionized Ca through the cell membrane; production of calbindin, which binds Ca ions as they pass through the enterocyte membrane; and plasma membrane Ca ATPase that works with a sodium/calcium exchanger that pumps intracellular Ca through the enterocyte basolateral membrane into blood by exchanging 2 moles of Ca for 3 moles of sodium (Na).
When dietary Ca is insufficient to meet the requirements of the animal, Ca will be withdrawn from bone to maintain a normal concentration of extracellular Ca. If dietary Ca is severely deficient for a prolonged period, the animal will develop osteoporosis to the point of developing fractures, but plasma Ca will only be slightly lower than normal. A sudden large increase in loss of Ca from the extracellular pool (e.g., initiation of lactation) can result in acute hypocalcemia before the Ca homeostatic mechanisms can act. This is discussed in the section on milk fever (see Chapter 12).
Requirement for Absorbed Calcium
A factorial system is used to estimate the amounts of Ca required for maintenance, growth, pregnancy, and lactation as in the previous NRC (2001) publication.
Maintenance
The maintenance requirement is the amount of absorbed Ca that is needed to replace endogenous losses in urine and feces. Urinary Ca losses are trivial, and no reliable methods are currently available to predict urinary losses and thus are ignored. Previous estimates for metabolic fecal Ca were based on the fecal appearance of intravenously injected radioisotopes of Ca (Visek et al., 1953; Hansard et al., 1957). The 2001 NRC committee set daily metabolic fecal losses at 0.0154 g Ca/kg body weight (BW) for growing heifers and dry cows (Visek et al., 1953; Hansard et al., 1957) and 0.031 g Ca/kg BW (Martz et al., 1990) in lactating cows. Metabolic fecal requirements for most minerals should be expressed as a function of dry matter intake (DMI) reflecting increased losses in feces with increased feed consumption. Data from Hansard et al. (1954, 1957), Visek et al. (1953), and Martz et al. (1990, 1999) were pooled and regressed on DMI. The regression equation included a nonsignificant intercept that was dropped resulting in an equation applied to all physiological states:
where maintenance requirement is equivalent to metabolic fecal Ca, g/d; DMI is dry matter intake, kg/d; R2 = 0.92; and standard error (SE) = 1.75 g/d.
In NRC (2001), the absorbed Ca requirements for maintenance for a 300-kg growing heifer and 700-kg dry and lactating cows were 4.6, 10.8, and 21.7 g/d, respectively. In the new system based on DMI and assuming intakes of 7, 13, and 25 kg/d, the new requirements would be 6.3, 11.7, and 22.5 g/d, respectively. Although the equation has changed from NRC (2001), the amounts of absorbed Ca required for maintenance are similar.
Growth
Ca deposition in bone is the primary factor that drives Ca requirement for growth. Growing cattle require more Ca when animals are young and actively accruing bone and less as they approach mature skeletal size. An allometric equation (AFRC, 1991) was used to estimate the Ca requirement of growing calves:
where MatBW is mature BW, kg; BW is current body weight, kg; and ADG is average daily gain, kg/d.
Based on that equation, absorbed Ca requirements per unit BW gain decrease with increasing BW. The NASEM (2016) estimates absorbed Ca requirements for growth as 71 g Ca/kg of body protein gain but cited more recent experiments that measured as much as 144 g Ca/kg protein gain. Growth requirements for Ca estimated using the equation above are generally 25 to 30 percent greater than the value used by NASEM (2016) at small BWs relative to mature weight, but as animals get closer to mature weight, the estimated requirements are much greater than those currently used for beef.
Pregnancy
The developing fetus requires a negligible amount of Ca until the last trimester of pregnancy (after day 190 of pregnancy), when the fetal skeleton begins to become calcified. Fetal skeletal calcification is especially great in the last weeks before parturition, where the absorbed Ca requirement for pregnancy can exceed 10 g/d. The absorbed Ca required for growth of the uterus and conceptus is best described by the exponential equation (House and Bell, 1993) for any given day of gestation beyond day 190 as
where t is day of gestation. The average cow in the House and Bell (1993) study weighed 715 kg; therefore, gestation requirement is scaled to that BW.
Lactation
In NRC (2001), the absorbed Ca requirements for lactation were 1.22 g Ca/kg milk for Holstein cows, 1.45 g Ca/kg for Jersey cows, and 1.37 g/kg for other breeds. Castillo et al. (2013) reported a median milk concentration of 1.01 mg Ca/kg in a survey of bulk tank milk concentrations representing more than 30,000 cows in 39 California dairy herds. In more limited data, Carroll et al. (2006) reported a mean milk Ca concentration of 1.10, 1.25, and 1.25 g/kg for Holstein, Jersey, and Brown Swiss cows, respectively. The mean bulk tank milk concentration for 29 Holstein and 3 Jersey herds was 1.04 and 1.13 g Ca/kg, respectively (Robinson et al., 2002). These results implied that the values from previous NRC publications are too high.
Sixty-five percent of milk Ca is contained in the casein micelles in milk (Gaucheron, 2005), and milk Ca concentrations are strongly correlated with milk casein concentrations (Bijl et al., 2012). Survey data from Dutch dairy herds over time have shown that milk Ca has increased from 1.15 to 1.30 g Ca/kg as milk casein has increased from 2.64 to 2.88 percent (Bijl et al., 2012). To correct for differences in milk protein concentration among breeds and to account for the large discrepancy between 2001 dairy NRC milk Ca requirements and more recently measured milk Ca concentrations, a regression equation that related milk Ca concentration to milk true protein was developed using herd means (Robinson et al., 2002; Castillo et al., 2013), treatment means (Kume et al., 1998; Knowlton and Herbein, 2002; Carroll et al., 2006), and extracted individual cow data (Bijl et al., 2012). The resulting equation after adjustment for study effects was
where Milk Ca is g/kg milk, root mean square error (RMSE) = 0.065, and R2 = 0.86.
Using published (Animal Improvement Laboratory, 2015) mean protein concentrations of 3.08 and 3.65 percent for Holsteins and Jerseys, respectively, the equation would predict milk Ca concentrations of 1.03 and 1.17 g Ca/kg, values that are more in line with recently reported values.
Body Tissue Mobilization and Replenishment
Mobilization of body tissue in support of lactation includes the mobilization of bone Ca to support the demand for milk Ca secretion. Each kilogram of body tissue mobilized includes 21 g ash, and Ca accounted for 53 percent of total bone ash in samples taken at 8 days and 11 weeks postpartum (Taylor et al., 2009). This suggests that 11 g of Ca would be provided for each kilogram of body tissue mobilized. Ca balances of −11 to −15 g/d were observed in cows fed 0.52 percent dietary Ca during the first 8 weeks postpartum (Taylor et al., 2009). Ca mobilized at the beginning of lactation needs to be replenished as cows regain mobilized tissue stores over the course of the lactation. Mobilization of skeletal Ca is almost inevitable during early lactation, and cows could lose 800 to 1,300 g of bone Ca in early lactation (Ellenberger et al., 1931). This would require up to 8 g of absorbed Ca/d during the last 20 weeks of lactation to replenish. However, because of the uncertainties involved, no provision for replenishment of skeletal Ca mobilized during early lactation was included in the model.
Calcium Absorption Coefficient
The amount of Ca that must be fed to meet the requirement for absorbed Ca is dependent on the availability of Ca from the diet. The amount of Ca absorbed will generally equal the requirement for Ca if the diet contains enough available Ca. The proportion of dietary Ca absorbed will decrease as dietary Ca increases above requirements. As vitamin D–mediated Ca absorption from the intestine is tightly regulated, the determination of the efficiency of absorption of Ca from a diet requires that animals be fed at or near their Ca requirement. This will ensure that intestinal Ca absorption mechanisms are fully activated. Few studies fulfill this criterion, suggesting that published absorption data may often underestimate the availability of Ca. Furthermore, measuring Ca availability of specific ingredients is extremely difficult, and data must be extrapolated from total diets.
Ca absorption is usually measured using digestion experiments in which apparent Ca absorption equals Ca intake minus fecal Ca, both expressed in g/d. Since fecal Ca includes both undigested feed and metabolic fecal Ca, true Ca absorption is estimated by adding metabolic fecal Ca to apparently absorbed Ca. The absorption coefficient (AC) for a diet or feed is calculated as true Ca absorbed (g/d) divided by Ca intake (g/d).
Previous committees that authored Nutrient Requirements of Dairy Cattle publications (NRC, 1971, 1978, 1989) used a single AC for all diets. The 1971 and 1978 publications assumed an AC of 0.45, whereas the 1989 publication used 0.38. The AC was reduced in the 1989 Nutrient Requirements of Dairy Cattle partly in response to reports that cows in early lactation were less able to utilize dietary Ca (Ramberg, 1974; van't Klooster, 1976), making use of a lower coefficient prudent. The 0.38 AC was based largely on a summary of 11 experiments with lactating dairy cows in which the average apparent absorption of dietary Ca was 0.38 (Hibbs and Conrad, 1983). In the majority of these 11 experiments, cows were fed diets well in excess of their Ca requirements, but in 3 of the experiments, the cows were in negative Ca balance, and the AC was still less than 0.40. In those experiments, alfalfa and brome hay supplied most of the Ca. The 2016 Nutrient Requirements of Beef Cattle uses a mean true Ca availability from the diet of 0.50, and the INRA system (INRA, 2018) uses values between 0.30 and 0.55. The 2001 Dairy NRC adopted a system where Ca availability was based on AC of individual feeds rather than using a single dietary average because availability of Ca from forages and individual mineral supplements varies widely (Hansard et al., 1957; Ward et al., 1972; Martz et al., 1990).
To accommodate a system based on AC of individual feeds, AC based on work of Hansard et al. (1957) and summaries of the relative availabilities of mineral supplements compared to either calcium chloride (CaCl2) or calcium carbonate (CaCO3) were adopted. A major factor limiting Ca absorption is the solubility of the Ca from the mineral source, and CaCl2 represents a source of highly soluble Ca. In NRC (2001), CaCl2 was assigned an AC of 0.95. That value was based on studies in which 45CaCl was used as a tracer to measure Ca absorption by young calves (Hansard et al., 1954). Hansard et al. (1957) demonstrated that CaCl2 is between 1.2 and 1.32 times more absorbable than CaCO3. Therefore, the efficiency of absorption of Ca from CaCO3 was set at 75 percent. Finally, a list of common supplemental sources of Ca and an estimate of the efficiency of absorption of Ca from each source were developed using data summarized by Soares (1995a) based on their efficiency of absorption relative to CaCO3. In summary, ACs for all mineral supplements were based the assumption that CaCl2 had an AC of 0.95. Unfortunately, there were very little data in the literature at the time with diets fed at near estimated Ca requirements to determine whether the adopted ACs agreed with actual measured values. However, since the 2001 publication, several appropriate experiments have been conducted.
To evaluate NRC (2001) ACs, 45 treatment means from experiments with high-producing cows in early lactation were assembled (Wohlt et al., 1986; Martz et al., 1999; Knowlton and Herbein, 2002; Moreira et al., 2009; Taylor et al., 2009). Apparent Ca absorption from those studies was converted to true Ca absorption by subtracting metabolic fecal Ca from fecal Ca output (metabolic fecal Ca, g/d = 0.9 × DMI, kg/d). Diet ingredient information from those experiments was entered into NRC (2001) software to generate a predicted AC for Ca for each diet, and those were compared with the actual measured values.
The mean 2001 NRC predicted and actual true ACs (based on measured apparent absorption and estimated metabolic fecal Ca) were 0.60 and 0.45, respectively. Interestingly, the mean (± SD) measured value (0.45 ± 0.047) for true Ca absorption was identical to the average value adopted by the 1971 and 1979 committees (NRC, 1971, 1978). The range in Ca concentration in the diets summarized was from 0.17 to 1.03 percent, and it is known that excess Ca intake will reduce Ca absorption. However, the within-study change in true Ca availability was from 0.8 to 5.9 g/100 g Ca intake, which is equivalent to a reduction in the ACs of 0.008 to 0.059 units/100 g Ca intake. This suggests that the effect of Ca intake on absorption was small. The predicted intercept at zero Ca intake was 50.2 (± 1.5) equivalent to a true AC of 0.50 and varied little among studies. These observations suggest that the previously adopted ACs for feedstuffs and mineral supplements were too high.
Part of the overprediction of Ca absorption likely stemmed from the use of 0.95 true AC for CaCl2 based on values observed in young (<30 days old) calves prior to weaning (Hansard et al., 1954). In that same study, subsequent measurements of absorption from CaCl2 were much lower in animals ranging in age from 6 to 190 months. The measured AC for Ca from CaCl2 was 0.60 and 0.53 in young and mature steers, respectively (Hansard et al., 1957). The relative availability of CaCO3 was based on the 0.95 AC of CaCl2 (which was too high), and the ACs for other mineral supplements were based on their availability relative to CaCO3, resulting in an overestimation of the Ca availability for all mineral supplements.
To correct the observed overprediction of Ca availability, the ACs for each of the feed classes and mineral supplements were reviewed and adjusted where deemed appropriate. A comparison of adjusted AC to the other coefficients and literature values (Hansard et al., 1957; NRC, 2001; Kiarie and Nyachoti, 2010) is shown in Table 7-1. The availability of CaCl2 was reduced from 0.95 to 0.60, which is more in line with measured values in functioning ruminants (Hansard et al., 1957). For most supplements, the ACs were reduced by about 25 percent. In the 2001 NRC, the AC for corn silage was set at 0.60, but the mean measured value across three treatments (Martz et al., 1990, 1999) was 0.425. Therefore, a more conservative value of 0.40 was assigned. Legume silages and hays because of their high Ca concentration can be significant contributors of Ca, but for reasons discussed above, the AC was held at 0.30. For all other forages, the AC was raised to 0.40, a value that is more consistent with values observed for grasses and hays reported by Hansard et al. (1957). The coefficient for cereal grains and protein supplements was maintained at 0.60. Although there is little experimental basis for assigning this value, these feedstuffs are generally low in Ca and are only minor contributors to overall Ca intake. Most nonforage feedstuffs will contain only small amounts of Ca. A notable exception is for Ca soaps of palm oil fatty acids (FAs), which can be 7 to 9 percent Ca. Although the FAs in this product are approximately 76 percent digestible and digestion can only occur following dissociation of the Ca from the palmitate in the small intestine, it is not likely that Ca absorption would exceed that of CaCl2, so an availability of 0.60 was adopted. The accuracy of the new ACs was examined by entering the values in the 2001 NRC software for each of the feed ingredients in the diets from experiments with high-producing cows in early lactation (Wohlt et al., 1986; Martz et al., 1999; Knowlton and Herbein, 2002; Moreira et al., 2009; Taylor et al., 2009) and then comparing true Ca absorption (measured apparent plus estimated metabolic fecal Ca) with predicted absorbed Ca (see Figure 7-1). Both sets of coefficients were correlated with measured Ca absorption. As discussed, use of the 2001 NRC ACs overpredicted Ca absorption at high intakes of absorbed Ca (slope = 0.67). After adjustment of the ACs (see Table 7-1), measured and predicted Ca absorption were in good agreement where the slope (0.97) and intercept of the predicted versus actual regression equation did not differ from 1 and 0, respectively. The Ca-to-phosphorus (P) ratio was once thought to affect absorption of Ca and P, but data suggest that the ratio is not critical, unless the ratio is >7:1 or <1:1 (ARC, 1980; Miller, 1983) and the model does not adjust AC for that ratio.
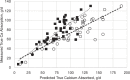
FIGURE 7-1
Comparison of true Ca absorption (g/d) (measured apparent absorption adjusted for estimated metabolic fecal Ca) versus predicted 2001 NRC values (open circles) and the adjusted (solid squares) absorption coefficients for individual feeds as shown in Table (more...)
TABLE 7-1Revised AC for Ca from Mineral Supplements and Feed Ingredient Classes
| AC of Ca | ||||
|---|---|---|---|---|
| Mineral Element Source | 2001 Dairy NRC AC of Primary Element | Adjusted ACs | Hansard et al. (1957) AC | Kiarie and Nyachoti (2010) Literature AC |
| Calcium Sources | ||||
| 0.95 | 0.60 | 0.61 | — |
| 0.75 | 0.50 | 0.46 | 0.59 |
| 0.95 | 0.60 | 0.57 | 0.63 |
| 0.95 | 0.60 | — | 0.63 |
| 0.55 | 0.60 | — | — |
| 0.50 | 0.33 | — | — |
| 0.95 | 0.60 | 0.56 | 0.55 |
| 0.70 | 0.60 | — | — |
| 0.70 | 0.45 | — | — |
| 0.94 | 0.60 | 0.47 | 0.73 |
| 0.60 | 0.35a | — | 0.50 |
| 0.70 | 0.45 | 0.41 | 0.50 |
| 0.70 | 0.45 | — | — |
| 0.75 | 0.50 | — | — |
| 0.70 | 0.45 | — | — |
| 0.30 | 0.22 | — | — |
| 0.30 | 0.22 | — | 0.48 |
| 0.30 | 0.22 | — | — |
| 0.86 | 0.55 | 0.52 | 0.57 |
| 0.30 | 0.30 | 0.36 | 0.58 |
| 0.60 | 0.40 | — | 0.52 |
| 0.30 | 0.40 | 0.45 | 0.04 |
| 0.30 | 0.40 | 0.45 | — |
- a
Absorption value for dolomite from Gerken and Fontenot (1967).
Although adjustments in the ACs improved the accuracy of prediction of Ca absorption, the committee recognizes that there is a dearth of measured availability data on individual feeds and mineral supplements. This is particularly important for comparing mineral supplements and for feeds that can provide substantial dietary Ca such as legume forages and canola meal. Major sources of variation for the AC of supplements have not been quantified. For example, particle size of limestone can affect rumen pH (Keyser et al., 1985), but it is unknown whether particle size (within typical ranges) affects the AC. The increased availability of stable isotopes of Ca such as 42Ca and 44Ca and higher sensitivity of mass spectrometer measurements should allow for improved estimates of Ca availability and metabolic fecal Ca losses.
Effects of Physiologic State
The amount of available Ca that will be absorbed varies with the physiologic state of the animal. Hansard et al. (1954) and Horst et al. (1978) reported that the efficiency of absorption of Ca decreases markedly as animals become older. As animals age, there is a decline in vitamin D receptors in the intestinal tract (Horst et al., 1990), which is thought to reduce the ability to respond to 1,25-dihydroxyvitamin D. The difference in efficiency of Ca absorption in beef steers from 1 to 6 years of age is nearly negligible (Hansard et al., 1954). Age was not included as a factor to adjust dietary Ca requirement in cattle >200 kg BW.
In early lactation, nearly all cows are in negative Ca balance (Ellenberger et al., 1931; Ender et al., 1971; Ramberg, 1974). As feed and Ca intake increase, most cows transition into positive Ca balance about 6 to 8 weeks into lactation (Ellenberger et al., 1931; Hibbs and Conrad, 1983). Cows in the first 10 days of lactation are at greatest risk of being in negative Ca balance (Ramberg, 1974), and many are subclinically hypocalcemic during this period (Reinhardt et al., 2011). Ramberg (1974) reported that the rate of entry of Ca into the extracellular fluid pool from the intestine increased about 1.55-fold from the day before parturition until 10 days in milk. Thereafter, the rate of entry of Ca into the extracellular pool from the intestine was constant. A study by van't Klooster (1976) demonstrated that Ca absorption increased from 22 percent in late gestation to 36 percent by day 8 of lactation, after which it remained relatively constant. This represented a 1.6-fold increase in efficiency of Ca absorption over this 8-day period. Regression analysis of data of Ward et al. (1972) predicted that cows need to be fed 5 g Ca/kg milk in early lactation to avoid negative Ca balance. However, there was no evidence to demonstrate that negative Ca balance in early lactation was detrimental to the cow provided the concentration of Ca in plasma remained normal (i.e., lactational osteoporosis ensures adequate entry of Ca from bone into the extracellular Ca pool).
Calcium Deficiency
A deficiency of dietary Ca in young animals leads to a failure to mineralize new bone and contributes to retarded growth. Rickets is more commonly caused by a deficiency of vitamin D or P, but a deficiency of Ca can contribute to rickets as well. In older animals, a deficiency of dietary Ca forces the animal to withdraw Ca from bone, which causes osteoporosis and osteomalacia, making bones prone to spontaneous fractures. The concentration of Ca in milk is not altered even during a severe dietary deficiency of Ca (Becker et al., 1933).
Excess Dietary Calcium
Feeding excessive dietary Ca is generally not associated with any specific toxicity. The maximum tolerable level (MTL) for Ca in ruminants was set at 1.5 percent of dietary dry matter (DM; NRC, 2005). Feeding excessive Ca could interfere with trace mineral absorption (especially zinc [Zn] and selenium [Se]) and dilutes energy and protein the animal might better utilize for increased production.
Phosphorus
Physiologic Roles
P has more known biological functions than any other mineral. About 80 percent of the body's P is in bones and teeth principally as apatite salts and as calcium phosphate. It is in every cell of the body, and almost all energy transactions involve formation or breaking of high-energy phosphate bonds (such as those in adenosine triphosphate [ATP]). Phosphorylation is a primary regulator of numerous enzymes. P also is involved in acid-base balance of blood and other bodily fluids, as well as in cell differentiation, and is a component of cell walls and cell contents as phospholipids and nucleic acids.
Normal P concentration in blood plasma of dairy animals is 1.3 to 2.6 mmol/L (4 to 8 mg/dL), and whole blood concentrations are six to eight times greater (Goff, 2004). Plasma concentrations decrease with increasing age and are lower in early lactation than later lactation (Forar et al., 1982). For a 600-kg cow, approximately 1 to 2 g of inorganic phosphate is circulating in blood plasma, 5 to 8 g of inorganic P is in the extracellular pool, and total intracellular P is about 155 g (Goff, 1998). The intracellular concentration of P is about 10 times greater than the concentration in plasma (Goff, 1998).
Rumen microorganisms also require P (Burroughs et al., 1951; Breves and Schröder, 1991), and it is supplied to the rumen by the diet and recycling via saliva. Using various techniques, estimates of P recycling in lactating dairy cows fed adequate or excess P range from about 30 to 75 g/d (Kebreab et al., 2005; Puggaard et al., 2011). Inadequate supply of P to the rumen can reduce fiber digestibility. A diet with 0.24 percent P had lower neutral detergent fiber (NDF) digestibility than a similar diet with 0.34 percent P when fed to dairy cows (Puggaard et al., 2011). Digestibility of NDF was reduced even though the concentration of P in rumen fluid was 3 to 4 mmol/L, which is greater than the concentrations (0.6 to 2.5 mmol/L) that maximized cellulose digestibility in vitro (Hall et al., 1961; Chicco et al., 1965). However, no evidence is available showing improved ruminal digestion once the cow's P requirement is met.
Phosphorus Homeostasis
Blood plasma P concentrations are controlled via alterations in intestinal absorption, P recycling via saliva, renal excretion, and bone resorption. Absorption of P from the intestines is much less regulated than absorption of Ca. Net absorption of P occurs mainly in the small intestine (Grace et al., 1974; Reinhardt et al., 1988), with only small amounts absorbed from the rumen, omasum, and abomasum. Absorption is thought to occur mainly in the duodenum and jejunum (Care et al., 1980; Scott et al., 1984); however, little is known about absorption anterior to the small intestine (Breves and Schröder, 1991). Presumably, as in nonruminants, absorption occurs via two distinct mechanisms. A saturable vitamin D–dependent active transport system is operative when animals are fed low P diets. Synthesis of 1,25-dihydroxyvitamin D can be stimulated when blood P is low, resulting in more efficient absorption (Horst, 1986). Feeding 25-hydroxyvitamin D increases circulating 1,25-dihydroxyvitamin D and plasma P concentrations (Wilkens et al., 2012; Weiss et al., 2015b), which could indicate increased intestinal absorption of P. Passive absorption predominates when adequate or excessive amounts of potentially absorbable P are consumed, and absorption is proportional to the concentration gradient between the lumen of the small intestine and blood plasma (Wasserman and Taylor, 1976). However, data suggest that this process is saturable (Mogodiniyai Kasmaei and Holtenius, 2013), and current ruminant P models use Michaelis–Menten kinetics to describe intestinal absorption (Kebreab et al., 2004; Hill et al., 2008).
Renal clearance is usually a minor contributor to P homeostasis, but both urinary concentration and excretion of P increase as the supply of absorbable P to dairy cows increases (Knowlton and Herbein, 2002; Guyton et al., 2003; Knowlton et al., 2005; Puggaard et al., 2011; Mogodiniyai Kasmaei and Holtenius, 2013). The increase in urinary P excretion as P intake increases is usually less than 10 percent of the increase in P intake. P recycling via saliva is the major homeostatic mechanism for P (Breves and Schröder, 1991). P absorbed from the intestine in excess of requirement elevates blood P, which is then transferred to saliva and reenters the rumen. Salivary and plasma concentrations of P have a strong positive correlation (Valk et al., 2002), but the mass of P recycled via saliva is not necessarily correlated with plasma P concentrations when cows are fed marginal amounts of P (Puggaard et al., 2011). Recycled P can be used by ruminal microorganisms; a portion of it will be reabsorbed by the intestines, and a portion will pass out in feces. Fecal excretion of recycled P is one reason why apparent absorption of P does not reflect true absorption of dietary P.
Requirements for Absorbed Phosphorus
As described previously (NRC, 2001), the factorial approach was used to estimate the requirement for absorbed P by summing requirements for maintenance, growth, pregnancy, and lactation.
Maintenance
The maintenance requirement of P is the endogenous fecal loss (inevitable fecal loss) plus endogenous urinary loss when P supply just meets the true requirement. Previously (NRC, 2001), endogenous urinary P was estimated as 2 mg P/kg BW. However, studies with dairy cows, steers, and goats fed diets that were at or below requirements consistently reported lower losses of P in urine than estimated by that equation (Bortolussi et al., 1996; Rodehutscord et al., 2000; Knowlton and Herbein, 2002; Kebreab et al., 2005; Puggaard et al., 2011). In those studies, urinary loss of P ranged from 0.2 to 0.9 mg/kg BW (mean = 0.5; SD = 0.28). For the three studies using lactating dairy cows, the range was 0.24 to 0.58 mg P/kg BW (mean = 0.4; SD = 0.17). Endogenous urinary P loss was set at 0.6 mg absorbed P/kg BW (i.e., the highest reported endogenous urinary P loss in dairy cows).
About half of the inevitable fecal loss of P is associated with microbial debris and purines and pyrimidines of nucleic acids. The other portion of endogenous fecal P includes sloughed cells, digestive secretions, and unabsorbed recycled P. As discussed previously (NRC, 2001), endogenous fecal loss should be expressed as a function of DMI. Although the amount of endogenous P derived from microbes may be more related to intake of fermentable organic matter than DMI (Rodehutscord et al., 2000), accurately estimating fermentable matter is difficult. Any gain in accuracy in estimating endogenous fecal P by calculating it from intake of fermentable matter may be lost by the error associated with estimating fermentability. Therefore, endogenous fecal P was estimated from DMI as done previously (NRC, 2001).
Based on data from growing bulls and steers (Bortolussi et al., 1996; Klosch et al., 1997), NRC (2001) set the absorbed P requirement for endogenous fecal P for growing cattle at 0.8 g/kg DMI. Using data from experiments (Speikers et al., 1993; Valk et al., 2002; Puggaard et al., 2011) in which lactating cows were fed at or below estimated P requirements, fecal excretion ranged from 0.95 to 1.4 g P/kg DMI (mean = 1.2, SD = 0.14 from 14 treatment means). If the absorbability of dietary P is assumed to equal 0.80, then endogenous fecal P equals 1.0 g/kg DMI, which is identical to the endogenous fecal P requirement from NRC (2001). Myers and Beede (2009) varied DMI of lactating dairy cows over a wide range and measured inevitable fecal loss of P. For cows fed ad libitum (ca. 25 kg/d DMI), endogenous fecal loss equaled 1.04 g P/kg DMI. In that study, inevitable fecal loss of P increased to 1.36 and 1.19 g P/kg DMI when intake was restricted to 50 and 75 percent of ad libitum DMI, respectively. The severe DMI restriction imposed likely affected feeding behavior, rate of eating, rumination time, and so on, which could affect salivary flow and intestinal secretion, so the data from cows fed the restricted treatments were not used in establishing endogenous fecal requirement. Limited data are available regarding endogenous fecal P excretion by dry cows. Based on isotope dilution, endogenous fecal P was 0.4 g P/kg DMI for a corn silage, corn cob diet and 0.5 g P/kg DMI for a diet with 90 percent corn silage (Martz et al., 1999). With more practical diets, endogenous fecal P for dry cows fed low P diets (assumed true absorption of dietary P was 0.8) averaged 1 g of absorbed P/kg DMI (two treatment means: 0.92 and 1.07 g/kg DMI) (Valk et al., 2002). The almost 2-fold difference in estimated endogenous fecal P between those methods is difficult to explain but was not caused by differences in DM digestibility (similar between studies). Because of the atypical diets used in the Martz et al. (1999) study, the data from Valk et al. (2002) were used to set the endogenous fecal P requirement for dry cows at 1.0 g absorbed P/kg DMI (i.e., the same as for lactating cows).
Maintenance requirement for absorbed P (endogenous fecal and urinary losses):
Growth
The requirement for growth is the amount of absorbed P accreted in soft tissues plus that deposited in skeletal tissue. Skeletal growth comprises a larger portion of live weight gain in younger heifers than in older heifers; therefore, the grams of P required per kilogram of growth are higher in younger animals. The 2016 beef cattle requirement (NASEM, 2016) for retained P was set at 3.9 g/100 g of retained protein. Because younger animals deposit greater amounts of protein/kg of ADG, this approach will result in higher P requirements for younger animals compared with older animals. The main problem with that approach is that protein concentration in live weight growth must be known or estimated accurately. Therefore, the allometric equation used previously (NRC, 2001) was retained:
where MatBW and BW are in kg; BW is current body weight, kg; and ADG is average daily gain, kg/d.
Pregnancy
No new data are available on conceptus P accretion; therefore, the NRC (2001) pregnancy requirement was retained. Quantitatively, the requirement for P for pregnancy is low until the last trimester. House and Bell (1993) measured accretion of P in conceptuses (fetus, fetal fluids, and membranes, placentomes, and uterine tissues) of 18 multiparous Holstein cows slaughtered at varying times from 190 to 270 days of gestation. Changes in fetal mass and P content across the sampling period were similar to earlier data (Ellenberger et al., 1950). The requirement for absorbed P to meet demands of the conceptus for any day beyond 190 days of gestation is
where t is day of gestation (House and Bell, 1993). The average cow weighed 715 kg in that study; therefore, the requirement was scaled to 715 kg.
Estimates of rates of P accretion in conceptuses of Holstein cows increase from 1.7 g/d at 190 to 5.4 g/d at 280 days of gestation. The P requirement of the conceptus at <190 days was set to zero in the model.
Lactation
The daily requirement for absorbed P for lactation is equal to the amount of P secreted in milk daily. Mean (e.g., treatment groups or farms) P content of milk ranged from 0.83 to 1.00 g/kg (Speikers et al., 1993; Wu et al., 2000; Castillo et al., 2013). For individual cows, milk concentration ranged from about 0.7 to 1.2 g P/kg (Klop et al., 2014). NRC (2001) used a value of 0.90 g P/kg of milk, and newer data (Klop et al., 2013) support that value. Concentrations of protein and P in milk are correlated, and milk P can be estimated from milk protein (Klop et al., 2013). The lactation requirement for absorbed P (g/d) is set at
Using Equation 7-8b (Klop et al., 2013) with an average milk true protein of 3.1 percent yields an estimated milk concentration of 0.88 g P/kg.
Dietary Requirement and Efficiency of Absorption
The dietary requirement is the total requirement for absorbed P divided by the AC for P from the diet. The use of feed (or feed class)–specific AC was introduced in NRC (2001), and that approach has been expanded. The AC for P in NRC (2001) was set at 0.64 for all forages except corn silage and 0.70 for all other feeds. The AC for P supplements ranged from 0.30 to 0.90, and the AC for total diets (weighted average from the dietary ingredients) was usually around 0.70.
To accurately determine the AC for a specific feedstuff or mineral source, P must be fed in an amount close to the animal's true requirement, and P recycling must be accurately quantified. Most studies do not satisfy these experimental specifications. Furthermore, even simple diets will contain multiple sources of P, and accurately partitioning the overall dietary AC into AC for ingredients is not possible. In the previous edition (NRC, 2001), the AC for P for all feedstuffs other than mineral supplements was based on data for alfalfa hay and corn silage. An alternative to assuming all feedstuffs have essentially the same AC is to analytically partition dietary P into fractions and estimate the AC for each fraction via modeling (Hill et al., 2008; Feng et al., 2015, 2016). This is the approach used for basal ingredients (described below).
The AC for P supplements from NRC (2001) was retained because newer data are not available. These values were tabulated from Soares (1995b), Peeler (1972), and other sources in the literature and are used in the model. Values determined using ruminants, especially cattle, were given preference whenever possible in tabulation. Dicalcium phosphate (calcium phosphate dibasic) with an AC of 0.75 in cattle (Tillman and Brethour, 1958; Challa and Braithwaite, 1988), phosphoric acid with an AC of 0.90 in cattle (Tillman and Brethour, 1958), and monosodium phosphate with an AC of 0.90 in sheep (Tillman and Brethour, 1958) were taken as reference standards. The ACs of P in other mineral sources were set based on these reference standards (Soares, 1995b).
Form of Dietary Phosphorus
For the current model, feed P is analytically partitioned into inorganic P (blue molybdovanadate method; AOAC, 2000) and organic P (total P − inorganic P). A model on P metabolism and absorption (Hill et al., 2008; Feng et al., 2015, 2016) also included a phytate P fraction, but its absorption coefficient was similar to that of the nonphytate organic P fraction (0.66 versus 0.7). Therefore, those two fractions were combined into organic P, which simplifies analytical requirements. Using the above P model, the AC is 0.84 for inorganic P and 0.68 for organic P fraction (i.e., average for phytate and nonphytate organic P).
The weighted average AC is then calculated based on the size of the two P fractions, which is the AC values in the feed library. For feeds that did not have P fraction data, AC values from similar feeds were used, or the AC was set at the default of 0.72. Additional analytical data are needed regarding P fractions of different feedstuffs. Feeds can be assayed for total P and inorganic P and those values entered in the feed library, but at the time of publication, most commercial labs did not conduct those assays. Factors other than form of P can affect AC; however, these effects have not been adequately quantified and cannot be modeled.
Phosphorus Intake
Although not as tightly regulated as Ca, true absorption of P decreases as P intake increases above requirements (Challa and Braithwaite, 1988; Challa et al., 1989; Martz et al., 1999); however, adequate data are not available to accurately quantify that effect. Because salivary P typically supplies at least 2-fold greater amounts of P to the lumen of the small intestine than does dietary P, the efficiency of absorption of salivary P is important. Salivary P is in the form of sodium and potassium phosphate salts. The AC of salivary endogenous P recycled to the small intestine was 0.68 to 0.81 in bull calves (Challa et al., 1989). Excessive dietary P relative to the requirement reduced the efficiency of absorption of inorganic or salivary P (Challa et al., 1989). The AC shown in Table 19-3 for mineral supplements and the AC values used for the various P fractions outlined above should be considered maximum absorption. If P is fed in excess of requirements, those ACs will overestimate actual absorption; however, because this occurs once the P requirement is met, it will not affect the amount of dietary P needed to meet requirements for absorbed P.
Use of Phytase
Phytate phosphorus (inositol polyphosphate) is the common storage form of P in many plants and usually comprises the largest proportion of organic P in concentrates (Nelson et al., 1968; Morse et al., 1992). Forages (or vegetative matter) usually have low concentrations of phytate. Normal ruminal metabolism breaks down most of the phytate; however, exogenous phytase can increase phytate breakdown in the rumen (Brask-Pedersen et al., 2013). Feeding supplemental phytase to dairy cows has not consistently reduced fecal excretion of P, and most studies reported no effect (Guyton et al., 2003; Kincaid et al., 2005; Knowlton et al., 2005; Knowlton et al., 2007).
Dietary Calcium
When cows are fed P at or above requirements, Ca intake ranging from deficient to excess usually has not affected efficiency of P absorption (Hibbs and Conrad, 1983; Moreira et al., 2009; Taylor et al., 2009; Herrera et al., 2010). Solubility of supplemental Ca (CaCl2 versus limestone) did not affect P absorption by dairy cows (Herrera et al., 2010). However, Ca and P apparent digestibility are positively correlated (Hibbs and Conrad, 1983).
Animal Responses to Varying Dietary Phosphorus
Production responses by growing and lactating cattle to differing dietary P concentrations were reviewed in the previous edition; therefore, only more recent studies will be reviewed in detail in this version. In growing heifers, diets with 0.3 to 0.34 percent P generally resulted in maximum gain, adequate blood P concentrations, and adequate bone strength compared with animals fed diets with lower concentrations of P (NRC, 2001). Newer data support that conclusion. A study with Holstein and Holstein × Jersey crossbred heifers that started at 4 months of age and ended at 22 months of age found no differences in growth (weight and stature), reproductive measures, or bone strength between heifers fed 0.3 or 0.4 percent P (Esser et al., 2009; Bjelland et al., 2011).
The review conducted previously (NRC, 2001) concluded that for lactating cows, diets with 0.32 to 0.42 percent P for the entire lactation were sufficient depending on milk yield potential. Furthermore, they concluded that no benefits on lactational performance occurred when cows were fed diets with >0.42 percent P. Because of the ability to mobilize P from bone, longer-term performance studies evaluating effects of differing concentrations of dietary P on lactating cows are more meaningful than short-term studies. Newer studies lasting from 9 weeks to two lactations largely support the conclusions reached by the previous committee. Grazing dairy cows were fed diets with approximately 0.22 or 0.31 percent P starting at about 30 days in milk through about 90 days in milk (Reid et al., 2015), and no effects on milk yield, milk composition, or feed intake were observed. Dietary P concentration of 0.33 or 0.42 percent did not affect milk yield (35.1 versus 35.4 kg/d), DMI, or milk composition of mid-lactation Holstein cows fed diets for 14 weeks (Wu et al., 2003). However, Holstein cows fed diets with 0.32 percent P had reduced yields of fat-corrected milk (40.3 versus 44.3 kg/d) and DMI (25.0 versus 26.5 kg/d) compared with cows fed diets with 0.44 percent P for 10 weeks. The diets with 0.32 percent P did not meet the P requirement based on the current model. In a 23-week experiment (Lopez et al., 2004a,b, DMI, milk yield (35.1 versus 34.9 kg/d), milk composition, health disorders (except occurrence of eye inflammation, which was statistically greater in cows fed high P), and reproductive measures did not differ between Holstein cows fed diets with 0.37 or 0.57 percent P starting immediately after parturition. Similar results were obtained when Swedish Red and White cattle were fed diets with 0.32 or 0.42 percent P during the first 4 months of lactation (Ekelund et al., 2006). Based on bone markers, cattle in both groups exhibited bone P resorption, but resorption was similar between treatment groups.
Two multilactation studies evaluated effects of varying dietary P concentrations on long-term health and production of dairy cows. In one study, dairy cows (breed not reported, approximately 600 kg BW) were fed diets with 0.24, 0.28, or 0.33 percent starting in mid-lactation and continuing through a dry period and then for the entire next lactation and the subsequent dry period (Valk and Sebek, 1999). No treatment effects were observed in the first lactation period on milk yield (26.8, 25.9, and 27.5 kg/d, respectively), milk composition, or DMI. During the first dry period, cows fed the lowest P diet had reduced DMI. During the second lactation, cows fed the lowest P diet produced significantly less milk, consumed less DM, and were losing BW, and because of animal welfare concerns, that treatment was terminated after cows were on the treatment for approximately 12 months. No differences in milk yield (33.0 versus 34.1 kg/d), DMI, milk composition, or BW were observed between cows fed 0.28 or 0.33 percent P during the second lactation of the experiment. In another study, milk production (36.4 versus 35.4) and milk composition did not differ between Holstein cows fed diets with 0.35 or 0.42 percent P (Odongo et al., 2007) over two lactations. However, first-lactation, but not multiparous, cows fed the low P diet had lower DMI than first-lactation cows fed 0.42 percent P. BW and body condition were also lower for first-lactation cows fed the low P diet, indicating 0.35 percent dietary P was not adequate for first-lactation cows. Data from that experiment could not be evaluated with the current model because adequate parity data were not included in the study. But overall, data from longer-term production studies support the P requirements calculated using the current model.
Phosphorus and Reproduction
The previous edition (NRC, 2001) reviewed published research reports from 1923 through 1999 to assess the effects of dietary P on reproductive performance of cattle, and studies published after 1999 have been added to this review. In some studies, but not all, severe deficiency of dietary P caused infertility or reduced reproductive performance of cattle (Alderman, 1963; Morrow, 1969; McClure, 1994). Typically, P concentration was <0.20 percent of dietary DM, the deficient diet was fed for an extended length of time (1 to 4 years), and where measured, feed intake was depressed, causing coincidental deficiencies of energy, protein, and other nutrients. Low body condition generally is considered the main cause of reduced reproductive efficiency in P-deficient cows (Holmes, 1981). Little (1975) demonstrated that deficiencies of P and protein were additive on failure to exhibit first postpartum estrus in grazing multiparous beef cows.
In growing heifers, experimentally induced reproductive failure caused by a dietary P deficiency has been very difficult to produce. The studies reviewed by NRC (2001) reported no adverse effects on reproduction in heifers when they were fed diets with as little as 0.15 percent P for several months (some studies lasted more than 1 year). With lactating dairy cows, evidence from available research to support feeding P in excess of requirements to improve reproduction is virtually nonexistent. Results of 10 studies can be summarized very succinctly (Steevens et al., 1971; Carstairs et al., 1980; Call et al., 1987; Brodison et al., 1989; Brintrup et al., 1993; Valk and Sebek, 1999; Wu and Satter, 2000; Wu et al., 2000; Lopez et al., 2004a,c Odongo et al., 2007). All measures of reproductive performance compared within each study were not affected by the concentration of dietary P with one exception. In the study by Steevens et al. (1971), services per conception were greater in the second year for cows fed 0.40 versus 0.55 percent P, but not in the first year of study. Among these seven studies, dietary P ranged from 0.24 to 0.62 percent of dietary DM, length of feeding different dietary P concentrations ranged from the first 12 weeks of lactation to as long as three consecutive lactations, and average milk yields ranged from 15 to 37 kg/d. As long as dietary P was greater than 0.31 percent, reproductive performance was normal and not improved with increased concentrations of P. Cows in some of the studies would not be considered high-producing cows by modern standards. However, the more recent studies used cows producing more than 35 kg, and no effects of dietary P on reproduction were observed in those studies. The preponderance of data does not support feeding dietary P at concentrations in excess of those needed to meet dietary requirements to improve reproductive performance.
Phosphorus Deficiency
Detailed description of occurrence, etiology, clinical pathology, diagnosis, and treatment of P deficiency in ruminants has been described (Goff, 1998). Signs of deficiency may occur rather quickly if dietary P is insufficient. Deficiency is most common in cattle grazing forages on soils low in P or in animals consuming excessively mature forages or crop residues with low P content. Dairy cows do not seem to have the ability to self-select appropriate intakes of P or other minerals (Muller et al., 1977). Hypophosphatemia can also occur when a cow develops a displaced abomasum (Grünberg et al., 2005). Nonspecific chronic signs of deficiency include unthriftiness, inappetence, poor growth, and reduced milk yields, but signs are often complicated by coincidental deficiencies of other nutrients such as protein or energy. Animals may be chronically hypophosphatemic (<4 mg/dL in plasma), but the concentration of P in milk remains within the normal range. Hemoglobinuria (Jubb et al., 1990) and liver dysfunction (Grünberg et al., 2005) are associated with hypophosphatemia. In severe deficiency cases, bone mass is lost, and bones become weak. Severe clinical manifestations of P deficiency include acute hypophosphatemia, rickets in young growing animals, and osteomalacia in adults. Cows may also exhibit pica.
Acute hypophosphatemia (less than 2 mg P/dL of plasma) may occur when cows are fed marginally low dietary P and challenged by extra demand for P in late pregnancy with accelerated fetal growth, especially with twin fetuses and with colostrum and milk formation during early lactation. The disease usually is complicated with concurrent hypocalcemia, hypomagnesemia, and possibly hypoglycemia.
Concentrations of P in plasma often fall below the normal range in the periparturient period (Grünberg, 2008). In other mammals, physiologic correction can occur rather rapidly as P absorption is responsive to renal production of 1,25-dihydroxyvitamin D, which is stimulated by low P in the blood (Reinhardt et al., 1988; Goff, 1998). Feeding peripartum dairy cows 25-hydroxyvitamin D increased plasma 1,25-dihydroxyvitamin D and elevated plasma P (Wilkens et al., 2012; Weiss et al., 2015b). Secretion of cortisol around parturition may depress concentrations of P in plasma. Intravenous Ca to correct hypocalcemia usually results in a rise in P in plasma because parathyroid hormone secretion is lowered, reducing urinary and salivary loss of P. It also stimulates resumption of gut motility, recycling of salivary P, and absorption. Oral or intravenous administration of a soluble form of P such as sodium monophosphate can help correct hypophosphatemia. In some cows with severe cases of clinical milk fever, protracted hypophosphatemia (P in plasma <1 mg/dL) occurs with recumbency; even with successful treatment for hypocalcemia, P in blood remains low. This disorder is not well understood. However, increasing the amount or concentration of P in the diet in excess of requirement in late pregnancy or early lactation will probably not correct hypophosphatemia in the periparturient period, as this disorder seems to occur secondary to hypocalcemia.
When young calves are fed P-deficient diets, rickets occurs from a failure of mineralization in osteoid and cartilaginous (growth plate) matrices during bone remodeling. In contrast, in mature animals (no active growth plates), osteomalacia occurs over time with P deficiency with failure of mineralization of the remodeled osteoid matrix. In the adult, P in bone released during remodeling is used to maintain concentrations of P in blood rather than being reincorporated into bone. In young animals, bone cartilage remains unmineralized, resulting in bone that can be flexed without breaking.
Maximum Tolerable Level
NRC (2005) set the MTL of P for cattle at 0.7 percent of diet DM. That concentration was chosen because studies feeding higher concentrations were lacking, not because data were available showing negative effects when cattle were fed diets with >0.7 percent P. Long-term feeding of excess P can cause problems with Ca metabolism, inducing excessive bone resorption and urinary calculi, secondary to the elevated concentrations of P in blood (NRC, 2005). Most often, P toxicity is complicated with low dietary Ca, but ruminants can tolerate a wide ratio of Ca-to-P as long as P and Ca are adequate. Supplemental phosphates given in large oral doses are not considered highly toxic but can result in mild diarrhea and abdominal distress. Dairy cattle are quite adept at excreting excess absorbed P to maintain concentrations of P in blood within a normal range via salivary secretion and fecal excretion (Challa et al., 1989). Urinary excretion of P also may increase, although its quantitative importance is small relative to fecal excretion. Feeding 0.69 percent P to Holstein–Friesian cows for 14 weeks prepartum through 22 weeks of lactation caused no problems or signs of toxicity (De Boer et al., 1981). In contrast, a meta-analysis determined that even moderate overfeeding of P during the prepartum period was a risk factor for hypocalcemia (Lean et al., 2006). High P intake (>80 g/d) by cows approaching parturition increased blood P and incidences of milk fever and hypocalcemia (Reinhardt and Conrad, 1980). High (0.64 percent versus 0.22 percent) dietary P reduced apparent absorption of magnesium (Mg) in pregnant dairy heifers (Schonewille et al., 1994).
Magnesium
Mg is a major intracellular cation that is a cofactor for enzymatic reactions in every major metabolic pathway. Extracellular Mg is vital to normal nerve conduction, muscle function, and bone mineral formation and is involved in Ca and P homeostasis. Low concentrations of serum Mg attenuate PTH release in response to low serum Ca (Takatsuki et al., 1980), and in humans and laboratory animals, low Mg status results in lower serum concentrations of 1,25-dihydroxyvitamin D and can result in vitamin D insensitivity and perhaps PTH insensitivity (Rude and Gruber, 2004; Sahota et al., 2006). The concentration of Mg in plasma of cows is normally between 0.75 and 1.0 mmol/L (1.8 and 2.4 mg/dL). In an adult cow, 60 to 70 percent of the body's Mg is in bone (200 to 250 g), a small amount is in the blood and other extracellular fluid (<4 g), and the remainder is inside cells (~90 g) (Storry and Rook, 1962). Bone is not a significant source of Mg that can be utilized in times of deficit. Maintenance of normal concentration of Mg in plasma is nearly totally dependent on absorption of dietary Mg.
Magnesium Requirement
A factorial approach was taken to describe the Mg requirements of dairy cattle.
Maintenance
Fecal loss of endogenous Mg was set at 0.3 g Mg/kg DMI as explained below. When cows display signs of clinical hypomagnesemia, urinary Mg loss is essentially zero, but for cows near the threshold of hypomagnesemia, urinary loss in adult dairy cows was approximately 0.0007 g Mg/kg BW (Schonewille et al., 2000b), which was set as the obligate urinary loss.
Growth
In heifers, the Mg content of the body decreases from about 0.65 g Mg/kg at birth to about 0.2 g/kg at 500 kg BW (Blaxter and McGill, 1956); therefore, the value of 0.45 g Mg/kg ADG used in the 2001 NRC is a reasonable average growth requirement.
Pregnancy
In pregnant animals, fetal-placental accretion of Mg is about 0.18 g/d in Holsteins from day 190 until the end of pregnancy (House and Bell, 1993). However, based on the Mg concentration in the body of a newborn calf (Blaxter and McGill, 1956), estimated accretion rate for Mg was about 0.3 g/d in late gestation. Considering the problems associated with hypomagnesemia at parturition, 0.3 g/d is used to describe the fetal requirement for Mg, and requirements are scaled to 715 kg maternal BW.
Lactation
Milk has an average Mg concentration of about 0.11 g (Hermansen et al., 2005; van Hulzen et al., 2009; Castillo et al., 2013). Colostrum contains about 0.38 g Mg/kg (see Chapter 12). Because cows have limited stores of labile Mg, diets for late-gestation cows must be formulated to provide adequate Mg for colostrum synthesis.
Summary of Equations (g absorbed Mg/d)
where DMI, ADG, and milk are in kg/d, and BW is in kg.
Absorption and Dietary Requirements
Dietary requirements, not absorbed requirements, are generally similar to NRC (2001); however, the previous version included a substantial safety factor. If a similar safety factor was included, dietary requirements would be approximately 25 percent greater than the previous version.
Mg is absorbed primarily from the small intestine of young calves. As the rumen and the reticulum develop, they become the main site for Mg absorption (Pfeffer et al., 1970; Martens and Rayssiguier, 1980), but some absorption may occur in the large intestine. In adult ruminants, the small intestine is a site of net secretion of Mg, but absorption may still occur in that site (Greene et al., 1983). Absorption of Mg from the rumen mostly occurs via two active mechanisms (Leonhard-Marek et al., 2010; Fach, 2015; Martens et al., 2018). One mechanism (potential difference-dependent uptake) is driven by an electrical gradient at the apical (luminal) membrane and is an active process inhibited by elevated potassium (K) concentrations in rumen fluid (Leonhard-Marek and Martens, 1996; Leonhard-Marek et al., 2010; Fach, 2015). This is a high-affinity, low-capacity transporter system. The second system (low affinity, high capacity) is driven by the Mg concentration gradient that can exist between the rumen contents and the epithelial cell and is independent of the electrical potential difference and not sensitive to K concentrations. This transport system is active and electrically neutral; therefore, it involves either cotransport of an anion (e.g., Cl− or HCO3−) or an exchange with intracellular protons (Leonhard-Marek et al., 2010; Fach, 2015). The exact mechanism is not known at this time.
Factors Affecting Absorption
Absorption of Mg does not appear to be under any type of hormonal regulation; excess absorbed Mg is filtered by the kidney and excreted. A major driver of Mg absorption is the gradient between intracellular Mg and rumen contents. An increase in Mg intake usually linearly increases the concentration of Mg in rumen fluid, which usually increases apparent and calculated true absorption of Mg (Jittakhot et al., 2004a,b,c). However, Mg absorption might be saturable. Increasing dietary Mg concentrations above 1.1 percent continued to increase the concentration of soluble Mg in rumen fluid but did not increase apparent or true absorption of Mg by dry dairy cows (Jittakhot et al., 2004b).
Martens et al. (2018) reviewed Mg absorption by ruminants and antagonists to absorptions in great detail. Dietary K is a significant antagonist to Mg absorption because ruminal K disrupts the electrical gradient needed to drive Mg absorption (Fisher et al., 1994; Ram et al., 1998; Schonewille et al., 1999, 2008; Jittakhot et al., 2004c; Weiss, 2004). Inadequate intake of Na increases the concentration of K in rumen fluid (Bailey, 1961; Martens et al., 1987) and reduces absorption of Mg (Martens et al., 1987). However, once the Na requirement is met, dietary Na does not appear to affect Mg absorption. High dietary P concentration (ca. 0.6 percent) reduced apparent Mg absorption in heifers by about 18 percent (Schnewille et al., 1994), but within typical dietary concentrations, effects of dietary P are probably small.
Abrupt elevation in concentrations of ruminal ammonia reduces Mg absorption; however, chronic elevation (i.e., several days) did not affect Mg absorption (Gäbel and Martens, 1986). High concentrations of ruminal ammonia reduce the electrical potential, but the change probably is not great enough to affect Mg absorption. The adaption response suggests the involvement of inducible transport proteins, and alteration of the Na/proton pump has been implicated (Fach, 2015). Dietary changes that cause an abrupt increase in ruminal ammonia (e.g., initial turnout onto high-protein pasture) should be avoided; however, once animals are adapted, high ruminal ammonia does not appear to affect Mg absorption.
Rumen pH is negatively correlated with Mg solubility and under in vitro and other experimental situations, a small drop in pH within the normal physiological range (6.5 to 5.5) has increased Mg solubility by more than 50 percent (Dalley et al., 1997). When rumen pH was reduced by more realistic diet manipulation (i.e., increased starch concentrations), ruminal Mg concentrations increased (Schonewille et al., 2000a), but the effect was less consistent than with in vitro systems. Furthermore, the effect of ruminal pH on absorption of Mg was less dramatic than changes in solubility (Horn and Smith, 1978). In addition to Mg solubility, pH may have direct effects on Mg absorption systems. In cell culture experiments, Mg permeability through a protein channel increased markedly as pH decreased below 7 (Li et al., 2007). Increasing the dietary concentration of readily fermentable carbohydrates can increase apparent absorption of Mg. Adding 30 percent starch to a diet increased apparent Mg absorption by 50 percent (0.37 versus 0.24) or 28 percent (0.25 versus 0.20) when goats were fed low (0.8 percent) or high (3.4 percent) K diets, respectively (Schonewille et al., 1997). However, apparent Mg absorption did not differ when dairy cows were fed diets with 10 or 20 percent starch (Schonewille et al., 2000a). More data with cattle are needed before the effects of dietary starch can be modeled.
Supplemental dietary fat can reduce apparent digestibility of Mg, but the reduction was not related to the concentration of supplemental fat in the diet (Jenkins and Palmquist, 1984; Rahnema et al., 1994). Apparent Mg absorption decreased about 20 percent when cattle were fed diets that contained 2.5 to 5 percent added fat compared with control diets with no added fat. Supplementing up to 5 percent added fat from whole cottonseed did not affect apparent Mg absorption (Smith et al., 1981). Although data are limited, assuming a 20 percent reduction in absorption of Mg when supplemental fat is fed is recommended but was not included in the software. Feeding ionophores increased apparent absorption of Mg by beef cattle and dairy cattle by 10 to 28 percent when magnesium oxide (MgO) was fed (Greene et al., 1986a; Spears et al., 1989; Tebbe et al., 2018). However, monensin reduced absorption of Mg by 23 percent when magnesium sulfate was fed (Tebbe et al., 2018). Effects of monensin on Mg absorption are not included in the model.
The availability of Mg from MgO is affected by particle size (smaller particles enhance absorption), calcination temperature, and origin (Jesse et al., 1981; van Ravenswaay et al., 1989; Xin et al., 1989; Hemingway et al., 1998). Particle size also likely affects Mg availability from magnesium carbonate (MgCO3), magnesium hydroxide (Mg(OH)2), and dolomitic limestone.
TABLE 7-2Description of Data Used to Generate Mg Equationsa
| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| 13.6 | 6.49 | 5.8 | 26.1 |
| 24.9 | 12.7 | 6.9 | 75.6 |
| 3.60 | 2.75 | 1.08 | 17.3 |
| 42.6 | 23.4 | 11.8 | 124.3 |
| 27.0 | 28.1 | 0 | 90 |
| 0.26 | 0.10 | 0.07 | 0.47 |
- a
Ninety-seven treatment means.
Quantifying Absorption
In NRC (2001), inadequate data were available for a rigorous evaluation of Mg absorption, but a substantial number of studies have since been published. However, quantitative estimates of the true absorption of Mg are still difficult to obtain because of the uncertainty regarding the daily loss of endogenous fecal Mg. Endogenous fecal Mg has been expressed relative to BW, and typical estimates were 2 to 5 mg Mg/kg BW (Greene et al., 1986b; NRC, 2001; Schonewille et al., 2008). However, saliva and digestive secretions are important contributors to endogenous fecal Mg, and these are related more to DMI than BW, especially when comparing across physiologic states (e.g., dry versus lactating cow). Therefore, data from two meta-analyses (Weiss, 2004; Schonewille et al., 2008) were used to estimate endogenous fecal Mg as a function of DMI. Dietary Mg (g/kg of diet DM) was regressed on concentration of apparently digested Mg (g/kg) with trial as a random effect, but because of the negative effect of K, only studies with dietary K ≤2 percent were used. The absolute value of the intercept, 0.3 g Mg/kg DMI, is an estimate of endogenous fecal Mg. In sheep, loss of endogenous fecal Mg was positively correlated with serum concentrations of Mg (Allsop and Rook, 1979). If this is true for dairy cattle, cows consuming less than adequate Mg could have a lower loss of endogenous fecal Mg than cows fed adequate Mg, but no adjustment was made to endogenous fecal Mg loss based on Mg status of the cow.
Adequate data were available to quantify the relationship between dietary K concentration and Mg absorption by dairy cows. Data from studies using heifers, dry cows, and lactating cows (Weiss, 2004; Holtenius et al., 2008; Schonewille et al., 2008) were combined (see Table 7-2). If the amount of supplemental Mg as a percentage of total diet Mg could not be calculated, the study was deleted. The final data set contained 97 treatment means from 23 studies. True absorption of Mg was calculated as described above, and only dietary K concentration and percentage of total Mg provided by supplemental sources (MgO was the source of supplemental Mg in all studies except for three) were statistically related to it. The effect of dietary K was not linear; transforming to the natural logarithm provided the best fit. The resulting equation (trial was included as a random effect) was
where K is expressed as g/kg total diet and Supplemental = percentage of dietary Mg provided by MgO. Standard errors associated with the coefficients are 4.8, 1.54, and 0.034 for intercept, K, and supplemental coefficients, respectively.
A potential problem with this equation is the collinearity between dietary Mg concentration and supplementation (r = 0.70); however dietary Mg concentration was not statistically related with true absorption of Mg. Setting supplemental Mg at 0 and basal dietary K as 12 g/kg of diet DM (approximate K requirement), true absorption of Mg from basal diet = 0.31, which was assigned as the default for all feeds. Setting supplemental Mg at 100 percent and dietary K at 12 g/kg yields an estimate of 0.23 as the default availability for Mg from MgO, which is 26 percent lower than true absorption of Mg from basal feeds. This agrees with individual studies (van Ravenswaay et al., 1989; Davenport et al., 1990; Holtenius et al., 2008) in which apparent absorption of Mg was measured for diets with and without supplemental MgO and with <20 g of K/kg DM. In those studies, true absorption of Mg from MgO (calculated using the difference method) was 22 to 45 percent lower than the true absorption of Mg from the basal diet. The prediction error associated with Equation 7-13 is high (95 percent prediction interval associated with estimated ACs is + 0.16); users may wish to adjust ACs based on risk tolerance. In the previous NRC, the default AC for basal ingredients was reduced by 1-SD unit from the mean.
Absorption coefficients for common Mg supplements are in Table 19-3 (see Chapter 19). The default value for MgO reflects the average of the MgO used in the experiments; however, substantial variation exists among MgO sources, which can influence Mg availability as discussed above. High-quality MgO (e.g., small particle size and proper calcination procedures) may have greater availability than the default value. The proportion of particles <0.25 mm in MgO is positively correlated, and the proportion of particles >1.0 mm is negatively correlated with apparent absorption of Mg. Solubility of Mg from MgO in various solutions (water, citric acid, weak hydrochloric acid, buffered rumen fluid) is positively correlated with Mg absorption, but current data are not adequate to use solubility to quantify or adjust the ACs.
Few data are available for other Mg supplements. Relative to MgO, calculated true absorption of Mg was 1.7 times greater (van Ravenswaay et al., 1989) for magnesium sulfate (MgSO4), about the same for Mg(OH)2 (Davenport et al., 1990; Hemingway et al., 1998) and reagent-grade MgCO3 (Ammerman et al., 1972), about 0.5 times for dolomite limestone (Gerken and Fontenot, 1967), and 0.2 times for magnesite (Ammerman et al., 1972). However, Tebbe et al. (2018) reported that in diets without monensin, apparent absorption of Mg when MgSO4 was fed was only about 10 percent greater than that from MgO. Based on available data and because efficiency of Mg absorption differs between sheep and cattle, data from dairy cows were given more weight than data from sheep, and the true absorption of Mg from MgSO4 was assumed to be 20 percent greater than that from MgO. With monensin, apparent absorption of Mg when MgSO4 was fed was about 30 percent lower than when MgO was fed (this effect is not included in the model). Data are not available for MgCl2, but because of similar solubility to MgSO4, they were assigned the same AC.
Magnesium Deficiency
A deficiency of Mg is of greater practical concern than deficiency of most other minerals because of the limited labile stores of Mg within the body and because of the commonly occurring antagonists of Mg absorption discussed above. A clinical deficiency of Mg results in muscle twitching, hyperexcitability, convulsions, and often death (Martens et al., 2018) and is commonly referred to as grass or lactation tetany because it often occurs in spring when cattle are first let out to graze, and it is more common in lactating than nonlactating cattle. The direct cause of clinical signs is low concentration of Mg in cerebrospinal fluid. Low concentrations of Mg in plasma (less than approximately 0.7 mmol/L) are not associated with any specific clinical signs but are a risk factor for clinical hypocalcemia (discussed in more detail in Chapter 12).
Maximum Tolerable Level
Cattle can excrete large amounts of Mg in urine, so Mg toxicity is not a practical problem in dairy cattle. Although an MTL of 0.6 percent has been established (NRC, 2005), negative effects in cattle have been observed only when dietary concentrations are >1 percent. The negative effects of high Mg are generally reduced feed intake, reduced diet digestibility, and osmotic diarrhea.
The Strong Ions: Sodium, Potassium, and Chloride
Na, K, and chloride (Cl−) are completely dissociated in body fluids (Stewart, 1978) and are the major contributors to blood and cellular strong ion difference. Their relative concentrations in various body tissues are tightly regulated since they serve as osmoregulators that modulate water absorption and movement between extracellular and intracellular fluids and across the rumen and intestinal wall, and they have large impacts on systemic acid-base balance (Hu and Murphy, 2004). The dietary strong ions are absorbed with true absorptions of 0.9 or greater. Therefore, fecal strong ion excretion is primarily of metabolic origin. Regulation of strong ion balance occurs mostly via the kidney through urinary excretion. When cattle are fed typical diets, strong cation (K+ and Na+) excretion far exceeds strong anion (Cl−) excretion. This results in increased urinary bicarbonate ion excretion to maintain electrochemical neutrality. Because of this, cattle and other ruminants generally excrete an alkaline urine (pH 7.5 to 8). When Cl− is fed in excess of needs and insufficient cations (Na+ and K+) are available to balance excretion of Cl−, there is a reduction in urinary bicarbonate excretion and urine pH decreases. Thus, shifts in the relative amounts of excess Na+, K+, and Cl− that are excreted in the urine can have profound effects on acid-base status. Dietary cation–anion difference (DCAD), measured in mEq/kg diet DM, is a frequently used measure of the relative balance among the strong cations Na+ and K+ and strong anions (Cl− and sometimes S−2) (Ender et al., 1971; Mongin et al., 1981). DCAD is strongly associated with urinary pH (Constable et al., 2009) and acid-base status of the cow (Hu and Murphy, 2004) and is used in transition cow feeding to reduce incidence of hypocalcemia at calving (see Chapter 12).
Because strong ion intakes in excess of the requirements are excreted in the urine, urine volume and, correspondingly, water intake are directly related to strong ion intake. Bannink et al. (1999) showed a direct linear relationship between urine volume and strong ion intake exists in lactating cows. The increased urine volume dilutes the nitrogen (N) concentration in urine. Correspondingly, increasing dietary sodium chloride (NaCl) (Spek et al., 2012) and potassium sesquicarbonate (Iwaniuk et al., 2014) linearly decreases milk urea N concentrations.
Fecal Sodium, Potassium, and Chloride
Ruminants evolved consuming forages that were high in K (>20 g K/kg DM), low in Na (≤1 g Na/kg DM), and moderate in Cl (3 to 6 g Cl/kg DM). Therefore, their requirements reflect the differences in relative K, Na, and Cl concentrations of feeds. Dairy cow feces contain approximately 85 percent water. Fecal water output was strongly related to the sum of Na, K, and Cl fecal excretion when expressed on an equivalent weight basis in 122 balance experiments with dairy cows with a mean fecal strong ion excretion rate of 3.47 (± 1.24) equivalents per day, where Fecal H2O, L/d = 15.5 (± 1.78) + 5.88 (± 0.385) × Fecal Strong Ions (Eq/d); RMSE = 3.89; R2 = 0.861; P< 0.001. Because of the relationship between strong ion and fecal water excretion, the committee suggests that metabolic fecal requirements for Na, K, and Cl are likely due to the need to maintain osmotic balance and consistent fecal moisture content.
Sodium
Cattle evolved on feeds that are low in Na; hence, they developed efficient absorptive processes and a tenacious ability to conserve Na via the kidney, but they have only a small reservoir of Na in a form that is readily available for metabolism.
Physiologic Roles
Na is the primary extracellular cation (Aitken, 1976). In addition, 30 to 50 percent of total body Na is in a nonexchangeable fraction in the crystalline structure of bone (Eldman et al., 1954). The exchangeable fraction of Na modulates extracellular fluid volume and acid-base equilibrium (Stewart, 1983; McKeown, 1986). Heart function and nerve impulse conduction and transmission are dependent on the proper balance of Na and K. Na also plays an indispensable role in sodium–potassium adenosine triphosphate enzyme (Na-K ATPase) responsible for creating electrical gradients for nutrient transport. The Na–K pump is essential for all eukaryotic cells, enabling transport of glucose, amino acids (AAs), and phosphate into cells and hydrogen (H), Ca, bicarbonate, K, and Cl ions out of cells (Lechene, 1988). Sodium bicarbonate (NaHCO3) is a major component of saliva that helps buffer acids produced during rumen fermentation (Erdman, 1988).
Typical Na concentrations in blood plasma are 150 mEq/L and 160 to 180 mEq/L in saliva. Na is the predominant cation in rumen fluid with a typical content of 80 to 90 mEq/L, but the range can be from 50 to 140 mEq/L (Bennink et al., 1978; Catterton and Erdman, 2016). Ruminal concentrations of Na and K are strongly negatively correlated (Catterton and Erdman, 2016). Increased dietary K results in increased rumen K concentrations, which stimulate Na absorption across the rumen wall and reduce rumen Na concentration to maintain electrical and osmotic neutrality (Martens and Blume, 1987).
Sodium Utilization and Homeostasis
Absorption occurs throughout the digestive tract, and dietary Na generally is assumed to be almost completely available. Absorption occurs by an active transport process in the reticulorumen, abomasum, omasum, and duodenum. Passive absorption also occurs through the intestinal wall, so there is a tendency toward equal concentrations in intestinal and fecal fluids. However, substantial active absorption against a sizable concentration gradient occurs in the lower small intestine and large intestine (Renkema et al., 1962).
Na and K can interchange such that in Na-deficient animals, K excretion is increased, providing a mechanism that helps ensure that ruminants can subsist on feeds low in Na over long periods of time. Na concentrations in blood and tissues are maintained principally via reabsorption and excretion by the kidneys. Excretion of Na, K, and Cl− is closely synchronized. Na is the central effector of ion excretion, and changes in renal reabsorption are chief determinants of Na excretion. Endocrine control via tissue receptors and the renin–angiotensin system, aldosterone, and atrial natriuretic factor monitor and modulate Na concentrations in various tissues, which consequently control fluid volume, blood pressure, K concentrations, and renal processing of other ions. When cattle are depleted of Na, salivary glands decrease secretion of Na in saliva. The decrease in Na content is replaced reciprocally by nearly the same concentration of K (van Leeuwen, 1970; Morris and Gartner, 1971).
Requirement for Absorbed Sodium
Maintenance
The factorial method was used to derive the absorbed Na requirement. The maintenance requirement for absorbed Na is equal to the inevitable losses in feces and urine of animals fed very near their true requirement. In the review of the literature on Na, the 2001 NRC committee recognized that previous suggested maintenance requirement (0.015 g Na/kg BW) used for growing heifers and mature cows would be insufficient for lactating cows and would result in clinical signs of deficiency or reduced milk yields. Therefore, the maintenance requirement was empirically set for mature cows at 0.038 g Na/kg BW.
Urinary excretion of Na is dependent on the relative excretion rates of the other strong ions (K+ and Cl−) to maintain electrochemical neutrality in the urine and the acid-base balance of the cow. Because of these interrelationships, it is not possible to develop a consistent estimate of endogenous urinary excretion of Na or strong ions. Therefore, the committee's estimate of the maintenance requirement is based solely on the inevitable losses of Na in feces. Metabolic fecal excretion of Na was estimated from the results of 137 individual Na digestibility measurements from eight experiments in which cows were fed diets ranging from 0.27 to 1.17 percent Na. The metabolic fecal requirement was determined by regression of absorbed Na on Na intake, both expressed as grams per kilogram (g Na/kg) of diet DM. The resulting regression equation was as follows: Absorbed Na = −1.45 (± 0.25) + 0.98 (± 0.036) Na Intake; RMSE = 0.52; R2 = 0.91; P < 0.001. Metabolic fecal Na equals 1.45 g/kg DMI. The slope was not different from 1; therefore, absorption efficiency is assumed to be 1.0 (see below).
This maintenance requirement was adopted for both growing and lactating animals. For a 650-kg cow consuming 28 kg of feed DM, the metabolic fecal requirement for Na would be 41 g of dietary Na/d. This is a higher but also a more theoretically based value compared with the previous 2001 NRC estimate of 30 g/d for a lactating cow of the same size (0.038 g Na/kg BW × 700 kg/0.90 AC). A 300-kg growing animal consuming 7 kg DM/d would have a maintenance requirement of 10 g/d (0.145 percent of diet DM) or double the maintenance requirement of 4.5 g from the 2001 NRC report. Maintenance requirements for dry cows are also about doubled compared with the previous version. A review of Na requirements for beef cattle suggested that Na requirements for lactating and growing beef cattle were 0.10 and 0.07 percent of diet DM, respectively (Morris, 1980). Since osmoregulation in the feces is being used as the basis for the new maintenance requirement and K can replace Na in that role, diets with lower Na concentrations (0.07 percent) can likely be fed without affecting animal performance, assuming that the diet contains more than adequate amounts of K.
Effect of Environmental Temperature
Sweating, for aid in heat balance, includes secretion of Na (Jenkinson and Mabon, 1973) and other electrolytes. Based on the Agricultural Research Council (ARC, 1980) recommendations, the previous committee suggested that an additional increment of 0.10 and 0.50 g Na/100 kg BW be fed to animals maintained at ambient temperatures of 25°C to 30°C and >30°C, respectively. For a 700-kg cow, this would translate into an additional 0.7 and 3.5 g/d of absorbed Na for cows housed at 25°C to 30°C and >30°C, respectively. The losses of electrolytes in sweat are dependent on an animal's sweating rate and the concentration of minerals in the sweat, which have been shown to change with the rate of secretion (Sonner et al., 2015). In humans, Na+ and Cl− content increases from 30 to 90 mEq/L as sweating rate increases, whereas K+ content decreases from 20 to 5 mEq/L (Sonner et al., 2015). There are little reliable data on the composition of sweat in cattle. Jenkinson and Mabon (1973) suggested that Na and Cl excretion rate decreased in relation to K in Ayrshire cattle, but reevaluation of their data suggested that there were no changes in sweat composition in animals that were actually heat stressed (>25°C, temperature humidity index [THI] ≥72). The mean Na+ concentration was 0.25 g/L (11 mEq/L).
Jenkinson and Mabon's (1973) Na excretion data were fit to an exponential equation related to THI, where Na excretion, g per/M2/d = 0.198e0.044 × THI, R2 = 0.9687. Using surface area calculated by the Brody (1945) equation (M2 = 0.147 × BW0.56), a 700-kg cow would be expected to have a surface area of 5.8 m2. Combined with the predicted Na excretion rate per unit body surface area, the expected Na excretion in sweat was small (0.64 to 1.10 g) per day in cows housed between 25° and 35°C and a THI from 72 to 85. The measured sweating rates in Jenkinson and Mabon's (1973) study ranged from 5 to 66 mL/m2/h (0.12 to 1.9 L/d). This is similar to the more recently reported range of sweating rates observed in lactating Holstein cows under heat stress with either a shade cloth (19 to 33 g/m2/h) (Dikmen et al., 2014) or evaporative cooling (5 to 25 g/m2/h) (Dikmen et al., 2015).
Those sweating rates are on the very low end of the reported range (14 to 600 g/m2/h) in a meta-analysis of sweating rates in cattle (Thompson et al., 2011). This suggests that actual Na loss in sweat could be as much as 5- to 10-fold greater in heat-stressed cows, assuming the Na content in sweat does not change. Based on reported sweating rates in dairy cows during heat stress that was abated by evaporative cooling (Dikmen et al., 2015), Na losses in sweat would be minimal. The committee emphasizes the need for more reliable data using simultaneously measured sweating rates and sweat composition before a Na requirement during heat stress can be established. No provision is provided in the model to do so.
Growth
The requirement of absorbed Na for growth was set at 1.4 g/kg of ADG for animals weighing between 150 and 600 kg live BW (Gueguen et al., 1989).
Pregnancy
Slaughter data from 18 multiparous pregnant Holstein cows were used to quantify the requirement for absorbed Na of the conceptus during the last trimester (House and Bell, 1993). Requirements for all mineral elements are negligible to about 190 days of gestation. The Na requirement of the conceptus is 1.4 g/d × (BW / 715) from 190 to 270 days of gestation (the BW term scales values to the average BW in that study) but should not be used to compute the Na requirement for days of gestation <190 (House and Bell, 1993).
Lactation
The previous report set the absorbed Na requirement for milk at 0.65 g/kg, which was based on the average Na concentration in milk from several studies (0.63 g/kg) as reported by the ARC (1965). However, the weighted average milk Na concentration summarized across several more recent studies (Fisher et al., 1994; Sanchez et al., 1994a,c, 1997; Silanikove et al., 1997; Kume et al., 1998; Robinson et al., 2002; van Hulzen et al., 2009; Castillo et al., 2013; Khelil-Arfa et al., 2014; Visentin et al., 2016) was 0.41 (± 0.037) g Na/kg, nearly 40 percent lower than the previous 2001 NRC value. Milk Na is related to incidence of mastitis and increases with elevated milk somatic cell count (SCC) (Harmon, 1994). With greatly improved management techniques for prevention of mastitis, milk Na concentrations would be expected to have decreased during the past 50 years. The absorbed Na requirement for lactation was set at 0.4 g/kg milk.
Summary of Equations (g absorbed Na/d)
where DMI, ADG, and milk are in kg/d, and BW is in kg.
Dietary Requirement and Efficiency of Absorption
The regression coefficient of 0.98 for absorbed Na versus dietary Na was not statistically different from 1, implying that true absorption is 100 percent. The previous committee (NRC, 2001) set the Na absorption rate at 90 percent, which seemed too low given the ruminant animals' ability to survive on extremely low Na diets. In addition, Na from typical feeds is solubilized and released in the liquid matrix of digesta and is readily available for absorption. Feedstuffs commonly used in diets for dairy cattle do not contain enough Na to meet requirements, and supplemental sources typically account for the majority of an animal's total Na intake.
NaCl is the most often used supplement, and its Na is considered 100 percent available. The efficiency of absorption of Na from other salts (e.g., NaHCO3, sodium carbonate [Na2CO3], sodium sesquicarbonate [Na3H (CO3)2]) is also considered essentially 100 percent. When some animal byproduct feedstuffs containing bone are fed, Na would be less available as it is tightly bound in the crystalline structure. However, these sources represent rare circumstances and are minor Na sources compared to typical supplemental Na salts. Therefore, the committee set the AC for Na at 1.00 for all feeds.
For a 650-kg cow consuming 28 kg/d of feed DM, the metabolic fecal requirement for Na (28 kg × 1.45 g Na/kg) would be 41 g of dietary Na/d. The milk production requirement for a cow producing 45 kg/d milk would be 18 g/d (45 kg milk × 0.4 g Na/kg in milk) for a total Na requirement of 59 g/d or 0.21 percent Na in the diet DM. This compares with the previous requirement of 36 g (50 × 0.65 / 0.90) for milk production and 27 g (700 × 0.038 / 0.90) for maintenance for a total of 63 g Na/d (0.25 percent of diet DM). While the maintenance requirement for Na has increased, the reduced requirement for Na in milk more than compensated, such that total Na requirements are slightly lower than in the 2001 NRC.
Lactational Responses to Varying Dietary Sodium Concentrations
Kemp and Geurink (1966) reported that 0.14 percent Na in grazed forage was sufficient to support more than 30 kg of milk production per day. However, feeding lactating dairy cows a diet with no supplemental NaCl (0.16 percent Na, dry basis) resulted in marked depressions in DMI and milk yield after just 1 to 2 weeks of feeding (Mallonee et al., 1982a). Empirical modeling of data from 15 experiments with lactating cows conducted in either cool or warm seasons suggested that DMI and milk yield were improved by dietary concentrations of Na well above those needed to meet requirements (Sanchez et al., 1994b,c. DMI and milk yield responses over a range of dietary Na concentrations (0.11 to 1.20 percent, dry basis) were curvilinear, with maximum performance at 0.70 to 0.80 percent Na. Concentrations of Na, K, Cl−, Ca, and P in diet DM ranged from below those needed to meet requirements to concentrations considerably higher. Thus, there is a potential confounding between Na and DCAD, and it is not known whether the optimal Na concentration would vary if the dietary concentrations of other macrominerals would have been closer to requirements. There were interactions of Na with K, Cl−, and P on DMI, indicating that responses to Na differed over the range of dietary concentrations to those minerals. In addition, interactions of dietary Na with K, Cl−, and P on DMI differed in experiments conducted in the cool or warm season. In hot weather, milk yield and DMI increased when Na increased from basal (0.18 percent Na, dry basis) to 0.55 percent dietary Na with either NaCl or NaHCO3; Cl− was equalized among diets (Schneider et al., 1986).
Little evidence exists for a Na-by-K interaction when dietary Cl− was held constant, and Na and K were fed at or above their estimated requirement. Na and K were equally effective when dietary anion cation difference was increased by addition of either cation. In only one study (Iwaniuk and Erdman, 2015) was Na more effective than K in maintaining milk fat, but cation had no effect on milk yield or intake. In other experiments (West et al., 1992; Sanchez et al., 1997; Hu and Kung, 2009), the K/Na ratio did not affect intake or milk production. Wildman et al. (2007) showed a quadratic effect of the K/Na ratio on milk production but no effect on intake or milk composition.
Sodium Deficiency
Babcock (1905) fed a diet very low in Na to dairy cows and described intense craving for salt, licking and chewing various objects, and general pica. Deficiency signs were manifested within 2 to 3 weeks. Na deficiency signs may not develop for weeks to months, depending on rate of milk production. However, feed intake and milk yield began to decline 1 to 2 weeks after cows were fed a diet without supplemental NaCl (0.16 percent Na), and pica and drinking of urine of other cows were observed (Mallonee et al., 1982a). Although dietary Cl− concentration was not measured in that study, potassium chloride (KCl) was supplemented (1.0 percent total dietary K), so Cl− deficiency was probably not the cause of the condition. The condition was reversed quickly by inclusion of NaCl in the diet. Other deficiency signs include loss of appetite; rapid loss of BW; an unthrifty, haggard appearance; lusterless eyes; and rough hair coat (Underwood, 1981). More extreme signs of deficiency include incoordination, shivering, weakness, dehydration, and cardiac arrhythmia leading to death.
Free-Choice Feeding of Sodium Chloride and Sodium (Sodium Chloride) Toxicity
Cattle consume salt liberally when it is available. Smith et al. (1953) found that lactating cows consumed more salt when provided free-choice in granular versus block form, but consumption of block was sufficient to meet needs for lactation. Demott et al. (1968) fed lactating cows 4 percent NaCl in a grain mix at 1 kg of grain for each 2 kg of 4 percent fat-corrected milk yield for 2 weeks without ill effects on milk yield, BW, or general health. Although total DMI was not measured, the Na concentration of the total diet DM would have been about 0.8 to 1.0 percent. High intake of NaCl can increase the incidence and severity of udder edema (Randall et al., 1974). Feeding diets with 0.88 percent Na from NaCl or NaHCO3 to mid-lactation Holstein cows did not cause toxicity or reduce feed intake and milk yield compared with 0.55 percent Na (Schneider et al., 1986).
A major factor influencing the degree of exhibition of NaCl toxicosis is the availability and quality of drinking water. Extensive discussion of the effects of high Na and Cl− concentrations in drinking water is provided in Chapter 9. NRC (2005) set the MTL of NaCl at 3 percent of diet DM for lactating cattle and 4.5 percent for growing cattle.
Chloride
The requirements for Cl− for various classes of dairy cattle are the least studied of any strong ion. Nonetheless, its physiologic roles and interrelationships with Na and K are extremely important. Typically, Cl− is provided in the diet as NaCl, which is solubilized, releasing the negatively charged Cl− ion for absorption. Cl− is functionally important because of its propensity to accept electrons during metabolism.
Physiologic Roles
Cl− is the major anion in the body involved in regulation of osmotic pressure, making up more than 60 percent of the total anion equivalents in the extracellular fluid. As a strong anion, it always is dissociated in solution. It is essential for transport of carbon dioxide and oxygen, the chief anion in gastric secretions, and accompanied by H+ in nearly equivalent amounts. It is needed for activation of pancreatic amylase, and chlorinated compounds are produced by some phagocytic cells to kill pathogens. Typical concentrations of Cl− are from 90 and 110 mEq/L in blood plasma and 10 to 30 mEq/L in ruminal fluid. The concentration of Cl− in cattle was estimated to be about 1.2 to 1.4 g/kg over the range of 100 to 500 kg empty body weight (EBW; ARC, 1980).
Utilization and Homeostasis
About 80 percent of the Cl− entering the digestive tract arises from digestive secretions in saliva, gastric fluid, bile, and pancreatic juice. Cl− is absorbed throughout the digestive tract. It, like Na, is absorbed mainly from the upper small intestine by passive diffusion following Na along an electric gradient. Cl− is transported across the ruminal wall to blood against a wide concentration gradient (Sperber and Hyden, 1952). Martens and Blume (1987) showed that Cl− was co-transported actively with Na across the rumen wall, although the exact mechanism is unclear. Substantial absorption of Cl− from gastric secretions (hydrochloric acid) occurs in the distal ileum and large intestine by exchange with secreted bicarbonate. Appreciable quantities of Cl− are excreted in the feces, in part to maintain osmatic balance along with the other strong ions (Na+ and K) to maintain fecal moisture content. In the short term, relatively large day-to-day differences in dietary intake of Cl− have little effect on the total Cl− entering the digestive tract. Much smaller amounts of Cl− are lost in sweat mainly as NaCl or KCl. Cl− fed in excess of needs for maintenance and milk production is primarily excreted in the urine.
Tight regulation of the concentration of Cl− in extracellular fluid and its homeostasis is coupled intimately to that of Na. The role of Cl− in maintaining ionic and fluid balance was thought to be passive to that of Na and K. However, Fettman et al. (1984b) showed that during Cl− deficiency, the ion functioned independently to mediate Cl− conservation. Cl− was conserved by reducing excretion by the kidney, as well as in feces and milk. Excess Cl− intake is excreted mainly in urine of steers and sheep (Nelson et al., 1955), but in lactating cows, a significant amount of Cl− is excreted via feces (Coppock, 1986). Normally, anion concentration in extracellular fluid is regulated secondarily to cation concentrations, and when the amount exceeds reabsorption capability of the kidney, excess Cl− is excreted in urine (Hilwig, 1976). Cl− excretion is tied to excretion of strong cations, acid-base balance, and maintenance of electrochemical neutrality of the urine (Stewart, 1981). Under normal circumstances, excess cations are secreted in conjunction with Cl−, and bicarbonate ion excretion increases to maintain electrochemical balance, resulting in alkaline urine. If bicarbonate or electrolyte cations need to be conserved in relation to Cl−, Cl− excretion is accompanied by ammonium ions and urine pH decreases to maintain systemic acid-base balance.
Requirement for Absorbed Chloride
Maintenance
The factorial method was used to derive the absorbed Cl− requirement. In the previous report (NRC, 2001), the maintenance requirement for Cl− was set at 2.25 g/100 kg BW. This requirement was based on the suggestion that inevitable endogenous losses of Cl− in feces and urine on a mass basis are about 50 percent higher than that of Na (Gueguen et al., 1989), but no experimental evidence for that was given. Fettman et al. (1984b) showed that urinary excretion of Cl− was minimal (<2 g/d) during Cl− deficiency. Urinary excretion of Cl− is dependent on the relative urinary excretion rates of K and Na (Stewart, 1981) such that it is not possible to develop a consistent estimate of endogenous urinary excretion of Na.
With the relationship between fecal water excretion and total strong ion excretion (see Na discussion), the committee's estimate of the maintenance requirement for Cl− is based on inevitable losses in feces. Metabolic fecal excretion of Cl− was estimated from the results of 144 individual Cl− digestibility measurements from nine experiments in which cows were fed diets ranging from 0.25 to 0.61 percent Cl−. The metabolic fecal requirement was determined by regression of apparently absorbed Cl− on Cl− intake, both expressed as g Cl−/kg of feed DM. The resulting equation was as follows: Absorbed Cl− = −1.11 (± 0.25) + 0.92 (± 0.075) Cl− Intake; RMSE = 0.52; R2 = 0.87; P < 0.001. This equation indicates a metabolic fecal requirement of 1.11 g Cl−/kg diet DM (0.11 percent Cl− in the diet DM) and an average AC of 0.92.
Using an AC of 92 percent, a 700-kg cow consuming 25 kg of feed DM would have a metabolic fecal Cl− requirement of 30.1 g/d. This value is more theoretically grounded but is also 76 percent greater than the NRC (2001) estimate for maintenance of 17.5 g/d.
Effect of Environmental Temperature
No provision was provided for Cl− losses in sweat during heat stress in the 2001 NRC. Reexamination of the data of Jenkinson and Mabon (1973) with Ayrshire calves suggested that the mean Cl− concentration in sweat was 0.28 g/L. Using the surface area calculations and the sweating rates described for Na, an estimated Cl− excretion rate is 0.198e0.045 × THI (R2 = 0.93), suggesting that Cl− losses are similar to Na. The projected Cl− losses would be 0.7 to 1.2 g/d for a 700-kg cow exposed to a THI ranging from 72 to 85. These losses are small and subject to substantial uncertainty; therefore, effects of temperature were not included in the model. Assuming a 5-fold increase in actual sweating rate reported by Thompson et al. (2011) compared to those by Jenkinson and Mabon (1973), Cl− losses would be much greater. More reliable data on sweating rates and the Cl− concentration in sweat are needed to establish a requirement for Cl− losses during heat stress. The loss would be negligible when effective heat abatement technologies are used.
Growth
For cattle with BW between 150 and 600 kg, the requirement for absorbed Cl− for growth was set at 1.0 g Cl/kg of ADG (Gueguen et al., 1989).
Pregnancy
No research is available to directly establish the requirement for absorbed Cl− for pregnancy. However, based on consideration of the daily Na accretion rate of the conceptus and the fetus separately (House and Bell, 1993), and assuming that the relative proportions of Cl− and Na in the fetus and in a newborn calf (41.5 percent Cl− and 58.5 percent Na; ARC, 1980) are similar, Adequate Intake (AI) for pregnancy from 190 days of gestation to parturition was set at 1.0 g/d × (BW / 715). The average BW in House and Bell (1993) was 715 kg, and requirements are scaled to that.
Lactation
Cl− exists in milk almost entirely as the free ion (Holt, 1985). Cl− is highest in colostrum, declines rapidly to average concentrations after lactation commences, and increases toward the end of lactation (Flynn and Power, 1985). The previous report set the absorbed Cl− requirement for milk at 1.15 g Cl/kg based on the average Cl− concentration in milk from several studies reported by ARC (1965). Milk Cl− is strongly related to incidence of mastitis and is elevated in cows with high milk SCC (Harmon, 1994). With improved management techniques for prevention of mastitis, milk Cl− concentrations would be expected to have decreased during the past 50 years. The weighted average milk Cl− concentration across several more recent studies (Fisher et al., 1994; Sanchez et al., 1994a, 1997; Silanikove et al., 1997; Kume et al., 1998; Robinson et al., 2002; van Hulzen et al., 2009; Castillo et al., 2013; Khelil-Arfa et al., 2014; Visentin et al., 2016) was 0.97 (± 0.06) g Cl/kg. Therefore, the absorbed Cl− requirement for milk production was set at 1.0 g Cl/kg.
Summary of Equations (g absorbed Cl−/d)
where DMI, ADG, and milk are in kg/d, and BW is in kg.
Dietary Requirement and Efficiency of Absorption
Little research has been done in ruminants to measure the true AC for Cl− principally due to the widespread availability of good, inexpensive inorganic sources (e.g., NaCl). Cl− from inorganic sources and common feedstuffs is freely released into the liquid phase of the digesta and readily absorbed (Underwood, 1981). Apparent absorption of Cl− in lactating cows fed fresh forage ranged from 71 to 95 percent and averaged 88 percent (Kemp, 1966). This is comparable to other estimates of absorption efficiency of 85 to 91 percent in cattle and sheep fed mixed diets (ARC, 1980). Paquay et al. (1969b) found that apparent absorption of Cl− was not influenced by intake of Cl− but was correlated negatively with intakes of DM, energy, and pentosan, as well as positively correlated with intakes of K and N. Factors such as lactation, pregnancy, and growth affecting the requirement for Cl− do not appear to alter the efficiency of Cl− absorption. Overall, the absorption efficiency for Cl− in ingredients commonly fed to dairy cattle is usually ≥90 percent (Henry, 1995b). The committee estimated AC from 144 Cl− balance studies was 92 percent. Therefore, an AC for Cl− of 0.92 was assigned for all dietary ingredients.
For a 650-kg cow consuming 28 kg/d DMI and producing 45 kg/d milk, the new requirement for Cl− includes 34 g/d for maintenance (28 kg DMI × 1.11 / 0.92) plus 49 g Cl− required for milk production (45 kg milk × 1 / 0.92) for a total of 83 g dietary Cl−. This compares with the previous dietary Cl− requirement of 81 g/d (NRC, 2001). While the maintenance requirement has increased, milk production requirements have decreased such that the total Cl− requirement for lactating cows has changed little compared to the previous NRC.
Lactation and Growth Responses to Varying Dietary Chloride
Coppock (1986) reviewed the estimated requirements of dietary Cl− for lactating dairy cows in studies in which milk production ranged from 24 to 32 kg/d. Holstein cows fed a diet with 0.18 percent Cl− conserved Cl− by dramatically reducing excretion of Cl− in urine and feces and tended to reduce Cl− output in milk; however, intakes of feed and water, as well as milk yield and composition, did not differ from cows fed 0.40 percent Cl− (Coppock et al., 1979). Half of the cows in each treatment group had free access to a trace-mineral salt block, and cows fed the diet low in Cl− consumed more of the salt block. Fettman et al. (1984a) fed diets containing 0.10, 0.27, and 0.45 percent Cl− for the first 8 to 11 weeks of lactation. Cows fed 0.10 percent Cl− rapidly exhibited clinical signs of Cl− deficiency and poor performance compared with those fed medium and high concentrations of dietary Cl−. Health, feed intake, and yield and composition of milk by cows fed the medium and high concentrations of dietary Cl− were similar. Empirical models with a large data set showed that increasing dietary Cl− over a range of 0.15 to 1.62 percent decreased DMI and milk yield of mid-lactation cows (Sanchez et al., 1994b). The negative effects of increasing dietary Cl− were more dramatic in hot summer weather than in winter. This is consistent with the results of Escobosa et al. (1984) showing profound exacerbating effects of high dietary Cl− on acid-base balance (metabolic acidosis) and lactation performance during heat stress. Adding Cl− to the diet when the other strong ions (Na+ and K+) are held constant reduces DCAD, and the decrease in DCAD was likely the cause of the negative effect of high Cl− (discussed in the DCAD section below).
Feeding diets with 0.038 percent Cl− for 7 weeks to male Holstein calves did not produce clinical deficiency or depress feed intake, growth rate, or digestibility of feed compared with calves fed 0.50 percent Cl− (Burkhalter et al., 1979). Calves fed the low Cl− diet adapted by reducing urinary excretion of Cl−, and their water intake and urine output were greater than that of calves fed more Cl−. Calves fed a low Cl− (0.038 percent) diet developed mild alkalosis, but it did not affect growth, and calves adapted to the low intake of Cl− (Burkhalter et al., 1980).
If NaCl is used to meet the Na requirement, generally the Cl− requirement is met or exceeded. However, if NaHCO3 or some other Na-containing salt is used to supply Na, it may be necessary to meet the Cl− requirement with another supplement (e.g., KCl). Research is needed to establish more accurate requirements and appropriate dietary concentrations of Cl− (and Na) for all classes of dairy cattle. If current estimates are too high, it could contribute to soil salinity when manure is applied (Coppock, 1986). Cl− in drinking water also may make a major contribution to intake of Cl−. In a survey of 39 California dairy herds by Castillo et al. (2013), inclusion of the Cl− in water increased estimated total Cl− intake by 6.5 percent.
Chloride Deficiency
Cl− deficiency was created in young calves (100 kg BW) by feeding a diet with 0.063 percent Cl− and removing about 600 g of abomasal contents daily (Neathery et al., 1981). Clinical signs were anorexia, weight loss, lethargy, mild polydipsia, and mild polyuria. In latter stages, severe eye defects and reduced respiration rates occurred, and blood and mucus appeared in feces. Deficiency of Cl− resulted in severe alkalosis and hypochloremia, which manifested in secondary hypokalemia, hyponatremia, and uremia. Control calves also had abomasal contents removed daily but were fed a diet with 0.48 percent Cl−, and they grew normally and showed no signs of deficiency. During the first 8 to 11 weeks of lactation, dairy cows fed low (0.1 percent, dry basis) Cl− exhibited dramatic and progressive declines in intakes of feed and water, BW, milk yield, and electrolyte concentrations in blood serum, saliva, urine, milk, and feces (Fettman et al., 1984b).
A significant decline of Cl− in blood serum was found within 3 days after switching cows from a diet containing 0.42 percent to a diet with 0.10 percent Cl− (Fettman et al., 1984b). Clinical signs of deficiency were depraved appetite, lethargy, hypophagia, emaciation, hypogalactia, constipation, and cardiovascular depression. Metabolic alterations were severe primary hypochloremia, secondary hypokalemia, and metabolic alkalosis (Fettman et al., 1984a,bc). Cl− deficiency, resulting from an inadequate dietary supply or loss of gastric juices, can lead to alkalosis due to an excess of bicarbonate, because inadequate Cl− is partially compensated for by bicarbonate.
Chloride Toxicity
High systemic concentrations of Cl−, in the absence of a neutralizing cation (e.g., Na+), can cause disturbance of normal acid-base equilibrium (Stewart, 1981; Escobosa et al., 1984), but the maximum tolerable concentration of Cl− in the diet has not been determined. The maximum tolerable concentration of dietary NaCl was set at 3.0 percent (dry basis) for lactating dairy cows and 4.5 percent for growing cattle (NRC, 2005).
Potassium
Physiologic Roles
K is the third most abundant mineral in the body. It must be supplied daily because there is little storage in the body and the animal's requirement for K is high. K is involved in osmotic pressure and acid-base regulation, water balance, nerve impulse transmission, muscle contraction, and oxygen and carbon dioxide transport; as an activator or cofactor in many enzymatic reactions; in cellular uptake of AAs and synthesis of protein; in carbohydrate metabolism; and in maintenance of normal cardiac and renal tissue (Stewart, 1981; Hemken, 1983). It is the major intracellular electrolyte with concentrations in the range of 150 to 155 mEq/L. In contrast to Na+ and Cl−, extracellular concentrations of K+ are low (about 5 mEq/L). Saliva typically contains <10 mEq/L, whereas concentrations in ruminal fluid range from 40 to 100 mEq/L (Bennink et al., 1978; Catterton and Erdman, 2016). Blood plasma contains 5 to 10 mEq/L. The vast majority of K in blood is located within red blood cells (Aitken, 1976; Hemken, 1983). About 80 percent of the K in the body is associated with lean tissue and bone. Gastrointestinal contents account for an additional 15 percent of body K and are affected by the K content of the diet (Belyea et al., 1978).
Potassium Utilization and Homeostasis
K is absorbed primarily in the duodenum by simple diffusion, and some absorption occurs in the jejunum, ileum, and large intestine. The main excretory route of excess absorbed K is via urine. This route is primarily under regulation by aldosterone, which increases Na reabsorption in the kidney with the concomitant excretion of K. Blood acid-base status also affects urinary excretion of K (McGuirk and Butler, 1980). With the onset of an alkalotic condition, intracellular H+ are exchanged with K+ in plasma as part of the regulatory mechanisms to maintain blood pH. A large gradient exists between intracellular renal tubule concentrations of K and that of luminal fluid (urine). This gradient affects the passage of K from the tubular cells into urine.
There is a distinct relationship between excess cations such as Na+ and K+ and urinary pH (Hu and Murphy, 2004). Excess K and Na are excreted in the urine and result in increased urinary bicarbonate secretion. Because K+ is the primary cation in dairy cattle diets, intake responses to K may be directly related to changes in urinary acid-base balance. Fecal K is primarily from endogenous losses as true digestibility of K approaches 100 percent.
Requirement for Absorbed Potassium
The factorial method was used to derive the absorbed K requirement. In the previous report (NRC, 2001), the maintenance requirement for K was set at 0.038 g K/kg BW plus 6.1 g K/kg DMI. These requirements were based on suggested endogenous urinary losses of 0.038 g K/kg BW and endogenous fecal losses of 2.6 g K/kg of dietary DM (Gueguen et al., 1989) coupled with an empirical adjustment of the endogenous fecal losses of an additional 3.5 g K/kg diet DM for a total 6.1 g K/kg. The empirical adjustment to fecal losses was added because based on production responses, the initial requirement was not adequate (Dennis et al., 1976; Dennis and Hemken, 1978; Erdman et al., 1980; Sanchez et al., 1994b,c. Generally, as dietary K increased from 0.5 to 1.2 percent of dietary DM, feed intake was consistently increased. The previous committee suggested that a higher maintenance requirement for absorbed K for lactating cows compared with nonlactating animals was justified based on K's role in dynamic processes associated with ruminal function at higher levels of feed intake and maintenance of systemic acid-base balance.
However, the adjustment was applied to the metabolic fecal K requirement. Applying the adjustment in this way implied much greater fecal losses than the actual measured losses. A metabolic fecal requirement of 6.1 g K/kg diet DM in a diet containing 1.2 percent K would have implied an apparent AC of 0.49, which is far below any measured values in the literature.
As it is difficult to formulate a diet with less than 1 percent K using traditional forages, the studies used to determine the intake and milk production responses to K often fed atypical diets that were high in cereal grains, by-product feeds such as brewers dried grains, distillers grains, and cottonseed hulls as a forage substitute to achieve a low K basal diet (Dennis et al., 1976; Dennis and Hemken, 1978; Erdman et al., 1980; Sanchez et al., 1994b,c. In most cases, the calculated DCAD of the basal diets was low (0 to 50 mEq per kilogram diet DM) using the Ender et al. (1971) equation, which includes sulfide (S2−). In addition, dietary K was increased by addition of KCl, which would not change the DCAD concentration. Therefore, the committee is uncertain whether these results could be applied to more typical diets where basal DCAD is 200 mEq/kg or greater. Since only dietary K and Na can be used to increase DCAD, the question is whether Na+ could replace K+ as a urinary cation to maintain an alkaline urine once the needs for metabolic fecal and milk K secretion have been met.
Metabolic fecal excretion of K was estimated from the results of 149 individual K digestibility measurements from nine experiments in which cows were fed diets ranging from 0.96 percent to 1.86 percent K. The metabolic fecal requirement was determined by regression of apparently absorbed K on K intake, both expressed as grams per kilogram of feed DM. The regression equation was as follows: Absorbed K = −2.48 (± 0.74) + 1.02 (± 0.056) K Intake; RMSE = 0.52; R2 = 0.93; P < 0.001. This equation suggests a metabolic fecal requirement of 2.48 g K/kg DMI and a true absorption of 1.02, which was not different from 1. Therefore, the metabolic fecal requirement was set at 2.5 g K/kg DMI, similar to previous estimates (Paquay et al., 1969a; Gueguen et al., 1989), and an AC of 1.0 was assigned to dietary K.
Urinary excretion of K is dependent on the relative excretion rates of Na and Cl− (Stewart, 1981). Dairy cattle typically secrete an alkaline urine with a pH of ~8.0 (Hu and Murphy, 2004) because of the need to excrete excess cations (K+ and Na+) in relation to anions (Cl−), which in turn increases urinary bicarbonate secretion to maintain the electrochemical balance in the urine. As previously indicated, a maintenance requirement based on the measured metabolic fecal K alone would result in a diet that is too low in K compared to the observed experimental responses to dietary K with respect to feed intake and milk production (Dennis et al., 1976; Dennis and Hemken, 1978; Erdman et al., 1980; Sanchez et al., 1994b,c. Therefore, the committee arbitrarily set an AI to meet the endogenous urinary K needs at 0.2 g/kg BW. This will usually result in a minimum dietary K concentration of 1.00 percent of diet DM for lactating dairy cows. The AI to meet endogenous urinary K need for growing heifers and dry cows was set at 0.07 g K/kg BW to maintain a minimum total dietary K of 0.60 percent.
Effect of Environmental Temperature
NRC (2001) set absorbed K requirements for thermoregulation (sweating) at 0.04 and 0.36 g/100 kg BW for cattle maintained at environmental temperatures of 25° to 30°C and >30°C, respectively. This is equivalent to 0.28 and 2.5 g/d, respectively, in a 700-kg dairy cow. Reexamination of the data of Jenkinson and Mabon (1973) with Ayrshire calves suggested that the mean K concentration in sweat was 0.45 g/L. Using the surface area calculations and the sweating rates previously described for Na, an estimated K excretion rate (g/M2/d) would be 0.08e0.091 × THI (R2 = 0.93), suggesting that K losses at the upper end of the THI range would be 0.8 to 2.5 g/d for a 700-kg cow exposed to a THI ranging from 72 to 85. At the upper end of the THI range, these losses are approximately 2-fold greater than those for Na and Cl−. Assuming a 5-fold increase in actual sweating rate reported by Thompson et al. (2011) compared to those reported by Jenkinson and Mabon (1973), K losses could be much greater. The measured average sweating losses from 0900 to 2200 h in heat-stressed Holstein cows (Mallonee et al., 1985) was 0.20 g/h. While estimated K+ losses are approximately 2-fold greater than those for Na and Cl−, these losses are negligible in relation to typical K intakes in lactating dairy cows of 250 to 350 g/d (<1 percent of K intake). These losses would be minimal where evaporative cooling was used as a means of heat abatement. The committee concluded that more reliable data on sweating rates and the K concentration in sweat are needed to establish a requirement for K losses during heat stress. Therefore, no provision is provided in the model for sweating losses for K.
Growth
The previous committee (NRC, 2001) set the requirement of absorbed K for growth at 1.6 g/kg ADG based on the estimate of Gueguen et al. (1989) for cattle with BW between 150 and 500 kg. That value seemed low when compared to measured values. The K content in mature Holstein heifers (Belyea et al., 1978) fed diets that varied in K was 2.0 (± 0.08) and 1.95 (± 0.06) g K/kg total BW and EBW, respectively. The K content in growing cattle decreased from 2.20 to 1.96 g/kg EBW as slaughter weight increased from 252 and 454 kg (Lohman and Norton, 1968), but there was no effect of slaughter weight on K concentration in the total body (2.49 g/kg). Since K retention at any given range includes retention in the gastrointestinal tract, the K requirement for growth was set at 2.5 g K/kg gain.
Pregnancy
Slaughter data from 18 multiparous pregnant Holstein cows were used to quantify the requirement for absorbed K for conceptus accretion during the last trimester of pregnancy (House and Bell, 1993). Requirement for K is negligible up until about 190 days of gestation. After 190 days of gestation, the requirement of the conceptus for absorbed K is 1.03 g/d × BW / 715 (the BW term scales values to the average cow in that study).
Lactation
The K concentration in milk is constant even under conditions of widely varying K intakes (Sasser et al., 1966). The previous report set the absorbed K requirement for milk at 1.5 g/kg. The weighted average milk K concentration summarized across several more recent studies (Fisher et al., 1994; Sanchez et al., 1994a, 1997; Silanikove et al., 1997; Kume et al., 1998; Robinson et al., 2002; van Hulzen et al., 2009; Castillo et al., 2013; Khelil-Arfa et al., 2014; Visentin et al., 2016) was 1.49 (± 0.11) g K/kg. Therefore, the absorbed K requirement for milk production was maintained at 1.5 g K/kg milk.
Summary of Equations (g absorbed K/d)
(Equation 7-22a)
(Equation 7-22b)
where DMI, ADG, and milk are in kg/d, and BW is in kg.
Dietary Requirement and Efficiency of Absorption
Hemken (1983) indicated that K is almost completely absorbed with a true digestibility of 95 percent or greater for most feedstuffs. Paquay et al. (1969a) found that the apparent absorption of K by dairy cows fed alfalfa silage, clover silage, and cabbage silage ranged from 87 to 94 percent. Apparent absorption was slightly lower in four tropical forages fed to sheep, but efficiency of absorption was not affected by maturity of the forage (Perdomo et al., 1977). Average apparent absorption of K in eight forages fed to cattle and sheep was 85 percent (Miller, 1995).
Because K is excreted mainly in urine, urinary excretion and apparent absorption are reliable criteria for estimation of efficiency of absorption. Supplemental K from inorganic sources such as potassium carbonate, KCl, and potassium sulfate is highly soluble and readily available for absorption (Peeler, 1972; Miller, 1995). In the model, an AC value of 1.00 for K was used for all feedstuffs and mineral sources.
For growing heifers weighing 300 kg, gaining 1 kg BW/d and consuming 7 kg DM/d, the previous requirements (NRC, 2001) was 35 g (0.49 percent K in diet DM). The new requirement is 41 g (0.59 percent K in diet DM). The dietary K requirement from the previous report (NRC, 2001) for a 650-kg lactating dairy cow producing 45 kg milk and consuming 28 kg diet DM/d was 265 g (0.94 percent K in diet DM). The new dietary requirement is 268 g (0.96 percent K in diet DM). Essentially, the total requirements for K have not changed substantially, but the route of excretion has shifted from metabolic fecal to endogenous urinary excretion.
Production Responses to Varying Concentrations of Dietary Potassium
Growth
Growth of dairy calves was maximized with 0.58 percent dietary K, and no benefits were noted with higher concentrations (Bigelow et al., 1984). Weil et al. (1988) found no differences in BW gain (average 0.73 kg/d) or DMI when feeding diets with 0.55 to 1.32 percent K (dry basis) to Holstein and Jersey calves starting at 4 weeks of age, but ADG and feed intake were greater for calves fed 0.58 percent K than for those fed 0.34 percent. Tucker et al. (1991) fed diets with 0.4 or 0.6 percent dietary K (supplemented from KCl) and 0 or 2.0 percent NaHCO3 to growing calves (76 kg BW) and found no effects on feed intake. However, ADG increased with higher dietary K and tended to be reduced by addition of NaHCO3. Feedlot cattle require 0.55 to 0.60 percent K (NASEM, 2016), but for cattle under range conditions with slower growth rates, 0.3 to 0.4 percent K appears adequate.
Lactation
The secretion of K in milk necessitates higher dietary concentrations for lactating cows compared with growing cattle. Early research indicated that 0.75 and 0.70 percent dietary K (dry basis) was sufficient for early and mid- to late-lactation cows averaging 24 and 29 kg/d milk production, respectively (Dennis et al., 1976; Dennis and Hemken, 1978). Feed intake generally increased as dietary K concentration was increased up to about 1.0 percent of diet DM, but milk responses were small (Dennis et al., 1976; Dennis and Hemken, 1978; Erdman et al., 1980; Mallonee et al., 1985). Sanchez et al. (1994b,c), using data from 15 experiments with mid-lactation dairy cows (1,444 cow-period observations) conducted in either cool or warm seasons, showed that intake and milk yield were improved with concentrations of dietary K well above those needed to meet requirements. Intake and milk yield responses over a range of dietary K concentrations (0.66 to 1.96 percent, dry basis) were curvilinear, with maximum performance at 1.50 percent K in the cool season. In the warm season, DMI and milk yield increased over the range of dietary K concentrations in the data set. Because of the numerous interactions observed, optimal concentration of K likely varies depending on other minerals. For example, higher dietary Cl− would result in an increased cation response from either Na or K due to effect of DCAD (Hu and Murphy, 2004; Iwaniuk and Erdman, 2015). Interactions of dietary K with Na and Cl− on DMI differed in cool versus warm season experiments. In a winter study in Florida, Mallonee (1984) found no benefit of increasing dietary K from 1.07 to 1.58 percent (dry basis) on intake or lactation performance of mid-lactation Holstein cows; however, there were interactions with dietary Na (0.16 to 0.70 percent). Feeding diets with excess K relative to requirement increased intake and milk yield in heat-stressed cows (Beede et al., 1983; Schneider et al., 1984; Mallonee et al., 1985; Schneider et al., 1986; West et al., 1987; Sanchez, 1994a). A dietary K concentration of 1.5 percent (dry basis) during heat stress maximized lactation performance (Beede and Shearer, 1991).
Potassium Deficiency
Signs of severe K deficiency were manifested in lactating dairy cattle fed diets with 0.06 to 0.15 percent K (Pradhan and Hemken, 1968; Mallonee et al., 1982b). Marked decline in feed and water intake, reduced BW and milk yield, pica, loss of hair glossiness, decreased pliability of the hide, lower concentrations of K+ in plasma and milk, and higher blood hematocrit readings occurred within a few days to a few weeks after cows were offered the K-deficient diets. Rate of occurrence and severity of deficiency signs appear to be related to rate of milk production, with higher-yielding cows affected more quickly and severely than lower-yielding cows. With severe K deficiency, cows will be profoundly weak or recumbent with overall muscular weakness and poor intestinal tone (Sielman et al., 1997). In this case, hypokalemia syndrome was associated with treatment of ketosis.
When diets contained 0.5 to 0.7 percent K, the only apparent sign of inadequacy in lactating cows was reduced feed intake with corresponding lower milk yield compared with cows fed adequate K. Because many forages contain high concentrations of K, severe K deficiency would be extremely rare. However, marginal deficiency can occur if corn silage is the sole forage and no supplemental K is fed.
Potassium Toxicity
The dietary concentration of K that leads to toxicity is not well defined (Ward, 1966b). K toxicosis is unlikely to occur under natural conditions but could occur as a result of excess supplementation. Acute toxicity (Ward, 1966a) and death (apparently cardiac arrest) occurred when 501 g of K as KCl was given by stomach tube to a cow (475 kg BW). This amount was approximately the daily amount consumed by similar cows fed 15 kg of alfalfa that was consumed without ill effects. Dennis and Harbaugh (1948) administered 182 and 240 g of K as KCl without detectable clinical signs of toxicity, but 393 g by stomach tube to cattle weighing about 300 kg resulted in one death, two that required treatment, and two exhibiting no signs of toxicity. When 4.6 percent dietary K (via supplemental potassium carbonate) was fed to cows during early lactation, feed intake and milk yield were reduced, and water intake and urinary excretion were increased (Fisher et al., 1994). NRC (2005) set the maximum tolerable concentration at 2.0 percent of diet DM based on indexes of animal health. However, cattle are known to tolerate high concentrations (>3 percent of DM) of K such as are seen in early spring pastures for extended periods of time. Dietary K depresses Mg absorption and is a risk factor for grass tetany. Feeding K in excess of that needed to meet requirements can present metabolic and physiologic challenges to cattle and will increase excretion of K into the environment.
Dietary Cation–Anion Difference
DCAD was discussed in the 2001 NRC, but no requirements were suggested. The DCAD is calculated as the difference between the sum of the major cations (Na+ and K+) and the sum of the major anions (Cl− and sometimes S2−) and is expressed in milliequivalents (mEq) per kg or per 100 g of diet DM. The simplest calculation of the DCAD equation (Mongin, 1981) includes dietary Na, K, and Cl− and was developed for use in poultry diets. Ender et al. (1971), in early work related to the use of DCAD for prevention of milk fever, proposed a DCAD equation that included sulfur (S) assumed to be S2−, as the second anion. When discussing specific dietary values, DCAD calculated using Ender et al. (1971) will be referred to as DCAD-S, whereas the term DCAD refers to the Mongin (1981) equation. For a diet containing 1.11 percent K, 0.25 percent Na, 0.33 percent Cl−, and 0.20 percent S, the DCAD and DCAD-S concentrations would be 300 and 185 mEq/kg DM, respectively.
Inclusion of other dietary cations (Ca2+ and Mg2+) and anions (P3−) have been suggested to be included in the DCAD equations. However, the contribution of those ions to urine net acid excretion is dependent on their relative absorption rates and the degree of urinary excretion. Constable et al. (2009) found that K+, Na+, Ca2+, Mg2+, Cl−, and sulfate (SO42−) ion accounted for most of the strong ion effects on urine pH in cattle. However, urinary Ca and Mg losses in lactating cows are minimal such that the relative amounts of K, Na, Cl−, and SO42− excretion have the greatest impact on urine pH.
Cattle routinely consume diets that are high in DCAD, resulting in urinary excretion of excess strong cations (primarily K+) relative to strong anions (Cl−). In high DCAD diets, urinary electrochemical neutrality is maintained by increased bicarbonate secretion. Addition of a dietary cation source such as NaHCO3 (Erdman et al., 1982) to lactating dairy cows resulted in decreased urinary net acid excretion, increased urinary bicarbonate secretion, and increased urine pH. Feed intake declined as urine pH dropped from 8 to 7 (Hu and Murphy, 2004) with decreasing DCAD. When negative DCAD diets are fed, urinary excretion of excess Cl− relative to K+ and Na+ occurs, resulting in decreased urinary bicarbonate secretion (Tucker et al., 1988) and reduced urinary pH. Reduction in urinary pH below 7 is associated with increased Ca excretion in the urine (Constable et al., 2009). Feeding low DCAD diets to prepartum dairy cows reduces prevalence of milk fever (see Chapter 12).
Much of the research on the effects of DCAD in lactating dairy cows has used cation sources such as sodium and potassium bicarbonate, carbonate, and sesquicarbonate salts to increase the DCAD concentration in the diet. The anion components of these salts can also act as buffering agents in the rumen and contribute to the increase in rumen pH and acetate/propionate ratio in part because of their buffering effect. A 100 mEq/kg DM in DCAD resulted in a linear (0.03) increase in rumen pH (Iwaniuk and Erdman, 2015). Thus, the overall effect of DCAD effects on animal performance is likely due to a combination of their effects on rumen fermentation and acid-base status of the cow.
Lactation Responses to Dietary Cation–Anion Difference
Maximum DMI, milk yield, and 4 percent fat-corrected milk yield occurred at a DCAD of 380 mEq/kg diet DM (Sanchez et al., 1994b). Similar results were observed when data were subdivided by season with maximal DCAD for maximal DMI and 4 percent fat-corrected milk yield. However, maximum response appeared to occur at a slightly higher DCAD in winter-fed versus summer-fed cows.
Since the last report (NRC, 2001), two meta-analyses of published literature on DCAD effects on lactating dairy cattle (Hu and Murphy, 2004; Iwaniuk and Erdman 2015) have been conducted. Hu and Murphy (2004) reported that DCAD had significant effects on production and acid-base responses in lactating cows. The data set included 17 experiments and 69 treatment means using DCAD calculated with the Mongin (1981) equation. Comparison of response curves for DMI and urine pH revealed a 1.5-kg/d decline in feed DMI and a 1 pH-unit drop in urine pH as DCAD decreased from 340 to 120 mEq/kg diet DM. The close relationship between reduced DMI and urine pH suggests that urinary acid-base status is related to DMI. The maximal intake and yields of milk, fat-corrected milk, and fat occurred at DCAD of 396, 336, 489, and 550 mEq/kg diet DM, respectively, and the DCAD at which 80 percent of the maximum response occurred was 200, 185, 190, and 305 mEq/kg DM, respectively.
Iwaniuk and Erdman (2015) conducted a much larger meta-analysis of data collected from 43 published studies that included 196 treatment means. This analysis included data previously summarized by Hu and Murphy (2004) and incorporated earlier and later published work where supplements such as sodium and potassium bicarbonate, carbonate, and sesquicarbonate salts were fed. Data for DMI, milk production, and 3.5 percent fat-corrected milk were fitted to an asymptotic model where Y = a + b (1 − e(−k × DCAD-S)), where a = intercept, b = maximal response to DCAD-S, and k is the rate constant for the effect of DCAD-S (mEq/kg diet DM) on the response.
For DMI, the response equation was DMI, kg/d = 18.44 (± 0.389) + 1.11 (± 0.468) (1 − e(−0.0038 (±0.002) × DCAD-S)), R2 = 0.41, RMSE = 0.53. Eighty percent and 66 percent of the maximal intake response to DCAD-S occurred at 425 and 290 mEq/kg diet DM, respectively. Milk production responses to DCAD-S were relatively small with a maximal response of 1.1 kg/d.
Milk fat percentage and milk fat yield increased linearly with increased DCAD with 0.1 percentage unit and a 39-g/d increase in fat percentage and fat yield per 100-mEq/kg diet DM increase in DCAD-S. Rumen pH (83 treatment means) increased linearly with increasing DCAD-S, with the changes in pH being consistent with the effects on biohydrogenation of FA intermediates that are known to inhibit milk fat synthesis (see Chapter 4).
The response function for 3.5 percent fat-corrected milk was 3.5 percent FCM, kg/d = 25.49 (± 0.751) + 4.82 (± 1.57) × (1 − e(−0.0024 (±0.001) × DCAD-S), R2 = 0.48, RMSE = 0.73. For 3.5 percent FCM, 80 percent and 66 percent of the maximal response to DCAD occurred at a DCAD-S of 675 and 450 mEq/kg diet DM, respectively. However, 675 mEq/kg was outside of the range of inference in the data set. The changes in 3.5 percent fat-corrected milk production reflected the curvilinear response in milk yield and the linear response in fat yield to increasing DCAD-S.
With fewer observations (52 and 42, respectively), a 100-mEq/kg diet DM increase DCAD-S resulted in a 0.73 and 1.54 percentage unit increase in DM and NDF digestibility, respectively. The change in NDF digestibility accounted for approximately two-thirds of the increase in DM digestibility.
Dietary Cation–Anion Difference Requirements for Lactating Cows
There is no fixed requirement for DCAD in lactating cows. Rather, the feeding level chosen should be determined based on the incremental production responses (milk and fat yield) in relation to the incremental added costs (DMI and mineral salt supplementation) according to the response equations outlined by Hu and Murphy (2004) and Iwaniuk and Erdman (2015).
An absolute practical minimum or AI for DCAD would be based on the minimum requirements for the minerals, K, Na, Cl−, and S. For example, a 700-kg dairy cow producing 50 kg milk will require a diet containing 1.11 percent, 0.25 percent, 0.33 percent, and 0.20 percent K, Na, Cl−, and S, respectively. The calculated DCAD-S using those requirements would be 174 mEq/kg diet DM or 301 mEq/kg using the Mongin (1981) equation. If measured Cl− and S concentration of the diet exceeds the minimum requirement, then additional K or Na may be needed for an acceptable DCAD and DCAD-S.
Growth Responses to Dietary Cation–Anion Difference
Few studies have examined the influence of DCAD on growth of calves. Calves (1 to 12 weeks of age) grew faster when fed a 200-mEq DCAD-S diet than calves fed a −100-mEq diet (Xin et al., 1991). In another study, Holstein and Jersey calves averaging 56 to 70 days of age were fed diets containing −180, 45, 225, and 383 mEq/kg DCAD-S (Jackson et al., 1992). Feed intake and ADG responded quadratically, being greatest at 225 mEq and lowest with −180 mEq. In a follow-up study, intake, growth rate, and Ca metabolism were compared for Holstein calves (56 to 70 days of age) fed diets with −180 or 130 mEq DCAD-S/kg of dietary DM in a factorial arrangement of treatments with 0.42 and 0.52 percent dietary Ca (Jackson and Hemken, 1994). Feed intake did not differ due to DCAD, but growth rate was increased with the 130 mEq/kg DCAD-S; dietary Ca had no effect. Urinary Ca excretion was greater for calves fed diets with −180 mEq compared with diets with 130 mEq. Breaking strength of the ninth rib was greater for calves fed the 130-mEq treatment compared with the −180-mEq treatment; breaking strength of the seventh rib was greater when calves were fed either higher DCAD or higher Ca. Based on these studies, the AI of DCAD-S for growing calves is 150 to 200 mEq/kg of diet DM, which is the approximate value obtained when calves are fed their minimum requirements for K, Na, and Cl−.
No studies were identified in which DCAD was varied in growing dairy heifers. Growing (Ross et al., 1994b) and finishing (Ross et al., 1994a) beef steers were fed diets containing 0, 150, 300, and 450 mEq/kg DCAD (Mongin, 1981 equation), and a DCAD of 150 mEq/kg maximized feed intake and growth rate. In the absence of experiments with growing dairy heifers, an adequate DCAD (Mongin, 1981 equation) would be 150 mEq/kg diet DM.
Sulfur
Function
About 0.15 percent of the body is S, predominantly in the form of S AAs (S-AAs) and the amino sulfonic acid, taurine, but S is also a component of thiamin and biotin, structural compounds (e.g., chondroitin sulfate), and other biologically important molecules. Met, thiamin, and biotin cannot be synthesized by cattle; they must either be supplied in the diet or synthesized by ruminal microbes. The sulfate ion (SO42−) is found in cellular and extracellular spaces, and concentrations are likely under homeostatic control via renal clearance and perhaps other mechanisms (Markovich, 2001). S is not a major factor in acid-base balance, but the primary function of SO42− is likely acid-base balance.
Requirement
The dietary requirement for S by the cow is primarily to provide adequate substrate to ensure maximal microbial protein synthesis, which in turn will increase the supply of the S-containing compounds required by cows. Based on in vitro and in vivo studies, maximum fiber digestibility usually occurs when diets contain 0.15 to 0.25 percent total S (Guardiola et al., 1983; Qi et al., 1994). Bouchard and Conrad (1973a,b) determined that 0.20 percent dietary S (supplemental S provided by Na, Ca, K, or MgSO4) was adequate to sustain maximal S retention in mid-lactation dairy cows producing 30 to 37 kg milk/d. Based on the lack of any newer data, the S requirement for all classes of dairy cattle (excluding preruminant calves) remained at 0.2 percent of diet DM or
where DMI is kg/d, and S is total dietary, not absorbed.
Historically, a dietary N to S ratio of 10:1 to 12:1 has been considered optimal (Bouchard and Conrad, 1973a). However, no evidence is available indicating that the ratio is important when the dietary S requirement is met. Low-protein diets may benefit from S supplementation, but that is because dietary S probably was also low.
Sources
The S content of feedstuffs is positively correlated to the protein concentration; however, the use of S-based fertilizers can increase S concentrations of forages without a concomitant increase in protein concentrations (Spears et al., 1985; Arthington et al., 2002). S is not routinely assayed by many feed-testing labs, and table averages will likely underestimate concentrations for forages that have been fertilized with S. Most of the S in plants is in S-containing AAs, and those AAs reach the intestine to be absorbed, are used by rumen microbes to synthesize bacterial AAs, or are degraded. Within the rumen, S is released from degradation of S-AAs, but the rate of release is much greater for cysteine than for Met (Bird, 1972b). Furthermore, in vitro ruminal degradation of Met is slower than breakdown of other AAs (Mbanzamihigo et al., 1997). Theoretically, a diet with very low protein degradability could be limiting in rumen-available S, even though total dietary S is adequate; however, under practical situations, this is unlikely to occur.
Cows can consume inorganic S (usually as sulfate, SO42−) via forages that have been fertilized with S-containing fertilizers, distillers grains (Nietner et al., 2015), water (see Chapter 9), and from S supplements (e.g., MgSO4, CaSO4, and K2SO4). Based on in vitro rumen measures, in vivo S balance, and ruminant growth studies (mostly with sheep), the different sources of inorganic Sr have similar biological value (Henry and Ammerman, 1995).
Within cells and extracellular spaces of the cow, SO42− is involved with acid-base balance and perhaps other functions. Oxidation of S-AAs within cells is a major source of SO42−, but SO42− transporters also exist in the intestine (Markovich, 2001), and SO42− can be absorbed by ruminants (Bird and Moir, 1971). However, much of the ingested SO42− is probably reduced to hydrogen sulfide within the rumen.
Excess Sulfur
Excess ingested S (includes S from the diet and drinking water) causes indirect and direct negative effects on cow health and productivity. Excess intake of S can lead to deficiencies or reduced status of many trace minerals. Providing approximately 0.2 percent added S from SO42− (total diet S at approximately 0.4 percent) reduces the absorption of copper (Cu) and Se (van Ryssen et al., 1998; Ivancic and Weiss, 2001; Richter et al., 2012), and newer data suggest it may also negatively affect manganese (Mn) and Zn retention in cattle (Pogge et al., 2014). Negative effects of dietary S probably occur at concentrations less than 0.4 percent of the diet. Increasing dietary S from 0.13 percent up to 0.35 percent by increasing dietary inclusion of distillers grains linearly decreased liver Cu concentrations in feedlot lambs (Felix et al., 2012).
Excess SO42− added to rations can reduce feed intake and performance without eliciting any signs of clinical toxicity (Kandylis, 1984) perhaps mediated via a reduction in DCAD (discussed above). Diets with 0.2 percent added SO42 − S reduced DMI by lactating dairy cows (Ivancic and Weiss, 2001; Tebbe et al., 2018). High concentrations of SO42− in water can reduce water intake (see Chapter 9).
Clinical S toxicity causes neurologic changes, including blindness, coma, muscle twitches, and recumbency (Kandylis, 1984). Many of those clinical signs are consistent with polioencephalomalacia (Gould, 1998). Postmortem examination reveals severe enteritis, peritoneal effusion, and petechial hemorrhages in many organs, especially kidneys (Bird, 1972a). Often the breath will smell of hydrogen sulfide (H2S)—which is likely the toxic principal in S toxicosis. Much of the ingested SO42− and S from ruminally degraded S-AAs is reduced to H2S. When dietary S concentrations are close to requirement (i.e., 0.2 percent), the H2S is used by ruminal bacteria to synthesize S-AAs and other organic S-containing compounds (i.e., assimilatory pathway), resulting in low ruminal concentrations of H2S. However, when higher concentrations of dietary S are fed (usually from SO42− sources), dissimilatory reduction of SO42− by SO42− reducing bacteria occurs, and ruminal H2S concentrations become elevated (see review by Drewnoski et al., 2014). The generally accepted etiology of H2S toxicity is that at low rumen pH (pKa of H2S is about 7), much of the H2S produced remains as H2S, which is volatile and can be eructated. After eructation, it can be inhaled, enter the circulation, and reach the brain, causing brain damage and polioencephalomalacia (Bird, 1972a). In beef cattle fed high-grain finishing diets without any forage, the risk of polioencephalomalacia increases greatly when total dietary S is greater than 0.42 percent (water assumed to provide trivial amounts of S), but when the diet contained 8 percent forage NDF, dietary S had to be closer to 0.6 percent to increase the risk (Nichols et al., 2012). Higher dietary fiber should increase ruminal pH, causing more of the H2S to be dissociated (HS−) and not volatile. Because diets fed to dairy cows typically have substantially more forage than feedlot diets, polioencephalomalacia is not likely to be observed in dairy cattle even at very high concentrations of dietary S. NRC (2005) set the MTL of dietary S (water assumed to be a trivial source of S) at 0.3 percent for diets with 85 percent concentrate and at 0.5 percent for diets with at least 40 percent forage (more representative of dairy cow diets).
Sulfate anions have been added to rations of dry cows before calving to decrease the DCAD to help prevent milk fever (see Chapter 12), often to levels above 0.5 percent S. That concentration of S in high-forage diets should not cause clinical toxicity; however, Se and Cu absorption will likely be reduced, but because these diets are typically only fed for a few weeks, the overall effect on Se and Cu status is small. Longer-term feeding of high S diets (e.g., 0.5 percent) is not recommended.
TABLE 7-3Concentrations of Cr in Common Feeds (mg Cr/kg DM)a
| Feedb | Mean | SD | Range |
|---|---|---|---|
| Alfalfa hay or silage* | 0.522 | 0.220 | 0.199–0.889 |
| Beet pulp* | 1.222 | 0.386 | 0.776–1.451 |
| Corn grain, ground* | 0.049 | 0.031 | 0.014–0.114 |
| Corn grain, whole | 0.026 | 0.015 | 0.008–0.054 |
| Corn silage* | 0.220 | 0.087 | 0.105–0.441 |
| Cottonseed* | 0.094 | 0.086 | 0.033–0.155 |
| Dried distillers grains | 0.160 | 0.056 | 0.084–0.238 |
| Grass hay* | 0.155 | 0.093 | 0.098–0.320 |
| Oats, whole | 0.025 | 0.008 | 0.021–0.034 |
| Soybean, whole | 0.069 | 0.035 | 0.034–0.122 |
| Soybean hulls, loose | 0.262 | 0.073 | 0.191–0.336 |
| Soybean hulls, pelleted* | 0.550 | 0.175 | 0.309–0.705 |
| Soybean meal | 0.208 | 0.050 | 0.154–0.286 |
| Wheat | 0.041 | 0.014 | 0.029–0.062 |
| Wheat middlings | 0.084 | 0.031 | 0.044–0.132 |
- a
Source of data: Spears et al. (2017).
- b
Feeds with an asterisk were ground through a Wiley mill prior to Cr analysis and contamination is likely (Spears et al., 2017).
MICROMINERALS
Chromium
Chromium (Cr) is an essential nutrient, although requirements have not been quantified for cattle. An AI for Cr of 20 to 44 μg/d has been established for humans (NRC, 2006). Several forms of supplemental Cr have been fed to cattle, including chromium chloride, chromium picolinate, chromium nicotinate, chromium-enriched yeast, chromium Met, and chromium propionate. At the present time (2021), the only approved Cr supplement that can be fed to cattle in the United States is chromium propionate, and the maximum legal rate is 0.5 mg supplemental Cr/kg of diet DM.
Reliable data on the Cr concentrations in feeds are difficult to obtain because concentrations are very low (μg/kg DM range) and contamination occurs readily during sample processing (e.g., using a steel grinder). For example, concentrations of Cr in ground corn grain and ground soybean seed samples were twice the concentration measured in their unground counterparts (see Table 7-3). The lack of reliable data on basal concentrations of Cr in diets greatly limits the ability to establish AI or requirements for Cr.
Biochemistry and Absorption
In mammalian tissues, the primary active form of Cr is as a component of a small peptide called chromodulin. Chromodulin contains only four AA residues but can bind 4 Cr (Cr3+) ions and has been isolated from bovine liver (Davis and Vincent, 1997) and colostrum (Yamamoto et al., 1988). This compound is thought to bind to insulin-activated insulin receptors and stimulate tyrosine kinase, resulting in enhanced responses to insulin (Vincent, 2000). It may have other functions related to insulin activity. Cr also is likely involved in gene regulation.
Little is known regarding the absorption mechanism for Cr, but in nonruminants, absorption is usually <1 percent of Cr intake (Lukaski, 1999). Absorption of Cr from organic sources in nonruminants is greater (~3 percent of intake) than that for inorganic sources (Cefalu and Hu, 2004). Potential antagonists to Cr absorption include high concentrations of dietary Fe and perhaps Zn and phytate (Pavlata, 2007). Organic sources of Cr may also be converted more quickly within the body to biologically active forms, and organic forms usually have greater biological effects than inorganic sources (Vinson and Hsiao, 1985; Balk et al., 2007). Quantitative data on absorption of any form of Cr by ruminants are not available.
Cattle Responses to Supplemental Chromium
An extensive review of animal responses to Cr was conducted in 1997 (NRC, 1997), and another review limited to cattle was published in 1999 (Kegley and Spears, 1999). This section will concentrate on more recent publications. Studies have evaluated the effects of supplemental Cr on production measures, glucose and lipid metabolism, and immune function. Source of Cr and supplementation rate varied among studies, but most studies used chromium picolinate, chromium Met, or chromium propionate, and supplementation rates were usually 4 to 10 mg/d (approximately 0.5 mg Cr/kg diet DM when fed to lactating dairy cows). The effect of supplemental Cr on insulin sensitivity depends on the physiological state of the animal. Within 1 week or so prior to parturition, supplemental Cr often reduces insulin sensitivity in multiparous cows, as measured by an increased plasma insulin to plasma glucose ratio (Hayirli et al., 2001; Pechova et al., 2002). With primiparous animals, supplemental Cr starting 6 weeks prepartum increased insulin sensitivity when measured 2 weeks prepartum but reduced it when measured 2 weeks postpartum (Subiyatno et al., 1996). Increased plasma insulin prepartum can stimulate lipogenesis and suppress lipolysis, which can explain why supplemental Cr prepartum often reduces plasma nonesterified fatty acids (NEFAs) (Hayirli et al., 2001; Bryan et al., 2004). Elevated plasma NEFA prepartum is a risk factor for several health disorders in dairy cows (Roberts et al., 2012; McArt et al., 2013; Qu et al., 2014). Cr supplementation of growing beef and dairy animals enhances insulin sensitivity (Sumner et al., 2007; Spears et al., 2012). This should result in increased glucose uptake and increased protein synthesis by skeletal muscle. In early lactation dairy cows, supplemental Cr increased insulin responsiveness in one study (Hayirli et al., 2001), but in a limited study, supplemental Cr reduced insulin sensitivity (Subiyatno et al., 1996). Supplementation rates were similar between studies, but source of Cr differed. Supplemental Cr (approximate intake of 6 to 9 mg/d) usually increases milk yields in early lactation, higher-producing (>30 kg/d) cows (NRC, 1997; Hayirli et al., 2001a; AlSaiady et al., 2004; Smith et al., 2005; Sadri et al., 2009; Vargas-Rodriguez et al., 2014), but not in lower (ca. 30 kg/d)–producing cows (Bryan et al., 2004).
Supplemental Cr has improved certain measures of immune function in beef and dairy cattle (reviewed by Weiss and Spears, 2005). The most consistent effect has been increased blastogenesis of cytotoxic T-lymphocytes, which may be modulated via reduced cortisol concentrations. Neutrophil function has not been affected by Cr (Weiss and Spears, 2005); however, concentrations of proinflammatory cytokines in activated neutrophils were greater when cows were supplemented with Cr (Yuan et al., 2014). This may enhance overall immune response, but in a clinical trial, Cr did not affect incidence of mastitis (Chang et al., 1996).
Although production and immune function responses to supplemental Cr are often positive and Cr has been shown to have metabolic effects, establishing a requirement for Cr is not possible because total intakes of Cr (basal plus supplemental) have not been measured. Glucose and insulin often respond to Cr supplementation, but results are not linear with dose. The lowest rate of supplementation (0.01 mg Cr/kg BW for growing cattle or 0.006 mg Cr/kg BW for lactating cow) usually was adequate for maximal response (Hayirli et al., 2001; Spears et al., 2012). Whether the changes observed in insulin and glucose represent improvements in metabolism are not known. Milk yield has responded linearly and quadratically to increasing supplemental Cr (Hayirli et al., 2001; Smith et al., 2008). The maximal response occurred at a supplementation rate of approximately 0.01 mg Cr/kg BW (~6 mg/d) but in the study that reported a linear response that was the highest rate tested. Although an AI for Cr cannot be established based on only two titration studies with lactating cows, supplementation of approximately 0.01 mg Cr/kg BW often increases milk yield in early lactation.
Maximum Tolerable Level
The maximum tolerable dietary concentration for Cr3+ from soluble forms was set at 100 mg Cr/kg of diet DM (NRC, 2005), but data are very limited regarding adverse responses to high dietary concentrations of Cr3+. Hayirli et al. (2001) reported that milk yield responded quadratically to increasing Cr and feeding cows 0.025 mg Cr/kg BW (15 mg/d) reduced milk yields to values similar to the control. Concentrations of Cr in milk, muscle, and body fat were not greater in cows fed 2 mg Cr/kg of diet DM compared with cows fed no supplemental Cr; however, Cr concentrations in liver and kidney were two to three times greater (Lloyd et al., 2010). In vitro, Cr picolinate can increase production of the hydroxyl radical, which can negatively affect immune function, damage DNA, and oxidize membrane FAs (Vincent, 2000). The concentration of Cr at which this occurs in vivo is unknown, and it is not clear whether this effect is unique to Cr picolinate.
Cobalt
Function
The primary function of cobalt (Co) is to serve as a precursor for vitamin B12 (cobalamin) synthesis in the rumen. Rumen microbes can usually produce adequate vitamin B12 if adequate Co is available in the diet (see Chapter 8 for details). In addition to synthesizing vitamin B12, bacteria can synthesize vitamin B12 analogues, which are not biologically active. The presence of these vitamin B12 analogues in liver and blood reduces the utility of vitamin B12 determination to assess the status of dietary Co (Halpin et al., 1984). However, hepatic vitamin B12 concentrations below 0.1 μg/g wet weight are indicative of Co deficiency (Smith, 1987). A portion of dietary Co can be absorbed in the cation form (Smith, 1987); however, it has no known function and, once absorbed, does not appear capable of reentering the rumen so microbes could use it. Most is excreted in the urine, and a smaller amount exits with the bile (Underwood, 1981).
Cobalt carbonate, chloride, nitrate, sulfate, and glucoheptonate all appear to be suitable sources of Co for ruminants. Cobalt oxide, which is much less soluble, resulted in much lower vitamin B12 concentrations during in vitro rumen fermentation (Kawashima et al., 1997), and was somewhat less available (Henry, 1995a). Cobalt oxide pellets and controlled-release glass pellets containing Co that remain in the rumen–reticulum have been used successfully to supply Co over extended periods of time to ruminants on pasture, although regurgitation can cause loss of some types of pellets (Poole and Connolly, 1967).
Deficiency
Ruminants appear to be more sensitive to vitamin B12 deficiency than nonruminants (see Chapter 8 for details). This is likely because vitamin B12 is a key component in the pathways for gluconeogenesis and de novo methyl group synthesis. Ruminants are dependent on gluconeogenesis for meeting needs of tissues for glucose with ruminally derived propionate serving as a primary glucose precursor. A breakdown in propionate metabolism where methylmalonyl-CoA is converted to succinyl-CoA is a primary defect arising from a deficiency of vitamin B12. Inadequate dietary Co elevates plasma homocysteine concentrations in growing beef cattle, suggesting a deficiency in methyl group availability for resynthesis of Met from homocysteine (Stangl et al., 2000). Stores of vitamin B12 in the liver of adult ruminants are usually sufficient to last several months when they are placed on a Co-deficient diet. Hepatic concentrations of vitamin B12, urinary and plasma concentrations of methylmalonic acid, and serum concentrations of homocysteine have been used to evaluate Co (and vitamin B12) status of cattle (Stangl et al., 2000).
Young animals are more sensitive to dietary insufficiency of Co because they have lower reserves of vitamin B12 in the liver. Early signs of a deficiency of Co include failure to grow, unthriftiness, and loss of weight (Smith, 1997). More severe signs include fatty degeneration of the liver, anemia with pale mucous membranes (Underwood, 1981), and reduced resistance to infection as a result of impaired neutrophil function (MacPherson et al., 1987; Paterson and MacPherson, 1990). Although cows may have adequate stores of vitamin B12 to last several months, ruminal microbes do not. Within a few days of feeding a diet deficient in Co, ruminal concentrations of succinate rise. This may be the result of a blockade of microbial conversion of succinate to propionate, or a shift in ruminal bacterial populations toward succinate production rather than propionate production (Kennedy et al., 1996).
Requirement
The dietary requirement for Co was previously estimated to be 0.11 mg/kg of dietary DM. This was based on the amount of Co that must be supplied to keep plasma concentrations of vitamin B12 above 0.3 μg/L (Marston, 1970). However, depending on the biochemical response criteria, adequate dietary Co (basal plus supplemental) ranged from 0.13 to 0.25 mg Co/kg diet DM for growing beef cattle (Stangl et al., 2000). Maximal growth in beef cattle occurred when the diet contained between 0.15 and 0.18 mg Co/kg DM (Schwarz et al., 2000; Tiffany et al., 2003). Liver vitamin B12 and folate concentrations were maximized at 0.24 and 0.19 mg Co/kg, respectively, whereas plasma vitamin B12 was maximized at a dietary Co concentration of 0.26 mg/kg (Stangl et al., 2000). The dietary Co concentrations required to minimize plasma homocysteine and methyl malonic acid concentrations were 0.16 and 0.12 mg/kg of diet DM, respectively. Milk yield responses to increasing dietary Co have not been finely titrated. Few positive production responses have been reported when Co was supplemented to dairy cows, but in those studies, the lowest concentration evaluated was approximately 0.20 mg Co/kg of diet DM (Kincaid et al., 2003; Kincaid and Socha, 2007; Akins et al., 2013). Feeds are not commonly assayed for Co (sensitivity is an issue), but in the studies above, basal concentrations averaged about 0.1 mg Co/kg DM. Because of the lack of adequate data on basal feed and the variable concentrations required for maximal growth and biochemical indicators responses, the AI of total Co (basal + supplemental) is set at 0.2 mg Co/kg of diet DM or
where DMI is kg/d.
Beef steers fed barley in high-grain diets (85 percent of diet DM) had lower concentrations of vitamin B12 in rumen fluid and in plasma than steers fed a corn-based diet at similar concentrations of supplemental Co (Tiffany and Spears, 2005). Whether type of grain affects Co conversion to vitamin B12 when fed in typical dairy cow diets (usually<40 percent starch grains) is unknown.
Special Properties of Cobalt
Dietary Co may also have some effects independent of its necessity for production of vitamin B12. Co fed at 0.25 to 0.35 mg/kg of dietary DM, well above those required for sufficient vitamin B12 synthesis, seems to enhance ruminal digestion of feedstuffs, especially lower-quality forages (Saxena and Ranjhan, 1978; Lopez-Guisa and Satter, 1992). This effect may be due to selection of certain microbial populations with a higher Co requirement or may be a result of the divalent Co cation forming crosslinks between negatively charged bacteria and negatively charged forage particles, which allows bacteria to attach to forage particles more efficiently (Lopez-Guisa and Satter, 1992). Cu, Ca2+, and Mg2+ are divalent cations that may have some of the same ability (Storry, 1961; Somers, 1983). Addition of Co has increased total anaerobic bacteria in the rumen by 50 percent and lactic acid production in the rumen by 86 percent (Young, 1979).These results suggest that ruminal microbes may require greater concentrations of dietary Co than the cow. However, the general lack of effects on DMI, milk composition, and milk yield when diets contained 0.15 to 0.20 mg Co/kg DM suggests that the AI of 0.20 mg Co/kg is also adequate for ruminal bacteria.
Toxicity
Co toxicity causes reduced feed intake, loss of BW, and eventually anemia—signs similar to those seen in Co deficiency (Ely et al., 1948; Keener et al., 1949; NRC, 2005). The maximal tolerable dietary Co concentration has been set at 25 mg/kg of dietary DM (NRC, 2005).
Copper
Function
Cu is a component of several proteins, including cytochrome c oxidase (required for aerobic respiration), lysyl oxidase (required for formation of collagen and elastin), and tyrosinase (necessary for production of melanin pigment). Cu is required for hemoglobin synthesis and is involved with iron (Fe) metabolism (e.g., as a component of ceruloplasmin). Cu, along with Zn, is a component of cytosolic superoxide dismutase, which protects cells from the toxic effects of reactive oxygen species (ROS). This is particularly important in phagocytic cells and may be a primary mode of action for reduced infectious disease when adequate Cu is fed.
Absorption of Dietary Copper
Intestinal absorption of Cu by humans and rodents appears to be under homeostatic control and is upregulated when low Cu diets are fed and downregulated with high intakes of Cu (Lönnerdal, 2008). Whether homeostatic regulation of absorption occurs in ruminants has not been determined. A weak negative relationship was observed between initial concentration of Cu in liver and rate of accumulation of liver Cu, but over a wide range of initial concentrations (60 to 230 mg Cu/kg of liver dry weight), rate of liver accumulation was essentially constant in nonlactating dairy cows (Balemi et al., 2010). That range encompasses what is considered adequate Cu status. Age of the animal, chemical form of dietary Cu, and the presence of antagonists affect intestinal absorption of Cu. In calves without functioning rumens, the Cu AC can be as high as 0.70. Bremner and Dalgarno (1973a,b) found that 50 to 60 percent of dietary Cu (supplied as Cu sulfate) was retained in liver for calves between 3 and 14 weeks of age. As the rumen starts functioning, absorption of Cu decreases substantially, and the AC for Cu is usually ≤0.05 in adult cattle.
Effect of Other Minerals on Copper Absorption
S, molybdenum (Mo) in combination with S, and Fe antagonize Cu absorption in ruminants. Zn concentrations need to be 10 to 20 times requirement before antagonism is observed in ruminants (Miller et al., 1989), so Zn antagonism is not of practical importance. Antagonism of Cu by Fe could occur via competition for intestinal binding sites (e.g., divalent metal transporter or DMT1), and Fe may exacerbate the reaction between Cu and S within the rumen (Gould and Kendall, 2011). Fe antagonism of Cu absorption usually requires supplemental Fe at concentrations exceeding 250 mg Fe/kg of diet DM (Chase et al., 2000; Mullis et al., 2003). Dietary Fe concentrations can be that high because of the Fe in forages. High Fe concentrations in forages are likely caused by soil contamination and may not greatly affect Cu absorption because the Fe is mostly in the form of ferric oxide, which is essentially inert. However, high-Fe soil has been implicated in reducing Cu status of grazing sheep (Suttle et al., 1984). Because of acid conditions in silages, over time, the Fe in silages may become more reactive (Hansen and Spears, 2009) and might cause increased antagonism. Data are not available showing lower Cu status in cattle fed silages with high concentrations of Fe.
Increased consumption of S (dietary and via drinking water) in the absence of high Mo concentrations reduces Cu status of cattle (Arthington et al., 2002; Pogge et al., 2014). The antagonism may occur because of formation of Cu sulfides within the rumen. The dietary concentration of S required to reduce Cu absorption or Cu status has not been titrated, but liver concentrations of Cu in beef cattle decreased when diets contained 0.5 to 0.6 percent total S (Arthington et al., 2002; Pogge et al., 2014). Water that contained approximately 500 mg S (as sulfate)/L also reduced liver Cu concentrations in growing beef heifers (Wright et al., 2000). Mo interacts with S and exacerbates the antagonism. Within the rumen, S and Mo can form thiomolybdates and bind soluble Cu, but thiomolybdates can be absorbed into the circulation and bind Cu compounds within the animal (Gould and Kendall, 2011). Evidence that dietary Mo in the absence of high dietary S interferes with Cu absorption is lacking (Gardner et al., 2003). Many of the negative responses observed when dietary Mo is elevated may actually be signs of molybdenosis. An equation to estimate absorption of Cu based on dietary S and Mo has been developed using data from sheep experiments (McLauchlan and Suttle, 1976):
where Cu absorption = g absorbed/g of total Cu; S = dietary S (g/kg of diet DM) and Mo = dietary Mo in mg/kg of diet DM.
The accuracy of this equation has not been evaluated with cattle and was not included in the software. However, based on that equation, Cu absorption from diets with low Mo (≤1 mg Mo/kg) and dietary S at concentrations of 0.3, 0.4, and 0.5 percent would be reduced approximately 15, 30, and 43 percent compared with a diet with 0.22 percent S. With 4 mg Mo/kg, estimated absorption would be reduced another approximately 25 percent at each of those S concentrations.
Other Factors Affecting Absorption of Copper
Soil consumption at rates that may occur during grazing reduced absorption of Cu by 50 percent in sheep (Suttle, 1975) and cattle (Dewes, 1996). The effect of grazing on Cu absorption is likely a function of stocking rate and height of the sward (lower sward and greater stocking rate will increase soil consumption) and type of soil (Grace et al., 1996). Soils with clays may have a greater negative effect on Cu absorption. The model does not include an adjustment of Cu availability for grazing cattle because of the numerous uncertainties; however, users may wish to increase intake of Cu by cattle grazing short swards on clay soils.
Breed differences exist among cattle in susceptibility to Cu toxicity. Jersey cattle fed the same diet as Holstein cattle accumulated more Cu in their livers (Du et al., 1996; Morales et al., 2000). Whether this reflects differences in feed intake, efficiency of Cu absorption, hepatic storage, or biliary excretion of Cu is not known. However, differences in abundance of a Cu transporter in intestinal cells are likely a major cause of differences in Cu metabolism among beef cattle breeds (Fry et al., 2013). Because of a lack of data on Cu absorption by different breeds, the model does not include breed effects when calculating Cu requirements or Cu supply. Requirements and supply were calculated largely using data from Holsteins; therefore, absorbed supply of Cu may be underestimated, and requirements may be overestimated for Jersey cattle.
Effect of Source of Copper
Cu is found in most common feedstuffs in the range of 4 to 15 mg Cu/kg DM, and true absorption of Cu in those feeds was set at 0.05 (Buckley, 1991), assuming total diet had <0.22 percent S and <1 mg Mo/kg. In the seventh revised edition (NRC, 2001), the AC for feedstuffs was set at 0.04. Inorganic Cu is usually supplemented in the sulfate, chloride, carbonate, or oxide forms. Several commercial products in which the Cu is chelated or associated with organic compounds (e.g., AAs or carbohydrates) are also available. Very little data on actual true absorption of Cu from these sources are available, but several studies have been conducted to evaluate relative bioavailability by comparing changes in hepatic Cu concentrations when cattle are fed different Cu sources. Other biomarkers have been used to calculate relative bioavailability, but their accuracy and sensitivity are uncertain. The AC for Cu from Cu sulfate (CuSO4) was set at 0.05 in the previous edition (NRC, 2001), and that value was retained. The AC for other Cu supplements was based on relative bioavailability studies using liver Cu concentrations when available.
The bioavailability of Cu from dietary copper oxide (CuO) is almost zero (Langlands et al., 1989; Kegley and Spears, 1994); however, ruminal boluses containing CuO can be effective sources of Cu (Parkins et al., 1994). The long-term exposure (weeks or months) of CuO in a bolus to the ruminal environment increases its availability, whereas finely ground CuO does not stay in the rumen long enough to be solubilized. The bioavailability in Cu from copper chloride (CuCl2) is similar to that of CuSO4 (Ivan et al., 1990). In many studies, indicators of Cu status were not different in cattle fed CuSO4 or proprietary Cu supplements, including Cu proteinates, Cu-AA complexes and tribasic CuCl2 (Wittenberg et al., 1990; Ward and Spears, 1993; Du et al., 1996; Arthington et al., 2003; Spears et al., 2004; Correa et al., 2014). However, in other studies, proprietary products were significantly more available than CuSO4 (Kincaid et al., 1986; Rabiansky et al., 1999; Hansen et al., 2008). Reasons for the inconsistent results are not clear, but relative availability of proprietary forms of supplemental Cu probably depends on the presence of antagonists (e.g., S and Mo), Cu status, and specific product being fed. For example, tribasic CuCl2 was almost twice as effective at increasing liver Cu concentration as CuSO4 in the presence of S and Mo, but when those Cu sources were fed to cattle in low Cu status without antagonists, they had equal bioavailability (Spears et al., 2004). Inadequate data are available to quantify factors affecting relative bioavailability of proprietary Cu supplements; therefore, the generic commercial Cu supplement in the feed library was assigned the same AC as CuSO4. Users can modify the value based on available data for the specific product and situation.
Copper Requirements
Endogenous losses of Cu were assumed to be predominantly via bile and are expressed relative to BW. Using Cu isotopes (Buckley, 1991), biliary and urinary loss of Cu was approximately 0.0145 mg Cu/kg BW. This is more than twice the value used in the seventh revised edition (NRC, 2001). Cu content of growing tissues, when the liver is included as part of the carcass, is 2 to 2.5 mg Cu/kg based primarily on studies of sheep and beef cattle (Grace, 1983; Miranda et al., 2006; García-Vaquero et al., 2011). Because of concerns with the consumption of excessive Cu, the growth requirement was set at 2.0 mg Cu/kg live weight change. Cu content of milk is about 0.04 mg/kg when diets contain typical concentrations of Cu (Castillo et al., 2013; Faulkner et al., 2017), but it can be as high as 0.2 mg Cu/kg when animals are fed a high Cu diet (Schwarz and Kirchgessner, 1978). The requirement for absorbed Cu for lactation was set at 0.04 mg Cu/kg milk produced. This is about a 70 percent decrease compared to the 0.15 mg Cu/kg milk produced used by the seventh revised edition (NRC, 2001). The conceptus at 190 days of gestation of Holstein cows (average BW = 715 kg) contained approximately 20 mg Cu (House and Bell, 1993). Because data are lacking, the committee assumed no Cu accumulated during the first trimester, and accumulation was linear between 90 and 190 days of gestation, so that gestation requirement for absorbed Cu was (0.2 mg Cu accumulated/d from 90 to 190 days of gestation or 0.3 μg Cu/kg maternal BW/d). From 190 days of gestation until calving, Cu accumulation in conceptus was 1.6 mg/d or 2.3 μg Cu/kg of maternal BW/d (House and Bell, 1993).
For an average lactating Holstein cow (35 kg of milk, 650 kg BW, 150 days pregnant), the total requirement for absorbed Cu is 11.0 mg/d compared with 11.4 mg obtained using the seventh revised edition (NRC, 2001). Assuming an intake of 23 kg and a dietary AC of 0.045, dietary Cu concentration of about 11 mg/kg would meet her requirement. Requirements for a 700-kg nonlactating cow at 260 days of gestation is 11.7 mg/d (approximately 20 mg dietary Cu/kg assuming an intake of 13.5 kg) compared with 7 mg/d calculating using the seventh revised edition (NRC, 2001).
Summary of Equations (mg absorbed Cu/d)
where ADG and milk are in kg/d, and BW is in kg.
Signs of Copper Deficiency
Clinical signs of Cu deficiency are generally nonspecific (i.e., reduced growth rate, ill-thrift, increased prevalence of disease, reduced reproductive efficiency), but Cu deficiency can result in a loss of hair pigment or loss of hair. Diarrhea can also occur with Cu deficiency, but that may be related to excess Mo. Anemia (hypochromic macrocytic), fragile bones and osteoporosis, and cardiac failure also are observed in Cu deficiency (Underwood, 1981). Inadequate supply of Cu reduces the killing ability of phagocytic cells of cattle, but responses to supplemental Cu on cellular and humoral immunity have been inconsistent (Weiss and Spears, 2005). Impaired immune function is likely the reason cows fed inadequate Cu have more severe mastitis than those fed adequate Cu (Scaletti et al., 2003). The control diets in essentially all studies that showed improved immune function and reduced infectious disease would not have met current Cu requirements.
Assessing Copper Adequacy
Dietary concentrations of Cu are of limited value in assessing adequacy of Cu supply because of variable dietary and water concentrations of antagonists (discussed above). Plasma concentrations of Cu <0.5 mg/L are generally considered indicative of clinical Cu deficiency. However, other than confirming overt Cu deficiency, plasma concentrations are not useful in assessing status, including situations with excessive stores of Cu in the liver (López-Alonso et al., 2006). Activities of Cu-containing proteins (e.g., ceruloplasmin or superoxide dismutase) are generally considered unreliable biomarkers of Cu status in cattle (Mulryan and Mason, 1992; López-Alonso et al., 2006; Hepburn et al., 2009). Concentrations of the Cu chaperon protein may have potential as a marker of Cu status, but additional research is needed (Hepburn et al., 2009). Concentrations of Cu in liver are the standard for assessing Cu status in cattle, although recommended reference values vary. Concentrations of Cu in liver <10 mg/kg on a DM basis are generally considered indicative of impending clinical deficiency, and values less than about 35 mg Cu/kg DM are generally considered suboptimal (Smart et al., 1992; Underwood and Suttle, 1999). However, in the presence of high dietary Mo and S, which promote formation and absorption of thiomolybdates into the blood, Cu in liver may not accurately reflect Cu status (Suttle, 1991). The concentration of Cu in liver indicative of excess has not been definitively identified, but field reports indicate Cu toxicity occurs with liver concentration of 300 to 350 mg Cu/kg dry weight (Auza et al., 1999; Grace and Knowles, 2015).
Copper Toxicity
Cu toxicosis can occur in cattle that consume excessive amounts of supplemental Cu or feeds that have been contaminated with Cu compounds used for other agricultural or industrial purposes (Underwood and Suttle, 1999). When cattle consume excessive Cu, they accumulate large amounts of Cu in the liver before toxicosis becomes evident. Stress or other factors may result in the sudden liberation of large amounts of Cu from the liver to the blood, causing a hemolytic crisis. Such crises are characterized by considerable hemolysis, jaundice, methemoglobinemia, hemoglobinuria, generalized icterus, widespread necrosis, and often death (Steffen et al., 1997; Underwood and Suttle, 1999; Johnston et al., 2014). Because of antagonists (e.g., S and Mo) and because Cu continues to accumulate in the liver when excess Cu is fed (Balemi et al., 2010), defining a dietary concentration that will result in toxicity is not possible. NRC (2005) set the MTL for dietary Cu for cattle at 40 mg Cu/kg DM. However, growth rate and feed conversion were reduced in beef cattle with liver Cu concentrations of 290 mg/kg dry weight and fed diets with 20 mg supplemental Cu/kg DM for a total dietary concentration of 30 mg Cu/kg (Engle and Spears, 2000), and fiber digestibility by dairy cows was reduced when CuSO4 was supplemented to increase total dietary Cu to 20 mg/kg (Faulkner and Weiss, 2017).
Iodine
Function
The sole role of iodine (I) as a required nutrient is for the synthesis of the thyroid hormones thyroxine (T4) and triiodothyronine (T3) that regulate energy metabolism. The amount of I incorporated into thyroid hormones was about 0.25 mg/d in calves weighing 45 kg and increased to 1.4 mg I/d in nonpregnant heifers weighing 400 kg (Mixner et al., 1966). Late-gestation cows incorporate about 1.5 mg I/d into thyroid hormone (Miller et al., 1988). Thyroid hormone production increases during lactation, especially in high-producing cows, and I incorporation into thyroid hormones may reach 4 to 4.5 mg I/d (Sorensen, 1962). Thyroid hormone production also is increased during cold weather to stimulate an increase in basal metabolic rate (Goodman and Middlesworth, 1980). About 80 to 90 percent of dietary I is absorbed, and most of the I not taken up by the thyroid gland is excreted in urine and milk (Miller et al., 1988). The I content of milk is a reasonable indicator of I status because it increases as dietary I intake increases (Berg et al., 1988). The availability of assays for thyroxine and thyroxine-stimulating hormone (TSH) might provide an accurate assessment of thyroid function and the causes of thyroid dysfunction. Alternatively, blood TSH concentrations might be used as a biomarker for thyroid function to reevaluate the minimum I requirements in dairy cattle.
When the I content of the diet is adequate or excessive, less than 20 percent of the dietary I will be incorporated into the thyroid gland (Sorensen, 1962). Under conditions where intake of dietary I is marginal, the thyroid gland will incorporate about 30 percent of the dietary I into thyroid hormones (Miller et al., 1975). When severely I deficient, the hyperplastic thyroid can bind up to 65 percent of the I consumed by the cow (Lengemann and Swanson, 1957).
Adequate Intakes
I requirements in previous reports were based on a limited number of thyroxine production rates measured in cattle during the 1960s and 1970s. Due to the time that has elapsed since those studies were conducted and the limited number of measurements, the committee concluded that there were insufficient data to determine an estimated average requirement for I. Therefore, AIs, rather than requirements, were determined.
Total daily thyroxine secretion rate (TSR) increased at a decreasing rate in growing heifers ages 77 to 686 days (Mixner et al., 1962) and varied from 0.008 to 0.0030 mg/kg BW. In lactating cows, the mean thyroxine secretion rate was about 0.30 mg/100 kg BW (Mixner et al., 1962; Miller et al., 1975). The previous report listed separate requirements for maintenance, pregnancy, and lactation. It was suggested that the thyroxine secretion rate was 2.5-fold greater in lactating cows than in dry cows (Sorenson et al., 1962), yet limited evidence (Swanson et al., 1972) showed only a 25 percent increase from late pregnancy to lactation. The primary determining factor for TSR was BW. Therefore, the AI for maintenance for all groups of animals is based on TSR related to BW. In a summary of the studies of Swanson et al. (1972) and Mixner et al. (1962), TSR, mg/d = 0.0653 × BW0.528 (R2 = 0.96). Thyroxine (T4) contains 66 percent I and Miller et al. (1975) suggested that under conditions where I was not limiting, 20 percent of dietary I was used by the thyroid gland to synthesize thyroxine. Therefore, the maintenance AI for I can be predicted by the following equation: I, mg/d = 0.216 × BW0.528.
In addition to thyroxine synthesis, I is also secreted in milk (discussed below), and for lactating cows, this loss must be replaced by dietary intake. At low I intake, milk contains about 0.05 mg I/L, and transfer of dietary I to milk is about 0.5 (Swanson et al., 1972). Therefore, the AI for lactation was set at 0.1 mg/L of milk. The equation for total AI for all classes of cattle except calves is
where BW is kg and milk is kg/d.
The AI for I for nonruminating calves was based on the AI established for human infants and was set at 0.8 mg I/kg DMI. Once calves are ruminating, Equation 7-34 should be used.
This amount of I will likely not be adequate when diets contain goitrogenic feeds such as canola meal (Pappas et al., 1979). Diets with canola meal decreased transfer of I into milk by 50 percent (discussed below). Assuming thyroxine synthesis is affected similarly, the AI for animals fed diets with goitrogenic feeds would be twice that calculated above. However, because of limited data, this adjustment is not included in the model, but users should consider adjusting supplementation when goitrogens are fed. Based on typical DMI, a dry cow (700 kg BW) and an average lactating cow (650 kg BW and 35 kg/d of milk) would need to be fed diets with 0.51 and 0.48 mg I/kg DM when diets did not contain goitrogens and 1.02 and 0.96 mg I/kg DM with goitrogenic diets. Because of human health concerns related to excess I intake and the fact that milk I concentrations increase with increased I concentration in the cow's diet, supplemental I should not exceed amounts deemed as AI for the cow (see toxicity section).
Factors Affecting Iodine Needs
Goitrogens are compounds that interfere with the synthesis or secretion of thyroid hormones and cause hypothyroidism. Goitrogens fall into two main categories: (1) cyanogenic goitrogens impair iodide (I−1) uptake by the thyroid gland. Cyanogenic glucosides can be found in many feeds, including raw soybeans, beet pulp, corn, sweet potato, white clover, and millet, and once ingested are metabolized to thiocyanate and isothiocyanate. These compounds alter I−1 transport across the thyroid cell membrane, reducing I retention; (2) Progoitrins and goitrins found in cruciferous plants (rape, kale, cabbage, turnips, and mustard) and aliphatic disulfides found in onions that inhibit thyroperoxidase prevent formation of mono- and diiodotyrosine (Ermans and Bourdoux, 1989). With goitrins, especially those of the thiouracil type, hormone synthesis may not be restored by dietary I supplementation, and the offending feedstuff needs to be reduced or removed from the diet.
Canola meal derived from low erucic acid varieties of rapeseed contains glucosinolates that can be converted into thiocyanate during seed processing. Recent studies (Franke et al., 2009a,b; Weiss et al., 2015a) demonstrated that when canola meal substitutes for soybean meal in diets, milk I concentrations are reduced. While milk I continued to increase with increasing dietary I concentration (up to 5 mg/kg diet DM) when diets contained canola meal, the rate of increase in milk I was reduced by 50 percent or more depending on the amount of canola meal fed (Franke et al., 2009a; Weiss et al., 2015a). However, blood serum I concentrations were similar to controls (Weiss et al., 2015a), and urinary I excretion was increased, suggesting I absorption is not impaired (Franke et al., 2009b). The negative effects of dietary goitrogens can be overcome by increasing the concentration of dietary I or removal of the feeds containing goitrogens. Diets that contain goitrogenic ingredients may need more I than the recommended AI.
Supplemental I in the form of ethylenediamine dihydriodide (EDDI) has been used to decrease foot rot in beef cattle (Maas et al., 1984) and more recently has been suggested as a possible treatment for digital dermatitis in dairy cattle (Gomez et al., 2014). Dietary EDDI may also have value in treating ringworm in young cattle (Cam et al., 2007). However, concerns about excessive milk I concentrations with supplemental I would prevent the use of EDDI for these purposes in lactating dairy cows.
Sources of Iodine
Most sources of I are readily absorbable, and the iodides of Na, K, and Ca are commonly used. Potassium iodide is easily oxidized and volatilizes before the animal can ingest it. Pentacalcium orthoperiodate and EDDI are more stable and less soluble and are commonly used in mineral blocks and salt licks exposed to the weather. Concentrations of I in forage are variable and dependent on the I content of the soil. Soils near the oceans tend to provide adequate I in plants. However, in the Great Lakes regions and Northwest United States, I concentrations in forages are generally low enough to result in a deficiency of I unless I is supplemented.
Milk Iodine
Typical range in milk I concentration is 100 to 300 μg/L (Flachowsky et al., 2014), and I in dairy products is readily absorbed by humans. Milk and dairy products are major sources of I intake in the United States and Europe, where dairy products make up a significant portion of the average diet. Dairy products may account for 30 to 74 percent of I intake in the United States (Murray et al., 2008). The recommended dietary allowance for I intake ranges from 70 μg/d in infants to 290 μg/d in pregnant and nursing women (Swanson et al., 2012). However, the upper tolerable limit for I for humans is only 2.2- to 3.5-fold greater than recommended intake. A suggested limit for milk is 500 μg I/kg. While there have been concerns about excess I intake from milk, more recent evidence based on urinary I excretion suggests that a significant portion of individuals with high I requirements (pregnant and nursing women) may not be consuming adequate I. In part, this is due to the reduced consumption of iodized table salt in the United States. Milk and dairy products will continue to make an important contribution to I intake in the general population.
Flachowsky et al. (2014) reviewed factors that affected milk I concentration. By far, dietary concentration of I is the primary factor that affects milk I concentration. Berg et al. (1988) demonstrated a linear increase in milk I excretion with increasing supplementation of I in the form of EDDI with 26 to 39 percent of the supplemental I excreted in the milk. Other factors such as I source, I antagonists (discussed above), farm management practices including teat dipping with I-containing substances, and the use of I sanitizers in milk processing can affect milk I concentrations (Flachowsky et al., 2014). I present in iodophor-containing teat dips and udder disinfectants were shown to be absorbed through the teat skin and markedly increased milk I concentrations. However, use of iodophors was discontinued in dairy teat dips and disinfectants in the late 1980s such that milk I concentrations have gradually declined (Flachowsky et al., 2014).
Deficiency Symptoms
I deficiency reduces production of thyroid hormones, slowing the rate of oxidation of all cells. Often the first indication of I deficiency is enlargement of the thyroid (goiter) of newborn calves (Miller et al., 1968). Calves also may be born hairless, weak, or dead. Fetal death can occur at any stage of gestation, but often the cows will appear normal (Hemken, 1970). In adult cattle, I deficiency can cause enlarged thyroid glands, reduced fertility (males and females), and increased morbidity. Under conditions of marginal or deficient dietary I, the maternal thyroid gland becomes extremely efficient in removing I from the plasma and recovering I during the degradation of spent thyroid hormone and thyroglobulin. Unfortunately, this leaves little I for the fetal thyroid gland, and the fetus becomes hypothyroid. The goiter condition is the hyperplastic response of the thyroid gland to increased stimulation of thyroid growth by thyroid-stimulating hormone produced in the pituitary gland. Under mild I deficiency, the hyperplastic thyroid gland can compensate for the reduced absorption of I (Hetzel and Welby, 1997).
Toxicity
The maximum tolerable limit was set at 50 mg I/kg of diet DM (NRC, 2005). Clinical signs of I toxicity in ruminants include excessive nasal and ocular discharge, hyperthermia, salivation, decreased milk production, coughing, and dry, scaly coats (Paulikova et al., 2002). As discussed earlier, high concentrations of dietary I in the diet increase I concentrations in milk, and because humans are much more sensitive to I toxicity than cows, the danger of excess dietary I fed to cattle is a public health issue (Hetzel and Welby, 1997). Current U.S. Food and Drug Administration (FDA) regulations set the maximum limit of I supplementation in cattle from EDDI at 50 mg/d, which in a lactating cow consuming 25 kg of DM/d would correspond to a maximum concentration of 2.0 mg I/kg DM, roughly three times the amount considered to be needed.
Iron
Functions and Measures of Adequacy
Fe has a multitude of functions within the body, including oxygen transport (component of heme found in hemoglobin and myoglobin), electron transport (e.g., ferredoxins and cytochrome P-450 enzymes), immunity (e.g., myeloperoxidase and catalase), energy metabolism (e.g., aconitase), and gene regulations (Beard, 2001; Templeton and Liu, 2003). Fe deficiency results in hypochromic microcytic anemia due to failure to produce hemoglobin. Light-colored veal is due to low muscle myoglobin as a result of restricted dietary Fe. In young dairy cattle (<24 months old), serum ferritin had a stronger correlation with concentrations of Fe in liver and spleen than did hemoglobin (Miyata and Furugouri, 1987). However, over a lactation cycle, serum ferritin did not appear to reflect changes in Fe stores in dairy cows (Furugouri et al., 1982). Other measures of Fe status (e.g., Fe binding capacity of serum, plasma Fe) can change in response to factors that are independent of Fe status such as inflammation (Baydar and Dabak, 2014) and parturition (Miltenburg et al., 1991).
Because Fe deficiency is very rare in adult cattle, data evaluating the accuracy and sensitivity of Fe status indicators are not available. Common measures of Fe status did not change over the dry and early lactation (up to 60 days in milk) periods and were not affected when dry and lactating cows were fed 30 mg/kg of supplemental Fe from and Fe-AA complex (Weiss et al., 2010). Fe supplementation did not affect any production measure other than a small, but statistically significant, decrease in milk SCC. In Fe-deficient calves, morbidity and mortality associated with depressed immune responses occurred prior to any observed change in packed cell volume (Mollerberg and Moreno-Lopez, 1975). In lactating goats, hemoglobin concentrations were negatively related to SCC (Atroshi et al., 1986). Beard (2001) suggested that changes in functional measurements such as immune measures and performance during exercise in humans occurred prior to changes in hemoglobin and other measures of Fe status.
Requirement
Because of the uncertainty regarding the true absorption of Fe from feeds and the amount of Fe needed for maintenance, the factorial approach was used to generate an AI. During tissue and protein turnover, the majority of Fe is effectively recovered and recycled so that maintenance requirements for Fe are negligible. Milk contains about 1 mg Fe/kg (Aleixo and Nóbrega, 2003; Schnell et al., 2015). The absorbed Fe requirement of the conceptus of the pregnant cow between day 190 of gestation and the day of calving has been estimated to be 0.025 mg Fe/kg maternal BW or 18 mg/d for a 715-kg late-gestation Holstein cow (House and Bell, 1993). Increased vascularization of the uterus and other reproductive organs and fetal hematopoiesis would increase the gestation requirement for Fe earlier than 190 days of gestation, but data are unavailable to quantify this. Estimates of Fe content in the body range from 18 to 34 mg Fe/kg BW of calves (Bremner and Dalgarno, 1973c). The absorbed Fe requirement for growth of cattle has been set at 34 mg Fe/kg ADG.
Summary of Equations (mg absorbed Fe/d)
where ADG and milk are in kg/d, and BW is in kg.
Absorption
Fe in the ferric form (Fe3+) is poorly absorbed from the intestinal tract; however, reductases exist on the surface of enterocytes that convert ferric Fe to ferrous (Fe2+), allowing for absorption. Fe-deficient calves absorbed Fe provided by ferric chloride but not from ferric oxide (Ammerman et al., 1967). Some Fe3+ can be reduced to Fe2+ on reaction with the acid of the abomasum (Wollenberg and Rummel, 1987). The concentration of soluble Fe in corn silage also increased markedly following in vitro simulated abomasal exposure (Hansen and Spears, 2009).
Form of dietary Fe affects absorption, but Fe absorption by animals is a tightly regulated process, and Fe status interacts with form of Fe to ultimately determine the amount of Fe absorbed. Absorption of radioactive Fe from ferric chloride was three to five times greater by Fe-depleted calves than by Fe-sufficient calves (Ammerman et al., 1967). Calves fed diets with 750 mg Fe (from ferrous sulfate)/kg DM downregulated expression of a duodenal Fe importer (DMT1) found on the luminal side of the enterocyte and ferroportin, an exporter of Fe found on the basal-lateral membrane of enterocytes (Hansen et al., 2010a). The DMT1 may also be involved in absorption of Cu and Mn, which could be a reason high Fe can antagonize absorption of those two metals. Based on changes in expression of many proteins involved with Fe metabolism and a decrease in plasma Fe concentrations (Hansen et al., 2010b), a diet that is deficient in Cu but extremely excess in Mn may induce Fe deficiency in ruminants. In nonruminants, dietary Zn (Lind et al., 2003), phytate (Gillooly et al., 1983), phosphate, and Ca (Monsen and Cook, 1976) can reduce Fe absorption, and dietary βcarotene, vitamin A, and vitamin C can increase Fe absorption (García-Casal et al., 1998). Elevated dietary P reduces hepatic Fe concentrations in steers (Standish et al., 1971), but whether the other factors listed above affect Fe absorption in ruminants is unknown.
Assigning ACs for Fe to basal ingredients and supplements is plagued by a lack of data and because absorption is dependent on Fe supply and Fe status of the animal. Maximum absorption efficiency occurs when animals are deficient in Fe, which is very rare in adult ruminants. Maximum absorption can occur in calves because diets are inherently low in Fe, and deficiency is possible if animals are not supplemented. Absorption of Fe by deficient calves fed low Fe liquid-based diets ranges from 0.55 to 0.72 (Matrone et al., 1957; Bremner and Dalgarno, 1973a,b; Miltenburg et al., 1993). An analysis of balance studies done in growing calves suggests that the AC of soluble Fe from liquid diets declined from 0.40 to 0.15 as dietary Fe increased from 40 to 100 mg/kg (ARC, 1980).
Once the animal is ruminating, the efficiency of Fe absorption is considerably lower, in part because diets are generally excess in Fe and because much of the dietary Fe is provided by forages. Mechanically harvested forages can be high in Fe because soil contamination occurs during harvest. Much of that Fe is likely ferric oxide, which, based on data from calves and sheep, is essentially unavailable (Ammerman et al., 1967; van Ravenswaay et al., 2001). Based on in vitro tests and solubility, Fe from soil contamination has low bioavailability, but after soil-contaminated forage is stored as silage (pH ~4), solubility of Fe was about 20 times greater than in preensiled samples (Hansen and Spears, 2009). Whether that results in increased absorption of Fe is unknown.
Studies using radioactive Fe determined that Fe absorption efficiency was less than 0.02 in adult cattle fed a diet that supplied much more Fe than was needed (van Bruwaene et al., 1984). When pregnant ewes were fed diets that contained 20 mg Fe/kg diet (much lower than most practical diets fed dairy cows), absorption of dietary Fe was 0.21 (Hoskins and Hansard, 1964). Because data are not available to contradict the value used previously (NRC, 2001) for Fe absorption from basal ingredients (0.10), that value was retained. However, because of the typically high concentrations of Fe in most dairy diets and because much of the Fe is likely from soil contamination, an AC less than 0.10 is probably more accurate. Based on limited data from calves and sheep, ferrous sulfate has the greatest relative bioavailability, followed by ferrous carbonate (Ammerman et al., 1967; van Ravenswaay et al., 2001). In NRC (2001), Fe supplements were given ACs of 0.4 to 0.6, which may be appropriate for preruminant cattle but are likely much too high for adult ruminants fed practical diets. No data are available on actual absorption of Fe by ruminants; ferrous sulfate was assumed to have an absorption of 0.20 based on data from Hoskins and Hansard (1964), and based on relative availability, ferrous carbonate was assigned an absorption value of 0.10. Many mineral supplements are contaminated with low concentrations of Fe, and because of a total lack of data, the Fe was assumed to be mostly Fe oxide and given an AC of 0.01.
A 650-kg cow producing 25 kg of milk/d at 205 days of gestation and consuming 20 kg/d DM needs to absorb only 41 mg Fe/d or be fed a diet (AC = 0.1) with just 20 mg Fe/kg DM. Most feedstuffs will contain adequate Fe to meet the Fe requirements of adult cattle. Milk-fed calves are the only group of cattle that ordinarily require Fe supplementation. Feeding veal calves <15 weeks of age as little as 39 mg Fe/kg DM will allow calves to grow at a normal rate, but the muscles remain pale and the animals remain slightly anemic (Bernier et al., 1984). A study by (Lindt and Blum, 1994) suggests that a 50-mg Fe/kg diet is adequate to maintain physiological function in growing veal calves.
Toxicity
NRC (2005) set the MTL for cattle at 500 mg Fe/kg of diet DM; however, that value is dependent on source. The MTL assumes the Fe is from a readily available source; therefore, if the Fe is predominantly from forage, diets in excess of that value likely will cause little problem. However, when readily available sources of Fe are fed, the MTL established by NRC (2005) may be too high. Milk yield and DMI decreased linearly when cows were fed diets with 0, 250, or 400 mg supplemental Fe from Fe sulfate (McCaughey et al., 2005). Diets supplemented with 500 mg Fe (from Fe sulfate) decreases measures of Cu status (Chase et al., 2000). Excess Fe can lead to oxidative stress by increasing the generation of ROS and reactive N species (Meneghini, 1997). High Fe (from Fe sulfate) reduced in vitro ruminal digestibility of DM (Harrison et al., 1992). Fe in water can affect water intake and increase measures of oxidative stress in dairy cows (see Chapter 9).
Manganese
Function and Measures of Adequacy
Mn is a cofactor in a host of enzymes and other proteins that are needed for normal metabolism of AAs, carbohydrates, and lipids and is required by every organ system in the body. Mn-dependent transferases are vital for cartilage production and bone development, and a common sign of Mn deficiency is skeletal abnormalities. Clinical signs of these abnormalities include enlarged joints, shortened and weak bones, superior brachygnathism, ataxia, and deafness and equilibrium problems caused by improper development of bones in the middle ear. The skeletal system of the gestating fetus is especially sensitive to Mn deficiencies, and the newborn calf can show clinical signs of deficiency while its dam appears normal (Hansen et al., 2006a). The majority of calves born from beef cows (Rojas et al., 1965) and heifers (Hansen et al., 2006a) fed diets with approximately 16 mg Mn/kg diet DM for the entire gestation had bone deformity signs indicative of a Mn deficiency, whereas the dams appeared clinically normal.
Reproductive efficiency can be reduced when cows are fed diets with inadequate Mn; however, the concentration of dietary Mn at which this occurs is poorly defined (Hidiroglou, 1979). In an old study (Bentley and Phillips, 1951), heifers fed diets with approximately 10 mg Mn/kg had poor fertility, and the calves that were born had bone disorders. When the diet contained 30 to 40 mg Mn/kg DM, fertility improved and no bone disorders were noted. More recently, beef heifers fed a basal diet with 16 mg Mn/kg had similar reproductive measures as did heifers fed diets with 26 mg Mn/kg; however, heifers fed diets with 56 to 66 mg Mn/kg had a substantial but statistically insignificant increase in pregnancy rate compared with heifers fed 16 or 26 mg Mn/kg (Hansen et al., 2006b).
At the current time, no sensitive indicators of Mn status have been identified for cattle. Manganese superoxide dismutase (Mn-SOD) is in the mitochondria and works in concert with other antioxidants to minimize accumulation of ROS. In some species, Mn-SOD activity responds to changes in Mn intake (de Rosa et al., 1980). Data are lacking relating Mn intake to Mn-SOD activity in cattle, but steers injected with a mix of trace minerals including Mn had higher Mn-SOD activity in red blood cells than steers injected with saline (Genther and Hansen, 2014). In humans, Mn-SOD activity of lymphocytes responded to changes in Mn intake (Davis and Greger, 1992), and in rats, Mn intake was related to several measures of immune function (Son et al., 2007). Arginase is a Mn-dependent enzyme in the urea cycle, and rats fed Mn-deficient diets had reduced arginase activity, which resulted in higher concentrations of plasma ammonia and lower concentrations of plasma urea (Brock et al., 1994). Plasma urea concentrations in cattle have not been shown to respond to changes in dietary Mn. Arginase activity also affects concentrations of nitric oxide in certain tissues, which could explain some of the effects Mn has on immune function (Chang et al., 1998). Concentrations of Mn in plasma or whole blood did not differ between cattle fed diets with wide ranges in Mn concentrations (Weiss and Socha, 2005; Hansen et al., 2006a,b), indicating that it is not a good indicator of status in clinically normal cattle. Newborn calves that were displaying clinical signs of Mn deficiency had lower whole-blood Mn than calves born from heifers fed adequate Mn during gestation (Hansen et al., 2006a). In beef cattle, the concentration of Mn in liver responded linearly to increasing dietary concentrations of Mn (16 to 66 mg/kg), but liver Mn was only about 10 percent greater (9.4 versus 8.2 mg Mn/kg of liver DM), whereas intake of Mn varied more than 4-fold (Hansen et al., 2006b). The lack of sensitive status indicators and established reference ranges for liver concentrations make quantifying the Mn requirement difficult.
Manganese Absorption and Homeostasis
Intestinal absorption of Mn is thought to occur mainly via DMT1, a protein that is also involved with Cu, Zn, and especially Fe absorption (Garrick et al., 2003). Expression of DMT1 in the duodenum of humans experiencing an Fe deficiency is increased (Zoller et al., 2001), and it is downregulated in calves fed high Fe (Hansen et al., 2010a). Whether Mn status affects expression is unknown. Although data with cattle are lacking, high Co, Fe, and Cu can inhibit uptake of Mn in other species (Garrick et al., 2003). In humans, high Ca reduced absorption of Mn, but P and Mg did not (Davidsson et al., 1991). In chickens, high Ca had no effect on Mn absorption, whereas phosphate significantly reduced Mn absorption (Wedekind et al., 1991). Whether any of these antagonisms of Mn absorption occur in cattle are unknown. Elevated dietary S reduced apparent absorption of Mn by steers (Pogge et al., 2014).
Measuring the true absorption of Mn is especially difficult because the majority of dietary Mn that is absorbed is removed from the portal circulation by the liver and is excreted back into the intestine via bile. Using radioactive Mn (MnCl2), Mn absorption by humans ranged from 1.0 to 2.5 percent (Davidsson et al., 1991; Finley et al., 1994). In rats, Mn (MnCl2) absorption averaged 1.8 percent (Chua and Morgan, 1997). In a very limited study (two cows), absorption of radioactive Mn (from MnCl2) averaged about 0.5 percent (Vagg, 1976), and in another limited study (two cows) based on portal vein data, Sansom et al. (1978) calculated an absorption rate of 0.5 percent for MnCl2. Across studies, the AC in cattle was about one-fourth the value measured in nonruminants. That may be a true species difference or it could be caused by differences in intake of Mn. Absorption and turnover of Mn are positively correlated (Britton and Cotzias, 1966). When Mn intake increases, more Mn is absorbed, but biliary excretion also increases so that very little net change in body Mn content occurs.
Data are not available regarding absorption of Mn from basal feeds or from inorganic sources other than MnCl2. In the previous edition (NRC, 2001), the AC for Mn from the Cl− and SO4−2 salts was set at 0.01 and 0.0075 for all basal feedstuffs. The same relative differences between supplemental Mn sources and basal feedstuffs were retained in this edition; however, AC of Mn from MnCl2 was set at 0.005 based on the limited cow data that are available. Manganese sulfate was assigned the same AC value (0.005), and basal ingredients were assigned an AC of 0.004 (i.e., 0.005 × 0.75). The ACs of other sources of supplemental Mn were set at Mn carbonate (0.0015) and Mn monoxide (0.003) based on relative bioavailability studies (Henry, 1995c).
Manganese Requirements
An AI is used for Mn because inadequate data are available to establish a requirement. The amount of Mn needed by a dairy cow for maintenance has not been experimentally measured. Approximate intakes of 250 to 300 mg total Mn by gestating cattle were not adequate to prevent deficiency signs in their offspring (Hansen et al., 2006a; de Carvalho et al., 2010). Assuming an AC for dietary Mn of 0.004 and that approximately 50 mg Mn was needed for gestation (see below), the maintenance requirement for absorbed Mn must be greater than 0.0016 to 0.002 mg Mn/kg BW. Weiss and Socha (2005) determined an intake of approximately 580 mg Mn/d was necessary to maintain lactating dairy cows in zero Mn balance. In that study, average secretion of Mn in milk was 0.6 mg/d, which equals 150 mg dietary Mn (assumed AC = 0.004). Subtracting that value from 580 mg yields an estimated maintenance requirement of 430 mg for a 650-kg cow or 0.0026 mg absorbed Mn/kg BW. Because of a lack of other data, that value was set as the maintenance AI for absorbed Mn. This is 30 percent higher than the requirement estimated in the previous version (i.e., 0.002 mg Mn/kg BW).
House and Bell (1993) determined that 0.3 mg Mn/d was deposited into the fetus from 190 days of gestation until parturition for Holstein cows weighing 715 kg. Therefore, the daily gestation requirement (starting at 190 days of gestation) for absorbed Mn was set at 0.00042 mg/kg BW of the dam. The lactation requirement is equal to the amount of Mn secreted in milk daily. The concentration in milk ranges from about 0.016 to 0.050 mg Mn/kg (Gunshin et al., 1985; Erdogan et al., 2004; Pechova et al., 2008; Castillo et al., 2013). The weighted average was 0.027, which is essentially equal to 0.03 mg Mn/kg, the value used in the previous edition (NRC, 2001). Therefore, the lactation requirement for absorbed Mn was set at 0.03 mg Mn/kg of milk. The concentration of Mn in carcasses of calves averages about 2.5 mg/kg of total carcass on a DM basis (Suttle, 1979). Assuming the carcasses used in these experiments were 27 percent DM, the Mn requirement for growth can be estimated to be 0.7 mg Mn/kg BW gain. No new data were found on tissue accretion of Mn or whole-body Mn concentrations in cattle; therefore, the growth requirement was not changed.
The AI for absorbed Mn for a late-gestation Holstein cow weighing 700 kg would be 1.8 mg/d for maintenance plus 0.3 mg/d for gestation for a total of 2.1 mg absorbed Mn. This is about 490 mg total dietary Mn (assumed AC = 0.0042). Assuming a DMI of 12.5 kg/d, a concentration of 40 mg total Mn/kg of diet DM would meet the requirement. This is substantially greater than in the previous version. For a 650-kg cow producing 45 kg of milk/d, the maintenance requirement would be 1.7 mg and 1.35 mg for lactation for a total absorbed Mn requirement of 3.1 mg/d. Assuming a DMI of 26 kg/d, dietary concentration of total Mn to meet the requirement would be approximately 27 mg Mn/kg (assumed AC of 0.0042).
Summary of Equations (mg absorbed Mn/d)
where ADG and milk are in kg/d, and BW is in kg.
Maximum Tolerable Level
Because absorption of dietary Mn is extremely low, Mn toxicity in ruminants is unlikely to occur, and the few documented incidences with adverse effects are limited to reduced feed intake and growth (Jenkins and Hidiroglou, 1991). The maximum tolerable amount of Mn as given by NRC (2005) is 2,000 mg Mn/kg of diet DM. However, diets with 500 mg Mn/kg exacerbated the negative effects of feeding a Cu-deficient diet (Hansen et al., 2009). Furthermore, cattle fed a Cu-deficient diet with 500 mg Mn/kg DM displayed some indications of an Fe deficiency (Hansen et al., 2010b).
Selenium
Functions and Animal Response
The only known nutritional function of Se is as a component of specific selenoproteins. Essentially, any protein can contain Se because of nonspecific incorporation of selenomethionine (replacing Met) into the polypeptide chain. However, selenoproteins require selenocysteine in a specific location within the peptide chain to be functionally active. Selenocysteine is identical to cysteine, except a Se molecule replaces the sulfur molecule. To be inserted into the proper location in a peptide, selenocysteine must be synthesized from serine that is joined to a specific transfer RNA (tRNA; UGA codon). Selenocysteine that is absorbed by the intestine cannot be directly inserted into the active site within a polypeptide chain. In humans, at least 25 genes for selenoproteins have been identified (Lu and Holmgren, 2009), but the functions of many of the resulting selenoproteins are unknown. Glutathione peroxidases (GPx) are a family of selenoproteins that reduce hydrogen peroxide to water or phospholipid hydroperoxides to phospholipids, and increasing Se intake of cattle often increases the activity of these enzymes. Iodothyronine deiodinases are a family of selenoenzymes that activates thyroxin by deiodinating T4 into T3, and Se supplementation has increased serum T3 concentrations in cattle (Awadeh et al., 1998; Contreras et al., 2002). The enzyme can also inactivate T3 by further deiodination. Thioredoxin reductases (TRx) are selenoenzymes that reduce thioredoxin, which is involved in regulation of the redox potential of cells. Two other selenoproteins (selenoprotein P and selenoprotein W) have been found in bovine tissue, but their functions are unclear.
White muscle disease or nutritional muscular dystrophy is the classical sign of a clinical Se deficiency. Signs of this disease include leg weakness and stiffness, flexion of the hock joints, and muscle tremors (NRC, 1983). Cardiac and skeletal muscles have chalky striations and necrosis, and animals often die from cardiac failure. In cattle, improved Se status has increased growth rates (Wichtel et al., 1994; Reis et al., 2008) and reduced prevalence of retained fetal membranes (reviewed by Hemingway [1999] and Jovanovic et al. [2013]). Most studies evaluating effects of Se on clinical and subclinical mastitis have reported positive results (Smith et al., 1985; Erskine et al., 1987, 1989, 1990; Weiss et al., 1990; Wichtel et al., 1994; Jukola et al., 1996; Malbe et al., 2003; Kommisrud et al., 2005). Other health problems that have responded to Se supplementation include metritis, cystic ovaries (Harrison et al., 1984; Enjalbert et al., 2006), and udder edema (Miller et al., 1993). The likely mode of action of Se for these health disorders is via regulation of cellular concentrations of ROS via GPx and TRx. Se supplementation of cattle has improved the function of immune cells, including neutrophil (Hogan et al., 1990; Cebra et al., 2003), macrophage (Ndiweni and Finch, 1995), and lymphocyte (Stabel et al., 1990; Cao et al., 1992; Pollock et al., 1994). Concentrations of specific ROS within cells affect inflammatory responses, arachidonic acid metabolism, and production of prostaglandins and numerous cytokines (Salman et al., 2009; Sordillo, 2013).
Sources
Concentrations of Se in plants are positively correlated with those in the soil (Whelan et al., 1989; Hall et al., 2011). In general, feeds grown in the central plains of the United States and Canada contain more than 0.1 mg Se/kg DM, and feeds grown east of the Mississippi River and west of the Rocky Mountains typically contain <0.1 mg Se/kg DM (NRC, 1983). Except for Se accumulator plants (e.g., Astragalus bisulcatus), the predominant form of Se in plants is selenomethionine plus minor amounts of selenocysteine and selenite (Whanger, 2002). Se concentration of feeds is positively correlated with protein concentrations, and plant parts that are higher in protein also are higher in Se. Leaves of forage plants contain 1.5 to 2 times more Se than do stems (Gupta and Winter, 1989).
Based on current regulations of the U.S. FDA (1997, 2003), the only forms of Se that can be added legally to diets in the United States are sodium selenite, sodium selenate, and Se-enriched yeast at levels not to exceed 0.3 mg supplemental Se/kg DM. Other sources of supplemental Se that have been evaluated in cattle include barium selenate (Ceballos et al., 2010) and Se dioxide (Grace et al., 1995).
Efficiency of Absorption
Apparent absorption of Se from diets without supplemental Se is between 0.30 and 0.60 for sheep, goats, and nonlactating dairy cows (Harrison and Conrad, 1984a,b; Aspila, 1988; Koenig et al., 1997; Gresakova et al., 2013). Apparent absorption of Se in diets that contain selenite and selenate in diets ranged from 0.36 to 0.51 (Harrison and Conrad, 1984b; Ivancic and Weiss, 2001; Gresakova et al., 2013), and for diets with Se yeast, apparent absorption ranged from about 0.57 to 0.62 (Walker et al., 2010; Gresakova et al., 2013). In a direct comparison, apparent absorption of Se from Se yeast was about 24 percent greater (0.62 versus 0.50) than that of selenite when fed to sheep (Gresakova et al., 2013). Because of endogenous fecal losses, true absorption of Se is greater than apparent absorption. True absorption estimated using the regression method averaged about 0.5 in dairy cows fed inorganic Se (Harrison and Conrad, 1984a; Ivancic and Weiss, 2001). No data are available on estimated true absorption of Se from yeast. Because Se from Se yeast is retained in cellular proteins to a greater extent than that from inorganic Se, endogenous fecal losses of Se when Se yeast is fed may be greater, but the true absorption would also be greater.
Factors Affecting Absorption
Intestinal absorption of selenate is greater than selenite in sheep and rat intestinal in vitro models (Ardüser et al., 1986) and in the human Caco-2 cell model (Gammelgaard et al., 2012); however, because most but not all of the selenate is reduced to selenite within the rumen, absorption of selenate is probably only slightly greater than selenite in cattle (Podoll et al., 1992). The predominant form of Se in Se yeast is selenomethionine, which is absorbed via the same mechanism as Met. Intestinal absorption of selenomethionine is greater than absorption of inorganic Se sources (Gammelgaard et al., 2012). Assuming differences in apparent absorption accurately reflect differences in true absorption, absorption of Se from high-quality (i.e., high proportion of Se as selenomethionine) Se yeast is at least 1.2 times that of inorganic Se. Se uptake by ruminal microorganisms is much greater when Se yeast is fed compared with selenite (Mainville et al., 2009), but form of Se did not affect measures of ruminal fermentation (Panev et al., 2013).
Dietary sulfate added to increase concentrations of dietary S by 0.2 and 0.4 percentage units (Ivancic and Weiss, 2001) and low (<0.6 percent of DM) and high (>1.0 percent of DM) concentrations of dietary Ca (Harrison and Conrad, 1984a) reduce absorption of inorganic Se by cattle. Supplementation of S from anionic salts (approximately 0.6 percent total diet S) for the last 3 weeks of gestation did not influence Se status of nonlactating cows (Gant et al., 1998). Long-term feeding of diets that contained approximately 0.3 percentage units of added sulfate–sulfur to beef cattle did not affect concentrations of Se in blood or the activity of GPx (Khan et al., 1987). In an in vitro sheep intestinal model, thiosulfate and molybdate reduced absorption of inorganic Se (Ardüser et al., 1986). In nonruminants, Se absorption was not affected by dietary Cu, Fe, Mo, and Mn over a wide range of concentrations (Abdel Rahim et al., 1986). In dairy cows, increased dietary Cu did not affect measures of Se status (Koenig et al., 1991). Rats that were Mg deficient had significantly lower Se absorption than rats adequate in Mg (Jiménez et al., 1997), and elevated dietary Zn reduced Se absorption in rats (House and Welch, 1989). Whether Mg or Zn affects Se absorption in cattle is unknown. Antagonists to absorption of inorganic Se may affect absorption of Se yeast differently.
Indicators of Selenium Status
Se status can be assessed by concentrations of Se in tissue and blood, activity of glutathione peroxidase, and various immune cell assays. Few differences in blood and tissue concentrations of Se occur between different inorganic sources of Se when fed to dairy cattle (Podoll et al., 1992; Gibson et al., 1993; Ortman and Pehrson, 1999; Ortman et al., 1999). Se from Se yeast or from basal ingredients with higher concentrations of Se usually increases concentrations of Se in blood and activity of GPx more than diets with inorganic Se (Conrad and Moxon, 1979; Ortman and Pehrson, 1997; Knowles et al., 1999; Ortman et al., 1999; Weiss and Hogan, 2005; Juniper et al., 2008; Phipps et al., 2008; Koenig and Beauchemin, 2009). Blood concentrations and GPx activity average 20 to 25 percent greater when Se yeast is fed (Weiss, 2003; Juniper et al., 2006, 2008; Phipps et al., 2008; Koenig and Beauchemin, 2009) similar to the difference in apparent absorption. On average, milk Se is about 1.9 times greater when Se yeast is fed compared with inorganic Se, but a meta-analysis determined that depending on supplementation rate, the difference could be more than three times (Ceballos et al., 2009). Feeding rumen-protected Met reduces the concentration of Se in milk when Se yeast is fed (Weiss and St-Pierre, 2009).
Requirements and Factors Affecting Requirements
The seventh revised edition (NRC, 2001) defined the Se requirement as 0.3 mg/kg of dietary DM for all classes of dairy cattle. No new data are available to dispute this requirement. However, most of the data supporting this requirement were generated from experiments in which selenite or selenate/kg of dietary DM (DM basis) was fed, and total dietary Se ranged from 0.35 to 0.40 mg/kg.
Establishing requirements for Se using the factorial approach is difficult because the deposition of Se in body tissues, conceptus, and milk is dependent on Se intake and Se source, and essentially no data are available on endogenous fecal and urinary losses. Assuming a cow is fed a diet with approximately 0.3 mg Se from inorganic sources/kg of dietary DM, the conceptus will accumulate 0.055 mg Se/d during the last trimester of gestation (House and Bell, 1994). Comparable data when Se yeast is fed are not available, but accumulation in swine fetuses when Se yeast was fed was approximately 1.3 times greater than when selenite was fed (Ma et al., 2014).
Se concentrations vary across tissues in cattle, with kidney usually having the highest concentrations and muscle having lower concentrations (Lawler et al., 2004; Juniper et al., 2008), but muscle contained about 0.3 mg Se/kg DM when growing cattle were fed a diet with 0.3 mg Se/kg from selenite (Juniper et al., 2008) and 0.4 to 0.8 mg Se/kg dry weight when Se yeast was fed (Juniper et al., 2008; Richards et al., 2011). Concentrations of Se in various tissues of growing beef animals were 1.25 times greater when Se yeast was fed compared with selenite (Juniper et al., 2008). Se concentration of milk averages about 0.02 mg/kg and 0.04 mg/kg when selenite and Se yeast is fed at approximately 0.3 mg Se/kg of diet, respectively (Ceballos et al., 2009). Using the regression method, endogenous fecal and urinary losses varied by more than a factor of two depending on the source of data (Harrison and Conrad, 1984b; Ivancic and Weiss, 2001). Endogenous cells sloughed by cows that are deficient in Se would likely have lower concentrations of Se than cells sloughed by cows adequate in Se. No data are available on endogenous losses when Se yeast is fed but would likely be greater because of greater Se concentrations in cells.
Current FDA regulations limit Se supplementation to 0.3 mg/kg of diet (assumed air dried or approximately 90 percent DM basis) in the United States (FDA, 1997), and in most situations, that amount of supplemental Se will maintain dairy cattle in good Se status. Based on the effect of Se on mastitis, concentrations of Se in whole blood should be greater than about 0.18 μg/mL or approximately 0.075 μg/mL for plasma when inorganic Se is fed (Jukola et al., 1996). Intake of approximately 6 mg/d of inorganic Se should maintain those blood concentrations (Maus et al., 1980). Less Se may be needed when Se yeast is fed; however, more Se is also secreted in milk and retained in the body (nonspecific proteins) when Se yeast is fed. Based on available data, the AI of Se for all classes of cattle was set at
where DMI is in kg/d, and basal diet is generally assumed to provide no Se.
Most of the studies with Se for dairy cattle were conducted in areas with low soil Se so that basal Se was usually <0.1 mg Se/kg DM. This means that intake of supplemental Se was approximately equal to intake of total Se. In areas where soil has higher Se concentrations (e.g., North and South Dakota), users are advised to analyze locally grown forages for Se and include basal Se in the calculation of Se supply. Since Se concentrations in feedstuffs are low, specialized equipment is needed for the assay, which many labs do not have. The Se concentrations in the feed library included in the model are means and can differ greatly from feeds grown on specific farms.
Toxicity
Alkali disease and blind staggers result from Se toxicity. Clinical signs include sloughing of hooves, lameness, loss of hair, and emaciation. Most cases of Se toxicity have been related to consumption of Se-accumulating plants (e.g., Astragalus sp.), and much of the Se in those plants is found in methyl-selenium compounds. Similar clinical signs were also caused by feeding high doses of selenomethionine (10 mg Se/kg diet DM) or selenite (25 mg Se/kg diet DM) to yearling cattle for 120 days (O'Toole and Raisbeck, 1995). Acute toxicity can occur when cows are fed 10 to 20 mg Se/kg BW from selenite. An injection of about 0.5 mg Se/kg BW to young cattle (ca. 200 kg) resulted in a 67 percent mortality rate (NRC, 1983). The current MTL for dietary Se is 5 mg/kg of diet DM (NRC, 2005) or about 16 times greater than the recommended dietary concentration.
Zinc
Function
Zn is a component of more than 200 enzymes, including oxidoreductases (e.g., Cu–Zn superoxide dismutase), transferases (e.g., RNA polymerase), hydrolases (e.g., alkaline phosphatase and carboxypeptidase), lyases (carbonic anhydrase), and ligases (e.g., tRNA synthetase) (Kidd et al., 1996). Zn is involved with macronutrient metabolism, numerous aspects of the immune system, gene regulation, hormonal regulation, neurotransmission, and apoptosis. The effects of Zn on immune function have received substantial attention (Fraker and King, 2004). Historically, these effects were thought to be manifested via Zn-containing enzymes such superoxide dismutase (which usually does not show a change in activity when dietary Zn intake is altered). However, accumulating data indicate that changes in Zn concentrations within immune cells are a major regulatory mechanism that may affect the entire immune system (Haase and Rink, 2009). The ubiquitous functions of Zn are likely the primary reason identifying markers of Zn status has been so difficult.
Absorption
Molecular and cellular mechanisms of absorption of dietary Zn have not been studied to any degree in the bovine, but substantial information is available from rodents and poultry models. Whether information from those species reflects mechanisms in cattle is unknown. In rats and poultry, Zn absorption occurs throughout the small intestine and perhaps in the large intestine by two different mechanisms: a saturable, transport-mediated absorption system and nonsaturable diffusion. In poultry, transport-mediated absorption occurred primarily in the duodenum and jejunum, and nonsaturable diffusion occurred primarily in the ileum (Yu et al., 2008). In rats, saturable absorption of Zn was found in all segments of the small intestine. The saturable transporters probably belong to the Zip family, and expression is downregulated when dietary Zn supply is high and upregulated when supply is low (Liuzzi and Cousins, 2004; Lichten and Cousins, 2009). Based on poultry and rodent data, at low dietary concentrations of Zn, high-affinity transporters become important (jejunum and ileum) but at high concentrations of Zn, diffusion in ileum and colon likely would predominate because of transporter saturation and downregulation. Although some regulation of Zn absorption probably occurs, based on the putative absorption processes, when excess-absorbable Zn is fed, cows will absorb Zn in excess of requirement. Export of Zn out of enterocytes appears to be regulated and is used to maintain Zn homeostasis. Expression of metallothionein genes or synthesis of the protein in intestinal cells is upregulated when excess Zn is provided (Tran et al., 1998; Shen et al., 2008), which likely is one mechanism of increased fecal excretion of endogenous Zn.
Factors Affecting Zinc Absorption
Measuring the true absorption of Zn or relative availability of Zn is exceedingly difficult because fecal excretion of Zn is used to maintain Zn homeostasis, and good markers of Zn status are lacking. Lactating cows that were adapted to a Zn-deficient diet (6 mg Zn/kg diet DM) absorbed nearly 50 percent of dietary Zn (Kirchgessner and Schwarz, 1976). However, maximizing Zn absorption by feeding deficient diets is clearly not desirable. Based on isotope studies, Zn absorption by ruminating cattle fed practical diets that were likely adequate in Zn ranged from 12 to 33 percent (Miller and Cragle, 1965; Hansard et al., 1968; Miller et al., 1968). However, these studies are decades old and were done with cattle with low DMI compared with modern lactating cows. Whether DMI affects Zn absorption is unknown, but a positive correlation between DM digestibility and Zn absorption has been observed (Miller and Cragle, 1965). Because new data are lacking, the AC for zinc chloride (ZnCl2; the Zn source used in the above studies) used in the previous version (NRC, 2001) was retained (i.e., 0.20). Absorption of Zn by rats fed radioactive Zn from ZnCl2 or from soy flour produced by plants fertilized with radioactive ZnCl2 were the same (Stuart et al., 1986). Similar results were found when preruminant calves were fed ZnCl2 or corn plants grown with radioactive Zn (Neathery et al., 1972). These studies, at least for nonruminants, indicate that Zn contained in basal ingredients has similar absorbability as ZnCl2 (ca. 0.20). In agreement, true absorption of Zn was 0.182 by adult goats fed a diet in which about 50 percent of the Zn was from basal ingredients and 50 percent from ZnCl2 (Hattori et al., 2010). Therefore, Zn in basal ingredients was assigned an AC of 0.20. Absorption coefficients for Zn from other supplements were estimated from studies measuring relative bioavailability. ZnCl2 and zinc sulfate (ZnSO4) had similar bioavailability in calves with a functioning rumen (Kincaid, 1979). Zinc oxide had a bioavailability of approximately 80 percent that of ZnSO4 (Sandoval et al., 1997) so that its AC was set at 0.16. Zinc carbonate had the same bioavailability as ZnSO4 in sheep (Sandoval et al., 1997). Several proprietary supplemental Zn sources are available, but published data on measures of relative bioavailability are limited (Cao et al., 2000). The limited data indicate slightly higher (10 to 20 percent) greater bioavailability for some proprietary compounds compared with ZnSO4. A problem with most relative bioavailability studies is that very high concentrations of Zn are fed, which can affect results.
Several dietary components can interfere with Zn absorption or increase body losses of Zn, but most of the studies have been done with nonruminants (Lönnerdal, 2000). Phytate clearly reduces Zn absorption in nonruminants. Zn absorption by calves fed milk averaged about 50 percent (Miller and Cragle, 1965) but was reduced by more than half when soybean protein was included in the diet, likely because of the phytic acid in the soybean product (Miller et al., 1968). Phytase has little effect on P absorption (see P section), suggesting that in functioning ruminants, phytic acid probably does not greatly affect Zn absorption. High-fiber diets can reduce Zn absorption in nonruminants, but that affect is often confounded with effects of phytate. Diets that differed greatly in concentration of undigested NDF did not affect apparent Zn absorption in dairy cows (Faulkner et al., 2017). Ca can reduce Zn absorption in nonruminants, but that may be an indirect effect caused by the effects of Ca on phytate (Lönnerdal, 2000). In cattle, supplementation of Ca was associated with a reduction of Zn in serum of yearling steers and calves (Mills et al., 1967; Perry et al., 1968), but no deleterious effects of increased dietary Ca on metabolism of Zn or growth in sheep were observed (Pond and Wallace, 1986).
Cadmium markedly reduces Zn absorption in rats (Evans et al., 1974). High dietary Fe (1,000 mg Fe/kg of diet provided by ferrous sulfate) reduced liver Zn concentration in steers by about 18 percent (Standish et al., 1971). Zn and Cu are antagonistic to one another. Very high Cu can interfere with Zn metabolism; however, very low Zn-to-Cu ratios (0.15:1) are likely necessary to produce antagonism (Oestreicher and Cousins, 1985). Diets with 40 mg Cu/kg and 50 mg Zn/kg (0.8:1 Cu-to-Zn ratio) did not reduce plasma Zn in growing steers (Gooneratne et al., 1994). Elevated dietary S (approximately 0.5 percent) has increased urinary loss of Zn and reduced Zn absorption in beef cattle (Gooneratne et al., 2011; Pogge et al., 2014). Conversely, feeding monensin may increase absorption of Zn (Spears, 1990), and with nonruminants, certain proteins such as whey or beef increase the absorption of Zn, but other proteins such as casein and isolated soy protein (phytase treated) reduce Zn absorption (Lönnerdal, 2000). Based on currently available information, most practical diets should not contain adequate concentrations of antagonistic substances to reduce Zn absorption with the possible exception of excess S.
Dietary Zinc Requirement
A factorial approach was taken to determine the dietary requirement for Zn. In the previous edition (NRC, 2001) endogenous fecal and the obligate urinary loss of Zn was set at 0.033 mg Zn/kg BW and 0.012 mg Zn/kg BW, respectively, for a total maintenance requirement of 0.045 mg Zn/kg BW. The data used to generate those equations came from a study with growing heifers (ca. 300 kg BW) fed radioactive Zn (Hansard et al., 1968). Newer data do not support the value used for the obligate urinary loss and bring into question the value used for endogenous fecal loss. Growing cattle (ca. 300 kg) fed low but not deficient Zn diets excreted 0.003 mg Zn/kg BW in the urine (Gooneratne et al., 2011; Pogge et al., 2014), and lactating cows fed low Zn diets (30 mg Zn/kg DM) excreted 0.0016 mg Zn/kg BW in urine (Faulkner et al., 2017). Because urinary excretion of Zn is so low, the obligate urinary loss was set at zero. A stable isotope study with goats fed at maintenance estimated endogenous fecal loss of Zn at 0.17 mg/kg BW (Hattori et al., 2010). An earlier study with 300-kg beef heifers estimated endogenous fecal loss at 0.027 mg Zn/kg BW (Hansard et al., 1968). On a DMI basis (intake was <2 percent of BW in both studies), endogenous fecal Zn ranged from 2.0 (heifers) to 8.6 (goats) mg Zn/kg DMI. The extremely limited data and the great differences between studies make it difficult to estimate this requirement. In addition, the diets were not typical of what is fed to dairy cows. One reason for the disparate results is that endogenous fecal loss of Zn reflects Zn status. As more Zn is fed, Zn bound to metallothionein is excreted via feces to maintain Zn homeostasis. Based on Zn intake, the goats (Hattori et al., 2010) were likely in greater Zn status than the heifers (Hansard et al., 1968). Data on endogenous fecal excretion of Zn (and most trace minerals) by dairy cows are clearly needed to improve estimates of maintenance requirements. Therefore, the committee decided to use the mean value (rounded to the nearest whole number) from the two experiments and set the endogenous fecal Zn requirement as
TABLE 7-4Comparison Between Current and Previous (NRC, 2001) Zn Requirements for Dry and Lactating Cows
| 650 kg Cow, 30 kg Milk/d, DMI = 22.4 kg | 650 kg Cow, 50 kg Milk/d, DMI = 29.4 kg | 700 kg Dry Cow, 270 Days of Gestation, DMI = 13 kg | ||
|---|---|---|---|---|
| Total absorbed requirement | ||||
| Current, mg/d | 232 | 347 | 65 | |
| NRC 2001, mg/d | 149 | 229 | 44 | |
| Total dietary requirementa | ||||
| Current, mg/d | 1,160 | 1,735 | 325 | |
| NRC, 2001, mg/d | 993 | 1,526 | 293 | |
- a
Absorption coefficient was 0.20 for current requirements and 0.15 for NRC (2001).
No new data are available on Zn accretion by the conceptus; therefore, the gestation requirement was not changed and set at 0.017 mg Zn/kg of maternal BW, which equals 12 mg Zn/d between day 190 of gestation and the end of gestation for a 715-kg Holstein cow (House and Bell, 1993). Newer data support retaining the lactation requirement for Zn at 4 mg/kg of milk, but concentrations can range from about 3 to 6 mg Zn/kg (Schwartz and Kirchgessner, 1975; Kinal et al., 2007; Castillo et al., 2013; Faulkner et al., 2017). The amount of Zn retained during growth of body tissues averages 24 mg Zn/kg ADG (range, 16 to 31 mg) (Miller, 1970; Kirchgessner and Neesse, 1976). Zn accretion in growing sheep averaged about 28 mg Zn/kg of empty BW but was 24 mg Zn/kg in young sheep (15 kg BW) and increased to 30 mg Zn/kg in sheep weighing about 50 kg (Bellof and Pallauf, 2007). Whether the growth requirement (per kg of BW) increases as growing cattle get larger is not known; therefore, a single growth requirement (24 mg Zn/kg daily gain) was used.
Summary of Equations (mg absorbed Zn/d)
where DMI, ADG, and milk are in kg/d, and BW is in kg.
The maintenance requirement for absorbed Zn was greatly increased compared with the previous version, but the AC for basal Zn was also increased. A comparison between NRC (2001) and current requirements is in Table 7-4.
Deficiency
Cattle that are deficient in Zn quickly exhibit reduced DMI and growth rates. With a more prolonged deficiency, the animals exhibit reduced growth of testes, weak hoof horn, and parakeratosis of the skin on the legs, head (especially nostrils), and neck. On necropsy, thymic atrophy and lymphoid depletion of the spleen and lymph nodes are evident (Miller and Miller, 1962; Mills et al., 1967; Mayland et al., 1980). A genetic defect that greatly reduces absorption of Zn has been identified in Dutch–Friesian cattle, and they become severely deficient in Zn unless fed extremely large amounts of dietary Zn (Flagstad, 1976). Marginal deficiency of Zn may increase the risk of mastitis and other infectious disease. Dairy cows fed diets with approximately 41 mg Zn/kg diet DM had higher milk SCC than cows fed diets with 63 mg/kg Zn (Cope et al., 2009). The diet with 41 mg Zn/kg would not meet the current Zn requirements.
Concentrations of Zn in serum are normally between 0.7 and 1.3 μg/mL, and concentrations below 0.5 μg/mL are often considered deficient. However, stress or disease can cause a rapid redistribution of Zn out of extracellular fluids, causing concentrations of Zn in serum to fall into the “deficient” range even when dietary Zn is adequate (Goff and Stabel, 1990). Liver Zn concentrations are not reflective of Zn intake but will decline with prolonged periods of dietary deficiency (Herdt and Hoff, 2011). Increased liver Zn concentrations have been observed in sheep fed supplemental Zn compared to no added dietary Zn (Cao et al., 2000), and the effects are greater when fed zinc lysine compared to zinc sulfate, zinc oxide, and zinc Met (Rojas et al., 1995). However, no differences were observed in liver Zn concentrations of adult cattle fed 360 mg/d of supplemental Zn compared to no supplemental Zn (Rojas et al., 1996), and source of supplemental Zn had no effect on liver Zn concentrations (Rojas et al., 1996; Siciliano-Jones et al., 2008). Liver Zn concentrations in cattle are also affected by age and perhaps other factors (Puschner et al., 2004). Carbonic anhydrase and alkaline phosphatase activities in blood have been used to assess Zn status, but these are difficult to interpret because concurrent disease can affect these enzymes as much as a deficiency of Zn (Mills, 1987). No widely agreed-on status indicator is available for Zn.
Toxicity
Cattle can generally tolerate high concentrations of dietary Zn. Clinical toxicity was observed in cattle fed a 900-mg Zn/kg diet (Ott et al., 1966a,b. Feed intake, milk production, and Cu status were reduced when cows were fed diets with 2,000 mg Zn/kg (from ZnSO4) but not when fed diets with 1,000 mg Zn/kg (Miller et al., 1989). NRC (2005) established an MTL for cattle at 500 mg Zn/kg diet DM. However, dairy cows can likely tolerate greater concentrations.
Arsenic, Molybdenum, Nickel, and Vanadium
These elements can be found in minute amounts in the tissues of animals. In rodents, some of these elements have been demonstrated to be essential. Data on essentiality in dairy cattle are nonexistent, and practical diets would not be expected to result in deficiency of any of these elements. Most of these elements are toxic at levels occasionally occurring under field conditions.
The current understanding of metabolism does not include any specific role for arsenic (As). Goats fed a diet with 0.35 mg As/kg DM had more kids, and more of the kids survived through weaning compared with goats fed a diet with 0.035 mg As/kg (Anke, 1986). Organic arsenicals, as well as inorganic forms of As, are well absorbed and can cause toxicosis when feedstuffs are accidentally contaminated with As. Inorganic arsenicals are more toxic than organic arsenicals. The maximal tolerable level was set at 30 mg As/kg diet DM (NRC, 2005).
Mo is an essential mineral and a cofactor of xanthine oxidase, aldehyde oxidase, and sulfite oxidase (Rajagopalan, 1988). However, clinical signs of a deficiency of Mo have not been produced in any animal, making supplementation unnecessary. Because of its antagonistic effects on Cu absorption (see discussion in Cu section above), clinical signs of Mo toxicity are similar to those of a Cu deficiency. Increasing dietary Cu concentrations will usually decrease or eliminate clinical signs of Mo toxicity.
Nickel (Ni) is an essential nutrient for ruminants, although deficiencies are extremely difficult to produce (Spears, 1984). One function of Ni is as a cofactor for some forms of urease. Ruminal urease activity was stimulated when lambs were fed a diet supplemented with 5 mg Ni/kg (from nickel chloride) compared to basal with 0.06 mg Ni/kg (Spears et al., 1977). Feeding diets with 0 to 3 mg/kg of supplemental Ni to young heifers (125 kg of BW) linearly increased plasma urease activity, DMI, and ADG (Singh et al., 2019). Studies on the effects of Ni at nutritionally relevant supplementation rates on lactating dairy cows are lacking. Ni is relatively nontoxic with maximal tolerable dietary concentrations of 100 mg Ni/kg for cattle (NRC, 2005). However, no effects were noted on performance variables when lactating cows were fed diets with 0, 50, or 250 mg Ni/kg DM (O'Dell et al., 1970).
Vanadium (V) may have insulin-like activity (Heyliger et al., 1985). When V (as vanadyl sulfate) was supplemented to dairy cow diets at 0 to 0.12 mg V/kg BW0.75 from 4 weeks prepartum to 4 weeks postpartum, milk yield increased quadratically with a maximum at 0.04 mg V/kg BW0.75 (Heidari et al., 2016). Milk composition, BW, and DMI were not affected. Blood glucose concentrations followed a pattern similar to milk yield, and the insulin-to-glucose ratio postpartum was reduced by all V treatments compared to control. The basal concentration of V was 0.89 mg/kg DM. In goats, supplemental V at 2 mg/d increased glucose clearance rate and increased average daily gain and feed efficiency (Zarqami et al., 2018). Although these studies show promise, inadequate data are available to determine an AI for V. Although it is poorly defined, the MTL is 50 mg V/kg of diet (NRC, 2005). However, ruminal function (DM digestibility) in lambs was disrupted with just 7 mg vanadium/kg of diet (Williams, 1973).
REFERENCES
- Abdel Rahim AG, Arthur JR, Mills CF. Effects of dietary copper, cadmium, iron, molybdenum and manganese on selenium utilization by the rat. J. Nutr. 1986;116:403–411. [PubMed: 3950767]
- AFRC (Agricultural and Food Research Council). Technical committee on responses to nutrients. Report 6. A reappraisal of the calcium and phosphorus requirements of sheep and cattle. Nutr. Abstr. Rev. Ser. B. 1991;61:573–612.
- Aitken FC. Sodium and Potassium Nutrition of Mammals. Bucksburn, Aberdeen, UK: Commonwealth Agricultural Bureau of Nutrition; 1976. (Technical Communication No. 26.).
- Akins MS, Bertics SJ, Socha MT, Shaver RD. Effects of cobalt supplementation and vitamin B12 injections on lactation performance and metabolism of Holstein dairy cows. J. Dairy Sci. 2013;96:1755–1768. [PubMed: 23312998]
- Alderman G. Mineral nutrition and reproduction in cattle. Vet. Rec. 1963;75:1015–1018.
- Aleixo PC, Nóbrega JA. Direct determination of iron and selenium in bovine milk by graphite furnace atomic absorption spectrometry. Food Chem. 2003;83(3):457–462.
- Allsop TF, Rook JA. The effect of diet and blood-plasma magnesium concentration on the endogenous faecal loss of magnesium in sheep. J. Agric. Sci. 1979;92:403–408.
- AlSaiady MY, AlShaikh MA, AlMufarrej SI, AlShoweimi TA, Mogawer HH, Dirrar A. Effect of chelated chromium supplementation on lactation performance and blood parameters of Holstein cows under heat stress. Anim. Feed Sci. Technol. 2004;117:223–233.
- Ammerman CB, Wing JM, Dunavant BG, Robertson WK, Feaster JP, Arrington LR. Utilization of inorganic iron by ruminants as influenced by form of iron and iron status of the animal. J. Anim. Sci. 1967;26(2):404–410. [PubMed: 6033928]
- Ammerman CB, Chicco CF, Loggins PE, Arrington LR. Availability of different inorganic salts of magnesium to sheep. J. Anim. Sci. 1972;34:122–126. [PubMed: 5059168]
- Animal Improvement Laboratory. USDA summary of 2015 herd averages. 2015. [September 27, 2017]. https://www
.uscdcb.com /publish/dhi/dhi16/hax.html . - Anke M. Arsenic. In: Mertz W, editor. Trace Elements in Human and Animal Nutrition. Orlando, FL: Academic Press; 1986. pp. 347–372.
- AOAC (Association of Official Analytical Chemists). Gaithersburg, MD: AOAC International; Official Methods of Analysis of AOAC International. (17th) 2000;1 and 2
- ARC (Agricultural Research Council). The Nutrient Requirements of Farm Livestock: No. 2. Ruminants. London, UK: ARC; 1965.
- ARC. The Nutrient Requirements of Ruminant Livestock. Slough, England: Commonwealth Agricultural Bureaux; 1980.
- Ardüser F, Wolffram S, Scharrer E, Schneider B. Transport of selenate and selenite across the brush border membrane of rat and sheep small intestine. Biol. Trace Element Res. 1986;9:281–290.
- Arthington JD, Rechcigl JE, Yost GP, McDowell LR, Fanning MD. Effect of ammonium sulfate fertilization on bahiagrass quality and copper metabolism in grazing beef cattle. J. Anim. Sci. 2002;80:2507–2512. [PubMed: 12413071]
- Arthington JD, Pate FM, Spears JW. Effect of copper source and level on performance and copper status of cattle consuming molasses-based supplements. J. Anim. Sci. 2003;81:1357–1362. [PubMed: 12817481]
- Aspila P. Metabolism of selenite, selenomethionine, and feed-incorporated selenium in lactating goats and dairy cows. J. Agric. Sci. 1988;63:1–74.
- Atroshi F, Sankari S, Lindström UB. Somatic cell count and milk yield in relation to hemoglobin concentration in Finnish dairy goats. Vet. Res. Commun. 1986;10:57–63. [PubMed: 3753810]
- Auza NJ, Olson WG, Murphy MJ, Linn JG. Diagnosis and treatment of copper toxicosis in ruminants. J. Am. Vet. Med. Assoc. 1999;214:1624–1628. [PubMed: 10363091]
- Awadeh FT, Kincaid RL, Johnson KA. Effect of level and source of dietary selenium on concentrations of thyroid hormones and immunoglobulins in beef cows and calves. J. Anim. Sci. 1998;76:1204–1215. [PubMed: 9581946]
- Babcock SM. Wisconsin Experiment Station 22nd Annual Report. Madison: University of Wisconsin; 1905. The addition of salt to the ration of dairy cows; pp. 129–156.
- Bailey CB. Saliva secretion and its relation to feeding in cattle: 4. The relationship between the concentrations of sodium, potassium, chloride and inorganic phosphate in mixed saliva and rumen fluid. Br. J. Nutr. 1961;15:489–498. [PubMed: 13864005]
- Balemi SC, Grace ND, West DM, Smith SL, Knowles SO. Accumulation and depletion of liver copper stores in dairy cows challenged with a Cu-deficient diet and oral and injectable forms of cu supplementation. N. Z. Vet. J. 2010;58:137–141. [PubMed: 20514087]
- Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: A systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154–2163. [PubMed: 17519436]
- Bannink A, Valk H, van Vuuren AM. Intake and excretion of sodium, potassium, and nitrogen and the effects on urine production by lactating dairy cows. J. Dairy Sci. 1999;82:1008–1018. [PubMed: 10342240]
- Baydar E, Dabak M. Serum iron as an indicator of acute inflammation in cattle. J. Dairy Sci. 2014;97:222–228. [PubMed: 24268402]
- Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001;131(2):568S–580S. [PubMed: 11160590]
- Becker R, Neal W, Shealy AL. Effect of calcium-deficient roughage upon milk production and welfare of dairy cows. Gainesville, FL: Florida Agricultural Experiment Station Bulletin. 1933;262
- Beede DK, Shearer JK. Nutritional management of dairy cattle during hot weather. Agri-Prac. 1991;12:5–12.
- Beede DK, Mallonee PG, Schneider PL, Wilcox CJ, Collier RJ. Potassium nutrition of heat-stressed lactating dairy cows. S. Afr. J. Anim. Sci. 1983;13:198–200.
- Bellof G, Pallauf J. Deposition of copper, iron, manganese and zinc in the empty body of growing lambs of the breed German Merino Landsheep. Animal. 2007;1:827–834. [PubMed: 22444746]
- Belyea RL, Martz FA, Young RD, Clark JL. Effects of dietary potassium and rumen contents upon 40K estimation of body composition in dairy cattle. J. Anim. Sci. 1978;46(1):320–327. [PubMed: 417054]
- Bennink MR, Tyler TR, Ward GM, Johnson DE. Ionic milieu of bovine and ovine rumen as affected by diet. J. Dairy Sci. 1978;61(3):315–323. [PubMed: 659685]
- Bentley OG, Phillips PH. The effect of low manganese rations upon dairy cattle. J. Dairy Sci. 1951;34:396–403.
- Berg JN, Padgitt D, McCarthy B. Iodine concentrations in milk of dairy cattle fed various amounts of iodine as ethylenediamine dihydroiodide. J. Dairy Sci. 1988;71:3283–3291. [PubMed: 3235730]
- Bernier JF, Fillion FJ, Brisson GJ. Dietary fibers and supplementary iron in a milk replacer for veal calves. J. Dairy Sci. 1984;67:2369–2379. [PubMed: 6094628]
- Bigelow AC, Hemken RW, Harmon RJ. Potassium requirement of the dairy calf. J. Dairy Sci. 1984;67(Suppl. 1):104.
- Bijl E, van Valenberg HJF, Huppertz T, van Hooijdonk ACM. Protein, casein, and micellar salts in milk: Current content and historical perspectives. J. Dairy Sci. 2012;96:5455–5464. [PubMed: 23849643]
- Bird P. Sulphur metabolism and excretion studies in ruminants: X. Sulphide toxicity in sheep. Aust. J. Biol. Sci. 1972a;25:1087–1098. [PubMed: 4663345]
- Bird P. Sulphur metabolism and excretion studies in ruminants: V. Ruminal desulphuration of methionine and cyst(e)ine. Aust. J. Biol. Sci. 1972b;25:185–193. [PubMed: 5017904]
- Bird P, Moir R. Sulphur metabolism and excretion studies in ruminants I: The absorption of sulphate in sheep after intraruminal or intraduodenal infusions of sodium sulphate. Aust. J. Biol. Sci. 1971;24:1319–1328. [PubMed: 5163489]
- Bjelland DW, Weigel KA, Hoffman PC, Esser NM, Coblentz WK. The effect of feeding dairy heifers diets with and without supplemental phosphorus on growth, reproductive efficiency, health, and lactation performance. J. Dairy Sci. 2011;94:6233–6242. [PubMed: 22118111]
- Blaxter KL, McGill RF. Magnesium metabolism in cattle. CAB Vet. Rev. 1956;2:35–56.
- Bortolussi G, Ternouth JH, McMeniman NP. Dietary nitrogen and phosphorus depletion in cattle and their effects on liveweight gain, blood metabolite concentrations, and phosphorus kinetics. J. Agric. Sci. 1996;126:493–501.
- Bouchard R, Conrad HR. Sulfur requirement of lactating dairy cows: I. Sulfur balance and dietary supplementation. J. Dairy Sci. 1973a;56:1276–1282. [PubMed: 4742111]
- Bouchard R, Conrad HR. Sulfur requirement of lactating dairy cows: II. Utilization of sulfates, molasses, and lignin-sulfonate. J. Dairy Sci. 1973b;56:1429–1434. [PubMed: 4796098]
- Brask-Pedersen DN, Glitsø LV, Skov LK, Lund P, Sehested J. Effect of exogenous phytase on degradation of inositol phosphate in dairy cows. J. Dairy Sci. 2013;96:1691–1700. [PubMed: 23312994]
- Bremner I, Dalgarno AC. Iron metabolism in the veal calf. Br. J. Nutr. 1973a;30:61–76. [PubMed: 4352722]
- Bremner I, Dalgarno AC. Iron metabolism in the veal calf. The availability of different iron compounds. Br. J. Nutr. 1973b;29:229–243. [PubMed: 4693559]
- Bremner I, Dalgarno AC. Iron metabolism in the veal calf: 2. Iron requirements and the effect of copper supplementation. Br. J. Nutr. 1973c;30:61–76. [PubMed: 4352722]
- Breves G, Schröder B. Comparative aspects of gastrointestinal phosphorus metabolism. Nutr. Res. Rev. 1991;4:125–140. [PubMed: 19094328]
- Brintrup R, Mooren T, Meyer U, Spiekers H, Pfeffer E. Effects of two levels of phosphorus intake on performance and faecal phosphorus excretion in dairy cows. J. Anim. Physiol. Anim. Nutr. 1993;69:29–36.
- Britton AA, Cotzias GC. Dependence of manganese turnover on intake. Am. J. Physiol. 1966;211:203–206. [PubMed: 5911039]
- Brock AA, Chapman SA, Ulman EA, Wu G. Dietary manganese deficiency decreases rat hepatic arginase activity. J. Nutr. 1994;124:340–344. [PubMed: 8120652]
- Brodison JA, Goodall EA, Armstrong JD, Givins DI, Gordon FJ, McCaughey WJ, Todd JR. Influence of dietary phosphorus on the performance of lactating dairy cows. J. Agric. Sci. 1989;112:303–311.
- Brody S. Bioenergetics and Growth with Special Reference to the Energetic Efficiency Complex in Domestic Animals. New York: Reinhold; 1945.
- Bronner F. Intestinal calcium absorption: mechanisms and applications. J. Nutr. 1987;117(8):1347–1352. [PubMed: 3305814]
- Bryan MA, Socha MT, Tomlinson DJ. Supplementing intensively grazed late-gestation and early-lactation dairy cattle with chromium. J. Dairy Sci. 2004;87:4269–4277. [PubMed: 15545390]
- Buckley WT. A kinetic model of copper metabolism in lactating dairy cows. Can. J. Anim. Sci. 1991;71:155–166.
- Burkhalter DL, Neathery MW, Miller WJ, Whitlock RH, Allen JC. Effects of low chloride intake on performance, clinical characteristics, and chloride, sodium, potassium, and nitrogen metabolism in dairy calves. J. Dairy Sci. 1979;62:1895–1901. [PubMed: 541461]
- Burkhalter DL, Neathery MW, Miller WJ, Whitlock RH, Allen JC, Gentry RP. Influence of a low chloride practical diet on acid-base balance and other factors of blood in young dairy calves. J. Dairy Sci. 1980;63:269–276. [PubMed: 7358885]
- Burroughs W, Latona A, DePaul P, Gerlaugh P, Bethke RM. Mineral influences upon urea utilization and cellulose digestion by rumen microorganisms using the artificial rumen technique. J. Anim. Sci. 1951;10:693–705.
- Call JW, Butcher JE, Shape JL, Lamb RC, Woman RL, Olson AE. Clinical effects of low dietary phosphorus concentrations in feed given to lactating cows. Am. J. Vet. Res. 1987;48:133–136. [PubMed: 3826833]
- Cam Y, Gumussoy KS, Kibr M, Amydin N, Atalay O. Efficacy of ethylene diamine dihydriodide for the treatment of ringworm in young cattle. Vet. Rec. 2007;160:408–410. [PubMed: 17384294]
- Cao Y, Maddox JF, Mastro AM, Scholz RW, Hildenbrandt G, Reddy CC. Selenium deficiency alters the lipoxygenase pathway and mitogenic response in bovine lymphocytes. J. Nutr. 1992;122:2121–2127. [PubMed: 1331381]
- Cao J, Henry PR, Guo R, Holwerda RA, Toth JP, Littell RC, Miles RD, Ammerman CB. Chemical characteristics and relative bioavailability of supplemental organic zinc sources for poultry and ruminants. J. Anim. Sci. 2000;78:2039–2054. [PubMed: 10947086]
- Care AD, Bartlet JP, Abdel-Hafeez HM. Calcium and phosphate homeostasis in ruminants and its relationship to the aetiology and prevention of parturient paresis. In: Ruckebusch Y, Thivend P, editors. Digestive Physiology and Metabolism in Ruminants. Lancaster, UK: MTP Press; 1980. pp. 429–446.
- Carroll SM, DePeters EJ, Taylor SJ, Rosenberg M, Perez-Monti H, Capps VA. Milk composition of Holstein, Jersey, and Brown Swiss cows in response to increasing levels of dietary fat. Anim. Feed Sci. Technol. 2006;131:451–473.
- Carstairs JA, Morrow DA, Emery RS. Postpartum reproductive function of dairy cows as influenced by energy and phosphorus status. J. Anim. Sci. 1980;51:1122–1130. [PubMed: 7204264]
- Castillo AR, St-Pierre NR, Silva del Rio N, Weiss WP. Mineral concentrations in diets, water, and milk and their value in estimating on-farm excretion of manure minerals in lactating dairy cows. J. Dairy Sci. 2013;96:3388–3398. [PubMed: 23477818]
- Catterton TL, Erdman RA. The effect of cation source and dietary cation-anion difference on rumen ion concentrations in lactating dairy cows. J. Dairy Sci. 2016;99(8):6274–6284. [PubMed: 27289159]
- Ceballos A, Sanchez J, Stryhn H, Montgomery JB, Barkema HW, Wichtel JJ. Meta-analysis of the effect of oral selenium supplementation on milk selenium concentration in cattle. J. Dairy Sci. 2009;92:324–342. [PubMed: 19109290]
- Ceballos A, Kruze J, Barkema HW, Dohoo IR, Sanchez J, Uribe D, Wichtel JJ, Wittwer F. Barium selenate supplementation and its effect on intramammary infection in pasture-based dairy cows. J. Dairy Sci. 2010;93:1468–1477. [PubMed: 20338424]
- Cebra CK, Heidel JR, Crisman RO, Stang BV. The relationship between endogenous cortisol, blood micronutrients, and neutrophil function in postparturient Holstein cows. J. Vet. Intern. Med. 2003;17:902–907. [PubMed: 14658729]
- Cefalu WT, Hu FB. Role of chromium in human health and diabetes. Diabetes Care. 2004;27:2741–2751. [PubMed: 15505017]
- Challa J, Braithwaite GD. Phosphorus and calcium metabolism in growing calves with special emphasis on phosphorus homeostasis: 1. Studies of the effect of changes in the dietary phosphorus intake on phosphorus and calcium metabolism. J. Agric. Sci. 1988;110:573–581.
- Challa J, Braithwaite GD, Dhanoa MS. Phosphorus homoeostasis in growing calves. J. Agric. Sci. 1989;112:217–226.
- Chang C-I, Liao JC, Kuo L. Arginase modulates nitric oxide production in activated macrophages. Am. J. Physiol. 1998;274(1):H342–H348. [PubMed: 9458885]
- Chang X, Mallard BA, Mowat DN. Effects of chromium on health status, blood neutrophil phagocytosis, and in vitro lymphocyte blastogenesis of dairy cows. Vet. Immunol. Immunopathol. 1996;52:37–52. [PubMed: 8807775]
- Chase CR, Beede DK, van Horn HH, Shearer JK, Wilcox CJ, Donovan GA. Responses of lactating dairy cows to copper source, supplementation rate, and dietary antagonist (iron). J. Dairy Sci. 2000;83:1845–1852. [PubMed: 10984161]
- Chicco CF, Ammerman CB, Moore JE, van Walleghem PA, Arrington LR, Shirley RL. Utilization of inorganic ortho-, meta- and pyrophosphates by lambs and by cellulolytic rumen microorganisms. J. Anim. Sci. 1965;24:355–363. [PubMed: 14329434]
- Chua ACG, Morgan EH. Manganese metabolism is impaired in the Belgrade laboratory rat. J. Comp. Physiol. B. 1997;167(5):361–369. [PubMed: 9265748]
- Condomina J, Zornoza-Sabina T, Granero L, Polache A. Kinetics of zinc transport in vitro in rat small intestine and colon: Interaction with copper. Eur. J. Pharm. Sci. 2002;16:289–295. [PubMed: 12208459]
- Conrad HR, Moxon AL. Transfer of dietary selenium to milk. J. Dairy Sci. 1979;62:404–411. [PubMed: 447890]
- Constable PD, Gelfert CC, Fürll M, Staufenbiel R, Stämpfli HR. Application of strong ion difference theory to urine and the relationship between urine pH and net acid excretion in cattle. Am. J. Vet. Res. 2009;70:915–925. [PubMed: 19566478]
- Contreras PA, Matamoros R, Monroy R, Kruze J, Leyan V, Andaur M, Bo Hmwald H, Wittwer F. Effect of a selenium-deficient diet on blood values of t3 and t4 in cows. Comp. Clin. Pathol. 2002;11:65–70.
- Cope CM, Mackenzie AM, Wilde D, Sinclair LA. Effects of level and form of dietary zinc on dairy cow performance and health. J. Dairy Sci. 2009;92:2128–2135. [PubMed: 19389970]
- Coppock CE. Mineral utilization by the lactating cow—chlorine. J. Dairy Sci. 1986;69:595–603. [PubMed: 3700798]
- Coppock CE, Aguirre RA, Chase LE, Lake GB, Oltenacu EA, McDowell RE, Fettman MJ, Woods ME. Effect of low chloride diet on lactating cows. J. Dairy Sci. 1979;62:723–731. [PubMed: 457994]
- Correa LB, Zanetti MA, Del Claro GR, de Paiva FA, da Luz e Silva S, Netto AS. Effects of supplementation with two sources and two levels of copper on meat lipid oxidation, meat colour and superoxide dismutase and glutathione peroxidase enzyme activities in Nellore beef cattle. Br. J. Nutr. 2014;112:1266–1273. [PubMed: 25313573]
- Dalley DE, Isherwood P, Sykes AR, Robson AB. Effect of in vitro manipulation of pH on magnesium solubility in ruminal and caecal digesta in sheep. J. Agric. Sci. 1997;129:107–111.
- Davenport GM, Boling JA, Gay N. Bioavailability of magnesium in beef cattle fed magnesium oxide or magnesium hydroxide. J. Anim. Sci. 1990;68:3765–3772. [PubMed: 2262427]
- Davidsson L, Cederblad A, Lönnerdal B, Sandström B. The effect of individual dietary components on manganese absorption in humans. Am. J. Clin. Nutr. 1991;54:1065–1070. [PubMed: 1957822]
- Davis C, Vincent JB. Isolation and characterization of a biologically active chromium oligopeptide from bovine liver. Arch. Biochem. Biophys. 1997;339:335–343. [PubMed: 9056266]
- Davis CD, Greger JL. Longitudinal changes of manganese-dependent superoxide dismutase and other indexes of manganese and iron status in women. Am. J. Clin. Nutr. 1992;55:747–752. [PubMed: 1550052]
- De Boer G, Buchanan-Smith JG, Macleod GK, Walton JS. Responses of dairy cows fed alfalfa silage supplemented with phosphorus, copper, zinc, and manganese. J. Dairy Sci. 1981;64:2370–2377.
- de Carvalho PR, Pita MC, Loureiro JE, Tanaka HR, Ribeiro JC. Manganese deficiency in bovines: Connection between manganese metalloenzyme dependent in gestation and congenital defects in newborn calves. Pakistan J. Nutr. 2010;9:488–503.
- de Rosa G, Keen CL, Leach RM, Hurley LS. Regulation of superoxide dismutase activity by dietary manganese. J. Nutr. 1980;110:795–804. [PubMed: 7365547]
- DeLuca HF. The vitamin D system in the regulation of calcium and phosphorus metabolism. Nutr. Rev. 1979;37(6):161–193. [PubMed: 542234]
- Demott BJ, Hinton SA, Swanson EW, Miles JT. Influence of added sodium chloride in grain ration on the freezing point of milk. J. Dairy Sci. 1968;51:1363–1365.
- Dennis J, Harbaugh FG. The experimental alteration of blood potassium and calcium levels in cattle. Am. J. Vet. Res. 1948;9:20–25. [PubMed: 20268043]
- Dennis RJ, Hemken RW. Potassium requirement of dairy cows in early and mid-lactation. J. Dairy Sci. 1978;61:757–761. [PubMed: 690302]
- Dennis RJ, Hemken RW, Jacobson DR. Effect of dietary potassium percent for lactating dairy cows. J. Dairy Sci. 1976;59:324–328. [PubMed: 1249300]
- Dewes HF. The rate of soil ingestion by dairy cows and the effect on available copper, calcium, sodium and magnesium. N. Z. Vet. J. 1996;44:199–200. [PubMed: 16031934]
- Dikmen S, Khan FA, Huson HJ, Sonstegard TS, Moss JI, Dahl GE, Hansen PJ. The SLICK hair locus derived from Senepol cattle confers thermotolerance to intensively-managed lactating Holstein cows. J. Dairy Sci. 2014;97:5508–5520. [PubMed: 24996281]
- Dikmen D, Wang XZ, Ortega MS, Cole JB, Null DJ, Hansen PJ. Single nucleotide polymorphisms associated with thermoregulation in lactating dairy cows exposed to heat stress. J. Anim. Breed. Genet. 2015;132:409–419. [PubMed: 26198991]
- Drewnoski ME, Pogge DJ, Hansen SL. High-sulfur in beef cattle diets: A review. J. Anim. Sci. 2014;92:3763–3780. [PubMed: 24981568]
- Du Z, Hemken RW, Harmon RJ. Copper metabolism of Holstein and jersey cows and heifers fed diets high in cupric sulfate or copper proteinate. J. Dairy Sci. 1996;79:1873–1880. [PubMed: 8923258]
- Ekelund A, Sporndly R, Holtenius K. Influence of low phosphorus intake during early lactation on apparent digestibility of phosphorus and bone metabolism in dairy cows. Livestock Sci. 2006;99:227–236.
- Eldman IS, James AH, Boden H, Moore FD. Electrolyte composition of bone and the penetration of radiosodium and deuterium oxide into dog and human bone. J. Clin. Invest. 1954;33:122–131. [PMC free article: PMC438482] [PubMed: 13130679]
- Ellenberger H, Newlander J, Jones CH. Calcium and phosphorus requirements of dairy cows; weekly balances through lactation and gestation periods. Vermont Agric. Exp. Station Bull. 1931;10:245–260.
- Ellenberger HB, Newlander JA, Jones CH. University of Vermont Agricultural Experiment Station Bulletin No. 558. Burlington: University of Vermont; 1950. Composition of the bodies of dairy cattle.
- Ely RE, Dunn KM, Huffman CF. Cobalt toxicity in calves resulting from high oral administration. J. Anim. Sci. 1948;7:239–243.
- Ender F, Dishington IW, Helegebostad IW. Calcium balance studies in dairy cows under experimental induction and prevention of hypocalcaemic paresis puerperalis: The solution of the aetiology and the prevention of milk fever by dietary means. Z. Tierphysiol. Tierernaehr. Futtermittelkund. 1971;28:233–256. [PubMed: 5141049]
- Engle TE, Spears JW. Effects of dietary copper concentration and source on performance and copper status of growing and finishing steers. J. Anim. Sci. 2000;78:2446–2451. [PubMed: 10985420]
- Enjalbert F, Lebreton P, Salat O. Effects of copper, zinc and selenium status on performance and health in commercial dairy and beef herds: Retrospective study. J. Anim. Physiol. Anim. Nutr. 2006;90:459–466. [PubMed: 17083426]
- Erdman RA. Dietary buffering requirements of the lactating dairy cow: A review. J. Dairy Sci. 1988;71:3246–3266.
- Erdman RA, Hemken RW, Bull LS. Effects of dietary calcium and sodium on potassium requirement for lactating dairy cows. J. Dairy Sci. 1980;63:538–544. [PubMed: 7189759]
- Erdman RA, Hemken RW, Bull LS. Dietary sodium bicarbonate and magnesium oxide for early postpartum lactating dairy cows: Effects on production, acid-base metabolism, and digestion. J. Dairy Sci. 1982;65(5):712–731. [PubMed: 6286736]
- Erdogan S, Celik S, Erdogan Z. Seasonal and locational effects on serum, milk, liver and kidney chromium, manganese, copper, zinc, and iron concentrations of dairy cows. Biol. Trace Element Res. 2004;98(1):51–61. [PubMed: 15051900]
- Ermans A, Bourdoux P. Antithyroid sulfurated compounds. Gaitan E, editor. Boca Raton, FL: CRC Press; Environmental Goitrogenesis. 1989:15.
- Erskine RJ, Eberhart RJ, Hutchinson LJ, Scholz RW. Blood selenium concentrations and glutathionine peroxidase activities in dairy herds with high and low somatic cell counts. J. Am. Vet. Med. Assoc. 1987;190:1417–1421. [PubMed: 3301763]
- Erskine RJ, Eberhart RJ, Grasso PJ, Scholz RW. Induction of Escherichia coli mastitis in cows fed selenium-deficient or selenium-supplemented diets. Am. J. Vet. Res. 1989;50:2093–2100. [PubMed: 2558602]
- Erskine RJ, Eberhart RJ, Scholz RW. Experimentally induced staphylococcus aureus mastitis in selenium-deficient and selenium-supplemented dairy cows. J. Am. Vet. Med. Assoc. 1990;51:1107–1111. [PubMed: 2389887]
- Escobosa A, Coppock CE, Rowe LD Jr, Jenkins WL, Gates CE. Effects of dietary sodium bicarbonate and calcium chloride on physiological responses of lactating dairy cows in hot weather. J. Dairy Sci. 1984;67:574–584. [PubMed: 6325517]
- Esser NM, Hoffman PC, Coblentz WK, Orth MW, Weigel KA. The effect of dietary phosphorus on bone development in dairy heifers. J. Dairy Sci. 2009;92:1741–1749. [PubMed: 19307656]
- Evans GW, Grace CI, Hahn C. The effect of copper and cadmium on 65Zn absorption in zinc-deficient and zinc-supplemented rats. Bioinorg. Chem. 1974;3:115–120. [PubMed: 4441547]
- Fach C. Ruminal magnesium absorption: Mechanisms, modulation and meaning for assessment of Mg intake. Freie Universität Berlin; Berlin, Germany: 2015. PhD diss.
- Faulkner MJ, Weiss WP. Effect of source of trace minerals in either forage- or by-product based-diets fed to dairy cows: 1. Production and macronutrient digestibility. J. Dairy Sci. 2017;100:5358–5367. [PubMed: 28457553]
- Faulkner MJ, St-Pierre NR, Weiss WP. Effect of source of trace minerals in either forage or by-product-based diets fed to dairy cows: 2. Apparent absorption and retention of minerals. J. Dairy Sci. 2017;100:5368–5377. [PubMed: 28456401]
- FDA (U.S. Food and Drug Administration). Food additives permitted in feed and drinking water of animals; selenium. Fed. Regist. 1997;62:44892–44894.
- FDA. Food additives permitted in feed and drinking water of animals; selenium yeast. Fed. Regist. 2003;68(170):52339–52340.
- Felix TL, Zerby HN, Moeller SJ, Loerch SC. Effects of increasing dried distillers grains with solubles on performance, carcass characteristics, and digestibility of feedlot lambs. J. Anim. Sci. 2012;90:1356–1363. [PubMed: 22147466]
- Feng X, Knowlton KF, Hanigan MD. Parameterization of a ruminant model of phosphorus digestion and metabolism. J. Dairy Sci. 2015;98:7194–7208. [PubMed: 26233448]
- Feng X, Jarrett JP, Knowlton KF, James RE, Hanigan MD. Short communication: Comparison of predicted ration phosphorus balance using bioavailabilities from the NRC (2001) and Virginia Tech models. J. Dairy Sci. 2016;99:1237–1241. [PubMed: 26709165]
- Fettman MJ, Chase LE, Bentinck-Smith J, Coppock CE, Zinn SA. Nutritional chloride deficiency in early lactation Holstein cows. J. Dairy Sci. 1984a;67:2321–2335. [PubMed: 6501651]
- Fettman MJ, Chase LE, Bentinck-Smith J, Coppock CE, Zinn SA. Effects of dietary chloride restriction in lactating cows. J. Am. Vet. Med. Assoc. 1984b;185:167–172. [PubMed: 6086555]
- Fettman MJ, Chase LE, Bentinck-Smith J, Coppock CE, Zinn SA. Restricted dietary chloride and sodium bicarbonate supplementation in early lactation Holstein cows: Cerebrospinal fluid electrolyte alterations. Am. J. Vet. Res. 1984c;45:1403–1408. [PubMed: 24049907]
- Finley JW, Johnson PE, Johnson LK. Sex affects manganese absorption and retention by humans from a diet adequate in manganese. Am. J. Clin. Nutr. 1994;60:949–955. [PubMed: 7985639]
- Fisher LJ, Dinn N, Tait RM, Shelford JA. Effect of level of dietary potassium on the absorption and excretion of calcium and magnesium by lactating cows. Can. J. Anim. Sci. 1994;74:503–509.
- G Flachowsky, K Franke, U Meyer, M Leiterer, F Schone. Influencing factors on iodine content of cow milk. Eur. J. Nutr. 2014;53:351–365. [PubMed: 24185833]
- Flagstad T. Lethal trait A46 in cattle intestinal zinc absorption. Nor-disk Veterinaermed. 1976;28:160–169. [PubMed: 1272757]
- Flynn A, Power P. Nutritional aspects of minerals in bovine and human milks. In: Fox PF, editor. Developments in Dairy Chemistry-3: Lactose and Minor Constituents. New York: Elsevier Applied Science; 1985. pp. 183–215.
- Forar FL, Kincaid RL, Preston RL, Hillers JK. Variation of inorganic phosphorus in blood plasma and milk of lactating cows. J. Dairy Sci. 1982;65:760–763. [PubMed: 7202021]
- Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr. 2004;24:277–298. [PubMed: 15189122]
- Franke K, Meyer U, Wagner H, Flachowsky G. Influence of various iodine supplementation levels and two different iodine species on the iodine content of the milk of cows fed rapeseed or distillers dried grains with solubles as the protein source. Dairy Sci. 2009a;92:4514–4523. [PubMed: 19700713]
- Franke K, Meyer U, Wagner H, Hoppen HO, Flachowsky G. Effect of various iodine supplementations, rapeseed application and two different iodine species on the iodine status and iodine excretion of dairy cows. Livestock Sci. 2009b;125:223–231.
- Fry RS, Spears JW, Lloyd KE, O'Nan AT, Ashwell MS. Effect of dietary copper and breed on gene products involved in copper acquisition, distribution, and use in angus and Simmental cows and fetuses. J. Anim. Sci. 2013;91:861–871. [PubMed: 23148247]
- Furugouri K, Miyata Y, Shijimaya K. Ferritin in blood serum of dairy cows. J. Dairy Sci. 1982;65:1529–1534. [PubMed: 7142530]
- Gäbel G, Martens H. The effect of ammonia on magnesium metabolism in sheep. J. Anim. Physiol. Anim. Nutr. 1986;55:278–287.
- Gammelgaard B, Rasmussen L, Gabel-Jensen C, Steffansen B. Estimating intestinal absorption of inorganic and organic selenium compounds by in vitro flux and biotransformation studies in caco-2 cells and ICP-MS detection. Biol. Trace Element Res. 2012;145:248–256. [PubMed: 21863324]
- Gant RG, Sanchez W, Kincaid RL. Effect of anionic salts on selenium metabolism in nonlactating pregnant dairy cows. J. Dairy Sci. 1998;81:1637–1642. [PubMed: 9684171]
- García-Casal MN, Layrisse M, Solano L, Barón MA, Arguello F, Llovera D, Ramírez J, Leets I, Tropper E. Vitamin A and β-carotene can improve nonheme iron absorption from rice, wheat and corn by humans. J. Nutr. 1998;128(3):646–650. [PubMed: 9482776]
- García-Vaquero M, Miranda M, Benedito JL, Blanco-Penedo I, López-Alonso M. Effect of type of muscle and Cu supplementation on trace element concentrations in cattle meat. Food Chem. Toxicol. 2011;49:1443–1449. [PubMed: 21443918]
- Gardner WC, Broersma K, Popp JD, Mir Z, Mir OS, Buckley WT. Copper and health status of cattle grazing high-molybdenum forage from a reclaimed mine tailing site. Can. J. Anim. Sci. 2003;83:479–485.
- Garrick M, Dolan K, Horbinski C, Ghio A, Higgins D, Porubcin M, Moore E, Hainsworth L, Umbreit J, Conrad M, Feng L, Lis A, Roth J, Singleton S, Garrick L. DMT1: A mammalian transporter for multiple metals. Biometals. 2003;16(1):41–54. [PubMed: 12572663]
- Gaucheron F. The minerals of milk. Reprod. Nutr. Dev. 2005;45:473–483. [PubMed: 16045895]
- Genther ON, Hansen SL. A multi-element trace mineral injection improves liver copper and selenium concentrations and manganese superoxide dismutase activity in beef steers. J. Anim. Sci. 2014;92:695–704. [PubMed: 24398829]
- Gerken HJ, Fontenot JP. Availability and utilization of magnesium from dolomitic limestone and magnesium oxide in steers. J. Anim. Sci. 1967;26:1404–1408. [PubMed: 6081420]
- Gibson CD, Coe PH, Ellis RG, Stowe HD, Bartlett PC, Naasz PE. Field trial to evaluate the effects of different forms of oral selenium supplementation on production in lactating Holstein cows. Agri-Practices. 1993;14:14–19.
- Gillooly M, Bothwell TH, Torrance JD, MacPhail AP, Derman DP, Bezwoda WR, Mills W, Charlton RW, Mayet F. The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br. J. Nutr. 1983;49:331–342. [PubMed: 6860621]
- Girard CL, Santschi DE, Stabler SP, Allen RH. Apparent ruminal synthesis and intestinal disappearance of vitamin B12 and its analogs in dairy cows. J. Dairy Sci. 2009;92:4524–4529. [PubMed: 19700714]
- Goff JP. Phosphorus deficiency. In: Howard JL, Smith RA, editors. Current Veterinary Therapy 4: Food Animal Practice. Philadelphia, PA: W.B. Saunders; 1998. pp. 218–220.
- Goff JP. Macromineral disorders of the transition cow. Vet. Clin. North Am. 2004;20:471–494. [PubMed: 15471621]
- Goff JR. Mineral absorption mechanisms, mineral interactions that affect acid-base and anti-oxidant status, and diet considerations to improve mineral status. J. Dairy Sci. 2018;101:2763–2813. [PubMed: 29397180]
- Goff JP, Horst RL. Oral administration of calcium salts for treatment of hypocalcemia in cattle. J. Dairy Sci. 1993;76:101–108. [PubMed: 8436668]
- Goff JP, Stabel JR. Decreased plasma retinol, α-tocopherol, and zinc concentration during the periparturient period: Effect of milk fever. J. Dairy Sci. 1990;73:3195–3199. [PubMed: 2273148]
- Gomez A, Bernardoni N, Rieman J, Dusick A, Hartshorn R, Read DH, Socha MT, Cook NB, Döpfer D. A randomized trial to evaluate the effect of a trace mineral premix on the incidence of active digital dermatitis lesions in cattle. J. Dairy Sci. 2014;97:6211–6222. [PubMed: 25087030]
- Goodman H, Middlesworth L. The thyroid gland. Mountcastle V, editor. St. Louis: C.V. Mosby; Medical Physiology. 1980;2:1495–1518.
- Gooneratne SR, Christensen DA, Bailey JV, Symonds HW. Effects of dietary copper, molybdenum and sulfur on biliary copper and zinc excretion in Simmental and Angus cattle. Can. J. Anim. Sci. 1994;74:315–325.
- Gooneratne SR, Laarveld B, Pathirana KK, Christensen DA. Effects of dietary Cu, Mo and S on urinary Cu and Zn excretion in Simmental and Angus cattle. Res. Vet. Sci. 2011;91:e116–e120. [PubMed: 21338998]
- Gould DH. Polioencephalomalacia. J. Anim. Sci. 1998;76:309–314. [PubMed: 9464912]
- Gould L, Kendall NR. Role of the rumen in copper and thiomolybdate absorption. Nutr. Res. Rev. 2011;24:176–182. [PMC free article: PMC3269883] [PubMed: 22296933]
- Grace ND. Amounts and distribution of mineral elements associated with fleece-free empty body weight gains in the grazing sheep. N. Z. J. Agric. Res. 1983;26:59–70.
- Grace ND, Knowles S. Taking action to reduce the risk of copper toxicity in cattle. Vet. Rec. 2015;177:490–491. [PubMed: 26564888]
- Grace ND, Ulyatt MJ, MacRae JC. Quantitative digestion of fresh herbage by sheep: 3. Movement of Mg, Ca, P, K, and Na in the digestive tract. J. Agric. Sci. 1974;82:321–330.
- Grace ND, Venning M, Mills AR, Death AF. The efficacy of selenium dioxide as a selenium supplement for dairy cattle. N. Z. Vet. J. 1995;43:77–78. [PubMed: 16031813]
- Grace ND, Rounce JR, Lee J. Effect of soil ingestion on the storage of se, vitamin B12, Cu, Cd, Fe, Mn, and Zn in the liver of sheep fed lucerne pellets. N. Z. J. Agric. Res. 1996;39:325–331.
- Greene LW, Fontenot JP, Webb KE Jr. Site of magnesium and other macromineral absorption in steers fed high levels of potassium. J. Anim. Sci. 1983;57:503–510. [PubMed: 6619018]
- Greene LW, Schelling GT, Byers FM. Effects of dietary monensin and potassium on apparent absorption of magnesium and other macroelements in sheep. J. Anim. Sci. 1986a;63:1960–1967. [PubMed: 3818469]
- Greene LW, Solis JC, Byers FM, Schelling GT. Apparent and true digestibility of magnesium in mature cows of five breeds and their crosses. J. Anim. Sci. 1986b;63:189–196.
- Gresakova L, Cobanova K, Faix S. Selenium retention in lambs fed diets supplemented with selenium from inorganic or organic sources. Small Ruminant Res. 2013;111:76–82.
- Grünberg W. Proceedings of the 17th Annual Tri-State Dairy Nutrition Conference. Fort Wayne, IN: Ohio State University, Michigan State University, and Purdue University; 2008. Phosphorus homeostasis in dairy cattle: Some answers, more questions; pp. 29–35.
- Grünberg W, Constable P, Schröder U, Staufenbiel R, Morin D, Rohn M. Phosphorus homeostasis in dairy cows with abomasal displacement or abomasal volvulus. J. Vet. Intern. Med. 2005;19:894–898. [PubMed: 16355687]
- Guardiola CM, Fahey GC, Spears JW, Garrigus US, Izquierdo OA, Pedroza C. The effects of sulphur supplementation on cellulose digestion in vitro and on nutrient digestion, nitrogen metabolism and rumen characteristics of lambs fed on good quality fescue and tropical star grass hays. Anim. Feed Sci. Technol. 1983;8:129–138.
- Gueguen L, Lamand M, Meschy F. Mineral requirements. In: Jarrige R, editor. Ruminant Nutrition: Recommended Allowances and Feed Tables. Paris: Institut National de la Recherche Agronomique; 1989. pp. 49–56.
- Gunshin H, Yoshikawa M, Doudou T, Kato N. Trace elements in human milk, cow's milk, and infant formula. Agric. Biol. Chem. 1985;49:21–26.
- Gupta UC, Winter MA. Effect of selenate vs. selenite forms of selenium in increasing the selenium concentration in forages and cereals. Can. J. Soil Sci. 1989;69:885.
- Guyton AD, McKinney JM, Knowlton KF. The effect of steam-flaked or dry ground corn and supplemental phytic acid on phosphorus partitioning and ruminal phytase activity in lactating cows. J. Dairy Sci. 2003;86:3972–3982. [PubMed: 14740835]
- Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu. Rev. Nutr. 2009;29:133–152. [PubMed: 19400701]
- Hall JA, Harwell AM, van Saun RJ, Vorachek WR, Stewart WC, Galbraith ML, Hooper KJ, Hunter JK, Mosher WD, Pirelli GJ. Agronomic biofortification with selenium: Effects on whole blood selenium and humoral immunity in beef cattle. Anim. Feed Sci. Technol. 2011;164:184–190.
- Hall OG, Baxter HD, Hobbs CS. Effect of phosphorus in different chemical forms on cellulose digestion by rumen microorganisms. J. Anim. Sci. 1961;20:817–819.
- Halpin CG, Harris DJ, Caple IW, Petterson DS. Contribution of cobalamin analogues to plasma vitamin B12 concentrations in cattle. Res. Vet. Sci. 1984;37:249–251. [PubMed: 6438747]
- Hansard SL, Comar CL, Plumlee MP. The effects of age upon calcium utilization and maintenance requirements in the bovine. J. Anim. Sci. 1954;13(1):25–36.
- Hansard SL, Crowder HM, Lyke WA. The biological availability of calcium in feeds for cattle. J. Anim. Sci. 1957;16(2):437–443.
- Hansard SL, Mohammed AS, Turner JW. Gestation age effects upon maternal-fetal zinc utilization in the bovine. J. Anim. Sci. 1968;27:1097–1102. [PubMed: 5744261]
- Hansen SL, Spears JW. Bioaccessibility of iron from soil is increased by silage fermentation. J. Dairy Sci. 2009;92:2896–2905. [PubMed: 19448021]
- Hansen SL, Spears JW, Lloyd KE, Whisnant CS. Feeding a low manganese diet to heifers during gestation impairs fetal growth and development. J. Dairy Sci. 2006a;89:4305–4311. [PubMed: 17033018]
- Hansen SL, Spears JW, Lloyd KE, Whisnant CS. Growth, reproductive performance, and manganese status of heifers fed varying concentrations of manganese. J. Anim. Sci. 2006b;84:3375–3380. [PubMed: 17093230]
- Hansen SL, Schlegel P, Legleiter LR, Lloyd KE, Spears JW. Bioavailability of copper from copper glycinate in steers fed high dietary sulfur and molybdenum. J. Anim. Sci. 2008;86:173–179. [PubMed: 17911232]
- Hansen SL, Ashwell MS, Legleiter LR, Fry RS, Lloyd KE, Spears JW. The addition of high manganese to a copper-deficient diet further depresses copper status and growth of cattle. Br. J. Nutr. 2009;101:1068–1078. [PubMed: 18775090]
- Hansen SL, Ashwell MS, Moeser AJ, Fry RS, Knutson MD, Spears JW. High dietary iron reduces transporters involved in iron and manganese metabolism and increases intestinal permeability in calves. J. Dairy Sci. 2010a;93:656–665. [PubMed: 20105537]
- Hansen SL, Trakooljul N, Liu H-CS, Hicks JA, Ashwell MS, Spears JW. Proteins involved in iron metabolism in beef cattle are affected by copper deficiency in combination with high dietary manganese, but not by copper deficiency alone. J. Anim. Sci. 2010b;88:275–283. [PubMed: 19820055]
- Harmon RJ. Physiology of mastitis and factors affecting somatic cell counts. J. Dairy Sci. 1994;77:2103–2112. [PubMed: 7929968]
- Harrison GA, Dawson KA, Hemken RW. Effects of high iron and sulfate ion concentrations on dry matter digestion and volatile fatty acid production by ruminal microorganisms. J. Anim. Sci. 1992;70:1188–1194. [PubMed: 1582949]
- Harrison JH, Conrad HR. Effect of calcium on selenium absorption by the nonlactating dairy cow. J. Dairy Sci. 1984a;67:1860–1864. [PubMed: 6480967]
- Harrison JH, Conrad HR. Effect of selenium intake on selenium utilization by the nonlactating dairy cow. J. Dairy Sci. 1984b;67:219–223. [PubMed: 6707305]
- Harrison JH, Hancock DD, Conrad HR. Vitamin E and selenium for reproduction of the dairy cow. J. Dairy Sci. 1984;67:123–132. [PubMed: 6707299]
- Hattori R, Torii S, Funaba M, Matsui T. Determination of true absorption and fecal endogenous loss of zinc in goats. Anim. Sci. J. 2010;81:564–568. [PubMed: 20887308]
- Hayirli A, Bremmer DR, Bertics SJ, Socha MT, Grummer RR. Effect of chromium supplementation on production and metabolic parameters in periparturient dairy cows. J. Dairy Sci. 2001;84:1218–1230. [PubMed: 11384049]
- Heidari SR, Ganjkhanlou M, Zali A, Ghorbani GR, Dehghan-Banadaky M, Hayirli A. Effects of vanadium supplementation on performance and metabolic parameters in periparturient dairy cows. Anim. Feed Sci. Technol. 2016;216:138–145.
- Hemingway RG. The influences of dietary selenium and vitamin E intakes on milk somatic cell counts and mastitis in cows. Vet. Res. Commun. 1999;23:481–499. [PubMed: 10672965]
- Hemingway RG, Parker ER, Parkins JJ, Fishwick G, Ritchie NS. Bioavailability assessments of granular calcined magnesites derived from magnesite rocks and of magnesium hydroxide powder in sheep. J. Agric. Sci. 1998;131:229–235.
- Hemken RW. Iodine. J. Dairy Sci. 1970;53:1138–1143. [PubMed: 4918953]
- Hemken RW. Sodium and Potassium in Ruminant Nutrition. West Des Moines, IA: National Feed Ingredients Association; 1983. Potassium in ruminant nutrition; p. 1.
- Henry PR. Cobalt bioavailability. In: Ammerman CB, Baker DH, Lewis AJ, editors. Bioavailability of Nutrients for Animals. New York: Academic Press; 1995a. pp. 119–126.
- Henry PR. Sodium and chlorine bioavailability. In: Ammerman CB, Baker DH, Lewis AJ, editors. Bioavailability of Nutrients for Animals. New York: Academic Press; 1995b. pp. 337–348.
- Henry PR. Manganese bioavailability. In: Ammerman CB, Baker DH, Lewis AJ, editors. Bioavailability of Nutrients for Animals. New York: Academic Press; 1995c. pp. 239–256.
- Henry PR, Ammerman CB. Sulfur bioavailability. In: Ammerman CB, Baker DH, Lewis AJ, editors. Bioavailability of Nutrients for Animals. New York: Academic Press; 1995. pp. 349–366.
- Hepburn JJ, Arthington JD, Hansen SL, Spears JW, Knutson MD. Technical note: Copper chaperone for copper, zinc superoxide dismutase: A potential biomarker for copper status in cattle. J. Anim. Sci. 2009;87:4161–4166. [PubMed: 19717775]
- Herdt TH, Hoff B. The use of blood analysis to evaluate trace mineral status in ruminant livestock. Vet. Clin. North Am. 2011;27:255–283. [PubMed: 21575769]
- Hermansen JE, Badsberg JH, Kristensen T, Gundersen V. Major and trace elements in organically and conventionally produced milk. J. Dairy Res. 2005;72:362–368. [PubMed: 16174368]
- Herrera D, Harris WG, Nair VD, Josan M, Staples CR. Effect of dietary modifications of calcium and magnesium on reducing solubility of phosphorus in feces from lactating dairy cows. J. Dairy Sci. 2010;93:2598–2611. [PubMed: 20494169]
- Hetzel B, Welby M. Iodine. In: O'Dell BL, Sunde RA, editors. Handbook of Nutritionally Essential Mineral Elements. New York: Marcel Dekker; 1997. pp. 557–581.
- Heyliger CE, Tahiliani AG, McNeill JH. Effect of vanadate on elevated blood glucose and depressed cardiac performance of diabetic rats. Science. 1985;227:1474–1477. [PubMed: 3156405]
- Hibbs JW, Conrad HR. The relationship of calcium and phosphorous intake and digestion and the effect of vitamin D feeding on utilization of calcium and phosphorous by lactating cows. Ohio Agric. Res. Dev. Center Res. Bull. 1983;1150:1–26.
- Hidiroglou M. Trace element deficiencies and fertility in ruminants: A review. J. Dairy Sci. 1979;62:1195–1206. [PubMed: 387829]
- Hill SR, Knowlton KF, Kebreab E, France J, Hanigan MD. A model of phosphorus digestion and metabolism in the lactating dairy cow. J. Dairy Sci. 2008;91:2021–2032. [PubMed: 18420632]
- Hilwig RV. Excretion and renal regulation of neutrality. Weinberg MS, Sheffner AL, editors. New York: Church and Dwight; P. Buffers in Ruminant Physiology and Metabolism. 1976;19
- Hogan JS, Smith KL, Weiss WP, Todhunter DA, Shockey WL. Relationships among vitamin E, selenium, and bovine blood neutrophils. J. Dairy Sci. 1990;73:2372–2378. [PubMed: 2258487]
- Holmes JHG. Phosphate deficiency in cattle on the Sepik Plains, Papua New Guinea. Trop. Anim. Health Prod. 1981;13:169–176. [PubMed: 7292616]
- Holt C. The milk salts: Their secretion, concentrations and physical chemistry. In: Fox PF, editor. Developments in Dairy Chemistry-3: Lactose and Minor Constituents. New York: Elsevier Applied Science; 1985. pp. 143–181.
- Holtenius K, Kronqvist C, Briland E, Spörndly R. Magnesium absorption by lactating dairy cows on a grass silage-based diet supplied with different potassium and magnesium levels. J. Dairy Sci. 2008;91:743–748. [PubMed: 18218762]
- Horn JP, Smith RH. Absorption of magnesium by the young steer. Br. J. Nutr. 1978;40:473–484. [PubMed: 31168]
- Horst RL. Regulation of calcium and phosphorus homeostasis in the dairy cow. J. Dairy Sci. 1986;69:604–616. [PubMed: 3517093]
- Horst RL, Deluca HF, Jorgensen NA. The effect of age on calcium absorption and accumulation of 1,25-hydroxyvitamin D3 in intestinal mucosa of rats. Metab. Bone Dis. Relat. Res. 1978;1(1):29–33.
- Horst RL, Goff JP, Reinhardt TA. Advancing age results in reduction of intestinal and bone 1,25-dihydroxyvitamin D receptor. Endocrinology. 1990;126(2):1053–1057. [PubMed: 2153518]
- Hoskins FH, Hansard SL. Placental transfer and fetal tissue iron utilization in sheep. J. Nutr. 1964;83(1):10–14. [PubMed: 14157087]
- House WA, Bell AW. Mineral accretion in the fetus and adnexa during late gestation in Holstein cows. J. Dairy Sci. 1993;76:2999–3010. [PubMed: 8227626]
- House WA, Bell AW. Sulfur and selenium accretion in the gravid uterus during late gestation in Holstein cows. J. Dairy Sci. 1994;77:1860–1869. [PubMed: 7929947]
- House WA, Welch RM. Bioavailability of and interactions between zinc and selenium in rats fed wheat grain intrinsically labeled with 65Zn and 75Se. J. Nutr. 1989;119:916–921. [PubMed: 2746373]
- Hu W, Kung JrL. Effect of dietary ratio of Na:K on feed intake, milk production, and mineral metabolism in mid-lactation dairy cows. J. Dairy Sci. 2009;92:2711–2718. [PubMed: 19448005]
- Hu W, Murphy MR. Dietary cation-anion difference effects on performance and acid-base status of lactating dairy cows: A meta-analysis. J. Dairy Sci. 2004;87:2222–2229. [PubMed: 15328236]
- INRA. INRA Feeding System for Ruminants. Wageningen, The Netherlands: Wageningen Academic; 2018.
- Ivan M, Proulx JG, Morales R, Codagnone HCV, Dayrell MDS. Copper accumulation in the liver of sheep and cattle fed diets supplemented with copper sulfate or copper chloride. Can. J. Anim. Sci. 1990;70:727–730.
- Ivancic J, Weiss WP. Effect of dietary sulfur and selenium concentrations on selenium balance of lactating Holstein cows. J. Dairy Sci. 2001;84:225–232. [PubMed: 11210037]
- Iwaniuk ME, Erdman RA. Intake, milk production, ruminal, and feed efficiency responses to dietary cation–anion difference by lactating dairy cows. J. Dairy Sci. 2015;98(12):8973–8985. [PubMed: 26409960]
- Iwaniuk ME, Weidman AE, Erdman RA. The effect of dietary cation-anion difference concentration and cation source on milk production and feed efficiency in lactating dairy cows. J. Dairy Sci. 2014;98:1950–1960. [PubMed: 25557895]
- Jackson JA Jr, Hemken RW. Calcium and cation-anion balance effects on feed intake, body weight gain, and humoral response of dairy calves. J. Dairy Sci. 1994;77:1430–1436. [PubMed: 8046082]
- Jackson JA Jr, Hopkins DM, Xin Z, Hemken RW. Influence of cation-anion balance on feed intake, body weight gain, and humoral response of dairy calves. J. Dairy Sci. 1992;75:1281–1286. [PubMed: 1597583]
- Jenkins KJ, Hidiroglou M. Tolerance of the preruminant calf for excess manganese or zinc in milk replacer. J. Dairy Sci. 1991;74:1047–1053. [PubMed: 2071705]
- Jenkins TC, Palmquist DL. Effect of fatty acids or calcium soaps on rumen and total nutrient digestibility of dairy rations. J. Dairy Sci. 1984;67:978–986. [PubMed: 6330189]
- Jenkinson DM, Mabon RM. The effect of temperature and humidity on skin surface pH and the ionic composition of skin secretions in Ayrshire cattle. Br. Vet. J. 1973;129:282–283. [PubMed: 4728197]
- Jesse BW, Thomas JW, Emery RS. Availability of magnesium from magnesium oxide particles of differing sizes and surfaces. J. Dairy Sci. 1981;64:197–205.
- Jiménez A, Planells E, Aranda P, Sánchez-Viñas M, Llopis J. Changes in bioavailability and tissue distribution of selenium caused by magnesium deficiency in rats. J. Am. Coll. Nutr. 1997;16:175–180. [PubMed: 9100219]
- Jittakhot S, Schonewille JT, Wouterse H, Focker EJ, Yuangklang C, Beynen AC. Effect of high magnesium intake on apparent magnesium absorption in lactating cows. Anim. Feed Sci. Technol. 2004a;113:53–60.
- Jittakhot S, Schonewille JT, Wouterse H, Uijttewaal AWJ, Yuangklang C, Beynen AC. Increasing magnesium intakes in relation to magnesium absorption in dry cows. J. Dairy Res. 2004b;71:297–303. [PubMed: 15354575]
- Jittakhot S, Schonewille JT, Wouterse H, Yuangklang C, Beynen AC. Apparent magnesium absorption in dry cows fed at 3 levels of potassium and 2 levels of magnesium intake. J. Dairy Sci. 2004c;87:379–385. [PubMed: 14762081]
- Johnston H, Beasley L, MacPherson N. Copper toxicity in a New Zealand dairy herd. Irish Vet. J. 2014;67:20. [PMC free article: PMC4181682] [PubMed: 25279139]
- Jovanovic IB, Velickovic M, Vukovic D, Milanovic S, Valcic O, Gvozdic D. Effects of different amounts of supplemental selenium and vitamin E on the incidence of retained placenta, selenium, malondialdehyde, and thyronines status in cows treated with prostaglandin F2a for the induction of parturition. J. Vet. Med. 2013;2013:6. [PMC free article: PMC4590878] [PubMed: 26464914]
- Jubb TF, Jerrett IV, Browning JW, Thomas KW. Haemoglobinuria and hypophoshatemia in postparturient dairy cows without phosphorus deficiency. Aust. Vet. J. 1990;67:86–89. [PubMed: 2375711]
- Jukola E, Hakkarainen J, Saloniemi H, Sankari S. Blood selenium, vitamin E, vitamin A, and β-carotene concentrations and udder health, fertility treatments and fertility. J. Dairy Sci. 1996;79:838–845. [PubMed: 8792283]
- Juniper DT, Bertin G, Jones AK, Phipps RH. Selenium supplementation of lactating dairy cows: Effect on selenium concentration in blood, milk, urine, and feces. J. Dairy Sci. 2006;89:3544–3551. [PubMed: 16899690]
- Juniper DT, Phipps RH, Ramos-Morales E, Bertin G. Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in beef cattle. J. Anim. Sci. 2008;86:3100–3109. [PubMed: 18567732]
- Kandylis K. Toxicology of sulfur in ruminants: Review. J. Dairy Sci. 1984;67:2179–2187. [PubMed: 6389627]
- Kawashima T, Henry PR, Bates DG, Ammerman CB, Littell RC, Price J. Bioavailability of cobalt sources for ruminants: 3. In vitro ruminal production of vitamin B12 and total corrinoids in response to different cobalt sources and concentrations. Nutr. Res. 1997;17(6):975–987.
- Kebreab E, Mills JAN, Crompton LA, Bannink A, Dijkstra J, Gerrits WJJ, France J. An integrated mathematical model to evaluate nutrient partition in dairy cattle between the animal and its environment. Anim. Feed Sci. Technol. 2004;112:131–154.
- Kebreab E, Shah MA, Beever DE, Humphries DJ, Sutton JD, France J, Mueller-Harvey I. Effects of contrasting forage diets on phosphorus utilisation in lactating dairy cows. Livestock Prod. Sci. 2005;93:125–135.
- Keener HA, Percival GP, Marrow KS. Cobalt tolerance in young dairy cattle. J. Dairy Sci. 1949;32:527.
- Kegley EB, Spears JW. Bioavailability of feed-grade copper sources (oxide, sulfate, or lysine) in growing cattle. J. Anim. Sci. 1994;72:2728–2734. [PubMed: 7883634]
- Kegley EB, Spears JW. Chromium and cattle nutrition. J. Trace Elements Exp. Med. 1999;12:141–147.
- Kemp A. Proceedings of the X International Grassland Congress. Helsinki: Finnish Grasslands Association; 1966. Mineral balance in dairy cows fed on grass, with special reference to magnesium and sodium; p. 411.
- Kemp A, Geurink JM. Further investigation on the sodium supply of lactating cows. Tijdschr Diergeneeskd. 1966;91:580–589.
- Kennedy DG, Kennedy S, Young PB. Effects of low concentrations of dietary cobalt on rumen succinate concentration in sheep. Int. J. Vit. Nutr. Res. 1996;66:86–92. [PubMed: 8698552]
- Keyser RB, Noller CH, Wheeler LJ, Schaefer DM. Characterization of limestones and their effects In vitro and In vivo in dairy cattle. J. Dairy Sci. 1985;68:1376–1389. [PubMed: 4019881]
- Khan AA, Lovejoy D, Sharma AK, Sharma RM, Prior MG, Lillie LE. Effects of high dietary sulphur on enzyme activities, selenium concentrations and body weights of cattle. Can. J. Vet. Res. 1987;51:174–180. [PMC free article: PMC1255298] [PubMed: 3607649]
- Khelil-Arfa H, Faverdin P, Boudon A. Effect of ambient temperature and sodium bicarbonate supplementation on water and electrolyte balances in dry and lactating Holstein cows. J. Dairy Sci. 2014;97:2305–2318. [PubMed: 24485695]
- Kiarie E, Nyachoti CM. Bioavailability of calcium and phosphorus in feedstuffs for farm animals. In: Kebreab E, Dorinha MS, editors. Phosphorus and Calcium Utilization and Requirements in Farm Animals. Cambridge, MA: CABI; 2010. pp. 76–93.
- Kidd MT, Ferket PR, Qureshi MA. Zinc metabolism with special reference to its role in immunity. World Poultry Sci. J. 1996;52:309–324.
- Kinal S, Korniewicz A, Slupczynska M, Bodarski R, Korniewicz D, Cermak B. Effect of the application of bioplexes of zinc, copper, and manganese on milk quality and composition of milk and colostrum and some indices of the blood metabolic profile of cows. Czech J. Anim. Sci. 2007;52:423–429.
- Kincaid RL. Biological availability of zinc from inorganic sources with excess dietary calcium. J. Dairy Sci. 1979;62:1081–1085. [PubMed: 512132]
- Kincaid RL, Socha MT. Effect of cobalt supplementation during late gestation and early lactation on milk and serum measures. J. Dairy Sci. 2007;90:1880–1886. [PubMed: 17369229]
- Kincaid RL, Blauwiekel RM, Cronrath JD. Supplementation of copper as copper sulfate or copper proteinate for growing calves fed forages containing molybdenum. J. Dairy Sci. 1986;69:160–163.
- Kincaid RL, Lefebvre LE, Cronrath JD, Socha MT, Johnson AB. Effect of dietary cobalt supplementation on cobalt metabolism and performance of dairy cattle. J. Dairy Sci. 2003;86:1405–1414. [PubMed: 12741565]
- Kincaid RL, Garikipati DK, Nennich TD, Harrison JH. Effect of grain source and exogenous phytase on phosphorus digestibility in dairy cows. J. Dairy Sci. 2005;88:2893–2902. [PubMed: 16027204]
- Kirchgessner M, Neesse KR. [Copper, manganese, and zinc contents in the whole body and in individual parts of veal calves at different weights (author's transl)] Z. Lebensm. Unfers. Forsch. 1976;161:1–6. [PubMed: 973442]
- M Kirchgessner, Schwarz WA. Effect of zinc deficiency and varying zinc supplements on absorption and retention in dairy cows (translation from German). Arch. Tierernahr. 1976;26(1):3–16. [PubMed: 962579]
- Klop G, Ellis JL, Bannink A, Kebreab E, France J, Dijkstra J. Meta-analysis of factors that affect the utilization efficiency of phosphorus in lactating dairy cows. J. Dairy Sci. 2013;96:3936–3949. [PubMed: 23567051]
- Klop G, Ellis JL, Blok MC, Brandsma GG, Bannink A, Dijkstra J. Variation in phosphorus content of milk from dairy cattle as affected by differences in milk composition. J. Agric. Sci. 2014;152:860–869.
- Klosch M, Richter GH, Schneider A, Flachowsky G, Pfeffer E. Influence of feeding on fecal phosphorus excretion of growing bulls varying in body weight. Arch. Anim. Nutr. 1997;50:163–172. [PubMed: 9227807]
- Knowles SO, Grace ND, Wurms K, Lee J. Significance of amount and form of dietary selenium on blood, milk, and casein selenium concentrations in grazing cows. J. Dairy Sci. 1999;82(2):429–437. [PubMed: 10068964]
- Knowlton KF, Herbein JH. Phosphorus partitioning during early lactation in dairy cows fed diets varying in phosphorus content. J. Dairy Sci. 2002;85:1227–1236. [PubMed: 12086059]
- Knowlton KF, Parsons CM, Cobb CW, Wilson KF. Exogenous phytase plus cellulase and phosphorus excretion in lactating dairy cows. Prof. Ani. Sci. 2005;21:212–216.
- Knowlton KF, Cobb C, Wilson KF, Taylor MS, Hill SR. Manure nutrient excretion by lactating cows fed exogenous phytase and cellulase. J. Dairy Sci. 2007;90:4356–4360. [PubMed: 17699056]
- Koenig KM, Beauchemin KA. Supplementing selenium yeast to diets with adequate concentrations of selenium: Selenium status, thyroid hormone concentrations and passive transfer of immunoglobulins in dairy cows and calves. Can. J. Anim. Sci. 2009;89:111–122.
- Koenig KM, Buckley WT, Shelford JA. True absorption of selenium in dairy cows: Stable isotope tracer methodology and effect of dietary copper. Can. J. Anim. Sci. 1991;71:175–183.
- Koenig KM, Rode LM, Cohen LM, Bucklet WT. Effects of diet and chemical form of selenium on selenium metabolism in sheep. J. Anim. Sci. 1997;75:817–827. [PubMed: 9078502]
- Kommisrud E, Osteras O, Vatn T. Blood selenium associated with health and fertility in Norwegian dairy herds. Acta Vet. Scand. 2005;46:229–240. [PMC free article: PMC1618966] [PubMed: 16398334]
- Kume S, Yamamoto E, Kudo T, Toharmat T, Nonaka I. Effect of parity and mineral concentrations in milk and plasma on Holstein cows during early lactation. Asian Australasian J. Anim. Sci. 1998;11:133–138.
- Langlands J, Donald G, Bowles J, Smith A. Trace element nutrition of grazing ruminants: 3. Copper oxide powder as a copper supplement. Aust. J. Agric. Res. 1989;40:187–193.
- Lawler TL, Taylor JB, Finley JW, Caton JS. Effect of supranutritional and organically bound selenium on performance, carcass characteristics, and selenium distribution in finishing beef steers. J. Anim. Sci. 2004;82:1488–1493. [PubMed: 15144091]
- Lean IJ, DeGaris PJ, McNeil DM, Block E. Hypocalcemia in dairy cows: Meta-analysis and dietary cation anion difference theory revisited. J. Dairy Sci. 2006;89:669–684. [PubMed: 16428636]
- Lechene C. Physiological role of the Na-K pump. In: Skou JC, Norby JG, Maunsbach AB, Esman M, editors. The Na, K-pump, Part B: Cellular Aspects. New York: Alan R. Liss; 1988. pp. 171–194.
- Lengemann FW, Swanson EW. A study of the secretion of iodine in milk of dairy cows, using daily oral doses of 131I. J. Dairy Sci. 1957;40:215–222.
- Leonhard-Marek S, Martens H. Effects of potassium on magnesium transport across rumen epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 1996;271:G1034–G1038. [PubMed: 8997247]
- Leonhard-Marek S, Stumpff F, Martens H. Transport of cations and anions across forestomach epithelia: Conclusions from in vitro studies. Animal. 2010;4:1037–1056. [PubMed: 22444608]
- Li M, Du J, Jiang J, Ratzan W, Su L-T, Runnels LW, Yue L. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J. Biol. Chem. 2007;282:25817–25830. [PMC free article: PMC3239414] [PubMed: 17599911]
- Lichten LA, Cousins RJ. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009;29:153–176. [PubMed: 19400752]
- Lind T, Lönnerdal B, Stenlund H, Ismail D, Seswandhana R, Ekström E-C, Persson L-A. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: Interactions between iron and zinc. Am. J. Clin. Nutr. 2003;77:883–890. [PubMed: 12663287]
- Lindt F, Blum JW. Growth performance, haematological traits, meat variables, and effects of treadmill and transport stress in veal calves supplied with different amounts of iron. Zentralbl. Veterinarmed. A. 1994;41:333–342. [PubMed: 7817636]
- Little DA. Effects of dry season supplements of protein and phosphorus to pregnant cows on the incidence of first postpartum estrus. Aust. J. Exp. Agric. Anim. Husb. 1975;15:25–31.
- Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu. Rev. Nutr. 2004;24:151–172. [PubMed: 15189117]
- Lloyd KE, Fellner V, McLeod SJ, Fry RS, Krafka K, Lamptey A, Spears JW. Effects of supplementing dairy cows with chromium propionate on milk and tissue chromium concentrations. J. Dairy Sci. 2010;93:4774–4780. [PubMed: 20855011]
- Lohman TG, Norton HW. Distribution of potassium in steers by 4~ measurement. J. Anim. Sci. 1968;27:1266.
- Lönnerdal B. Dietary factors influencing zinc absorption. J. Nutr. 2000;130:1378S–1383S. [PubMed: 10801947]
- Lönnerdal B. Intestinal regulation of copper homeostasis: A developmental perspective. Am. J. Clin. Nutr. 2008;88:846S–850S. [PubMed: 18779306]
- Lopez H, Kanitz FD, Moreira VR, Satter LD, Wiltbank MC. Reproductive performance of dairy cows fed two concentrations of phosphorus. J. Dairy Sci. 2004a;87:146–157. [PubMed: 14765821]
- Lopez H, Kanitz FD, Moreira VR, Wiltbank MC, Satter LD. Effect of dietary phosphorus on performance of lactating dairy cows: Milk production and cow health. J. Dairy Sci. 2004b;87:139–145. [PubMed: 14765820]
- Lopez H, Wu Z, Satter LD, Wiltbank MC. Effect of dietary phosphorus concentration on estrous behavior of lactating dairy cows. Theriogenology. 2004c;61:437–445. [PubMed: 14662142]
- López-Alonso M, Crespo A, Miranda M, Castillo C, Hernández J, Benedito JL. Assessment of some blood parameters as potential markers of hepatic copper accumulation in cattle. J. Vet. Diagn. Invest. 2006;18:71–75. [PubMed: 16566259]
- Lopez-Guisa JM, Satter LD. Effect of copper and cobalt addition on digestion and growth in heifers fed diets containing alfalfa silage or corn crop residues. J. Dairy Sci. 1992;75:247–256. [PubMed: 1311728]
- Lu J, Holmgren A. Minireview: Selenoproteins. J. Biol. Chem. 2009;284:723–727. [PubMed: 18757362]
- Lukaski HC. Chromium as a supplement. Annu. Rev. Nutr. 1999;19:279–302. [PubMed: 10448525]
- Ma YL, Lindemann MD, Pierce JL, Unrine JM, Cromwell GL. Effect of inorganic or organic selenium supplementation on reproductive performance and tissue trace mineral concentrations in gravid first-parity gilts, fetuses, and nursing piglets. J. Anim. Sci. 2014;92:5540–5550. [PubMed: 25403188]
- Maas J, Davis LE, Hempstead C, Berg JN, Hoffman KA. Efficacy of ethylenediamine dihydriodide in the prevention of naturally occurring foot rot in cattle. Am. J. Vet. Res. 1984;45(11):2347–2350. [PubMed: 6151816]
- MacPherson A, Gray D, Mitchell GB, Taylor CN. Ostertagia infection and neutrophil function in cobalt-deficient and cobalt-supplemented cattle. Br. Vet. J. 1987;143:348–353. [PubMed: 3620893]
- Mainville AM, Odongo NE, Bettger WJ, McBride BW, Osborne VR. Selenium uptake by ruminal microorganisms from organic and inorganic sources in dairy cows. Can. J. Anim. Sci. 2009;89:105–110.
- Malbe M, Klaassen E, Kaartinen L, Attila M, Atroshi F. Effects of oral selenium supplementation on mastitis markers and pathogens in Estonian cows. Vet. Ther. 2003;4:145–154. [PubMed: 14506590]
- Mallonee PG. Potassium and sodium nutrition and metabolism in lactating dairy cows. University of Florida; Gainesville: 1984. MS thesis.
- Mallonee PG, Beede DK, Wilcox CJ. Lactational and physiological responses of dairy cows to varying potassium and sodium quantities and ratios in complete mixed diets. J. Dairy Sci. 1982a;65(Suppl. 1):212. (Abstr.)
- Mallonee PG, Beede DK, Schneider PL, Caputo SJ, Wilcox CJ. Acute response of lactating Holstein cows to dietary potassium deficiency. J. Dairy Sci. 1982b;65(Suppl. 1):112.
- Mallonee PG, Beede DK, Collier RJ, Wilcox CJ. Production and physiological responses of dairy cows to varying dietary potassium during heat stress. J. Dairy Sci. 1985;68:1479–1487. [PubMed: 4019886]
- Markovich D. Physiological roles and regulation of mammalian sulfate transporters. Physiol. Rev. 2001;81:1499–1533. [PubMed: 11581495]
- Marston HR. The requirement of sheep for cobalt or for vitamin B12. Br. J. Nutr. 1970;24:615–633. [PubMed: 5273954]
- Martens H, Blume I. Studies on the absorption of sodium and chloride from the rumen of sheep. Comp. Biochem. Physiol. 1987;86A:653–656. [PubMed: 2882895]
- Martens H, Rayssiguier Y. Magnesium metabolism and hypomagnesemia. In: Ruckebusch Y, Thivend P, editors. Digestive Physiology and Metabolism in Ruminants. Lancaster, UK: MTP Press; 1980. p. 447.
- Martens H, Kubel OW, Gabel G, Honig H. Effects of low sodium intake on magnesium metabolism in sheep. J. Agric. Sci. 1987;108:237–243.
- Martens H, Leonhard-Marek S, Rontgen M, Stumpff F. Magnesium homeostatsis in cattle: Absorption and excretion. Nutr. Res. Rev. 2018;31:1–17. [PubMed: 29318981]
- Martz FA, Belo AT, Weiss MF, Belyea RL, Goff JP. True absorption of calcium and phosphorus from alfalfa and corn silage when fed to lactating cows. J. Dairy Sci. 1990;73:1288–1295. [PubMed: 2365887]
- Martz FA, Belo AT, Weiss MF, Belyea RL. True absorption of calcium and phosphorus from corn silage fed to nonlactating, pregnant dairy cows. J. Dairy Sci. 1999;82:618–622. [PubMed: 10194682]
- Matrone G, Conley C, Wise GH, Waugh RK. A study of iron and copper requirements of dairy calves. J. Dairy Sci. 1957;40(11):1437–1447.
- Maus RW, Martz FA, Belyea RL, Weiss MF. Relationship of dietary selenium to selenium in plasma. J. Dairy Sci. 1980;63:532–537. [PubMed: 7381079]
- Mayland HF, Rosenau RC, Florence AR. Grazing cow and calf responses to zinc supplementation. J. Anim. Sci. 1980;51:966–974. [PubMed: 7462120]
- Mbanzamihigo L, Vandycke E, Demeyer DI. Degradation of methionine by rumen contents in vitro and efficiency of its protection. Anim. Feed Sci. Technol. 1997;67:339–347.
- McArt JAA, Nydam DV, Oetzel GR. Dry period and parturient predictors of early lactation hyperketonemia in dairy cattle. J. Dairy Sci. 2013;96:198–209. [PubMed: 23102961]
- McCaughey KM, DePeters EJ, Robinson PH, Santos JEP, Taylor SJ, Pareas JW. Impact of feeding whole upland cottonseed, with or without cracked Pima cottonseed with increasing addition of iron sulfate, on milk and milk fat composition of lactating dairy cattle. Anim. Feed Sci. Technol. 2005;123–124:667–685.
- McClure TJ. Nutritional and Metabolic Infertility in the Cow. Oxon, UK: CAB International; 1994.
- McGuirk SM, Butler DG. Metabolic alkalosis with paradoxic aciduria in cattle. J. Am. Vet. Med. Assoc. 1980;177:551–558. [PubMed: 7440350]
- McKeown JW. Disorders of Na metabolism. In: Kakko JP, Tannen RL, editors. Fluids and Electrolytes. Philadelphia, PA: W.B. Saunders; 1986. p. 63.
- McLauchlan M, Suttle NF. Predicting the effects of dietary molybdenum and sulphur on the availability of copper to ruminants. Proc. Nutr. Soc. 1976;35:22A–23A. [PubMed: 940815]
- Meneghini R. Iron homeostasis, oxidative stress, and DNA damage. Free Radic. Biol. Med. 1997;23:783–792. [PubMed: 9296456]
- Miller ER. Potassium bioavailability. In: Ammerman CB, Baker DH, Lewis AJ, editors. Bioavailability of Nutrients. New York: Academic Press; 1995. pp. 295–301.
- Miller JK, Cragle RG. Gastrointestinal sites of absorption and endogenous secretion of zinc in dairy cattle. J. Dairy Sci. 1965;48:370–373. [PubMed: 14292223]
- Miller JK, Miller WJ. Experimental zinc deficiency and recovery of calves. J. Nutr. 1962;76:467–474. [PubMed: 14474041]
- Miller JK, Swanson EW, Spalding GE. Iodine absorption, excretion, recycling, and tissue distributions in the dairy cow. J. Dairy Sci. 1975;58:1578–1593. [PubMed: 1184809]
- Miller JK, Ramsey N, Madsen FC. The trace elements. In: Church DC, editor. The Ruminant Animal: Digestive Physiology and Nutrition. Englewood Cliffs, NJ: Prentice-Hall; 1988. pp. 342–400.
- Miller JK, Brzezinska-Slebodzinska E, Madsen FC. Oxidative stress, antioxidants, and animal function. J. Dairy Sci. 1993;76:2812–2823. [PubMed: 8227685]
- Miller WJ. Zinc nutrition of cattle: A review. J. Dairy Sci. 1970;53:1123–1135. [PubMed: 4918951]
- Miller WJ. Georgia Nutrition Conference for the Feed Industry. Athens: University of Georgia; 1983. Using mineral requirement standards in cattle feeding programs and feed formulations; pp. 69–74.
- Miller WJ, Martin YG, Gentry RP, Blackmon DM. 65zn and stable zinc absorption, excretion and tissue concentrations as affected by type of diet and level of zinc in normal calves. J. Nutr. 1968;94:391–401. [PubMed: 5642204]
- Miller WJ, Amos HE, Gentry RP, Blackmon DM, Durrance RM, Crowe CT, Fielding AS, Neathery MW. Long-term feeding of high zinc sulfate diets to lactating and gestating dairy cows. J. Dairy Sci. 1989;72:1499–1508. [PubMed: 2760310]
- Mills CF. Biochemical and physiological indicators of mineral status in animals: Copper, cobalt and zinc. J. Anim. Sci. 1987;65:1702–1711. [PubMed: 3327848]
- Mills CF, Dalgarno AC, Williams RB, Quarterman J. Zinc deficiency and the zinc requirements of calves and lambs. Br. J. Nutr. 1967;21:751–768. [PubMed: 6052889]
- Miltenburg GAJ, Wensing T, van Vliet JPM, Schuijt G, van de Broek J, Breukink HJ. Blood hemoglobin, plasma iron, and tissue iron in dams in late gestation, at calving, and in veal calves at delivery and later. J. Dairy Sci. 1991;74:3086–3094. [PubMed: 1779062]
- Miltenburg GAJ, Wensing T, Breukink HJ, Marx JJM. Mucosal uptake, mucosal transfer and retention of iron in veal calves. Vet. Res. Commun. 1993;17(3):209–217. [PubMed: 8284898]
- Miranda M, Alonso MJ, Benedito JL. Copper, zinc, iron, and manganese accumulation in cattle from Asturias (Northern Spain). Biol. Trace Element Res. 2006;109:135–143. [PubMed: 16444003]
- P MixnerJ, Szabo KT, Mather RE. Relation of thyroxine secretion rate to body weight in growing female Holstein-Friesian cattle. J. Dairy Sci. 1966;49:199–201. [PubMed: 5906156]
- Miyata Y, Furugouri KT. The relationship between serum ferritin concentration and tissue non-heme iron or tissue ferritin in dairy cattle. Jpn. J. Vet. Sci. 1987;49:1157–1159. [PubMed: 3430929]
- Mogodiniyai Kasmaei K, Holtenius K. Phosphorus net absorption in dairy cows subjected to abomasal infusion of inorganic phosphorus—a pilot study. J. Anim. Physiol. Anim. Nutr. 2013;97:599–603. [PubMed: 22404274]
- Mollerberg L, Moreno-Lopez J. The response of normal and iron anemic calves to nasal infection with an attenuated strain of para-influenza-3 virus. Acta Vet. Scand. 1975;16:186–196. [PMC free article: PMC8396005] [PubMed: 170811]
- Mongin P. Recent advances in dietary anion-cation balance in poultry. In: Haresign W, editor. Recent Advances in Animal Nutrition. London, UK: Butterworths; 1981. pp. 109–119. [PubMed: 7301833]
- Monsen ER, Cook JD. Food iron absorption in human subjects: IV. The effects of calcium and phosphate salts on the absorption of nonheme iron. Am. J. Clin. Nutr. 1976;29:1142–1148. [PubMed: 973603]
- Morales MS, Palmquist DL, Weiss WP. Milk fat composition of Holstein and Jersey cows with control or depleted copper status and fed whole soybeans or tallow. J. Dairy Sci. 2000;83:2112–2119. [PubMed: 11003245]
- Moreira VR, Zeringue LK, Williams CC, Leonardi C, McCormick ME. Influence of calcium and phosphorus feeding on markers of bone metabolism in transition cows. J. Dairy Sci. 2009;92:5189–5198. [PubMed: 19762837]
- Morris JG. Assessment of sodium requirements of grazing beef cattle: A review. J. Anim. Sci. 1980;50(1):145–152. [PubMed: 6991467]
- Morris JG, Gartner RJW. The sodium requirements of growing steers given an all-sorghum ration. Br. J. Nutr. 1971;25:191–205. [PubMed: 5548488]
- Morrow DA. Phosphorus deficiency and infertility in dairy heifers. J. Am. Vet. Med. Assoc. 1969;154:761–768. [PubMed: 5812997]
- Morse D, Head HH, Wilcox CJ. Disappearance of phosphorus in phytate from concentrates in vitro and from rations fed to lactating dairy cows. J. Dairy Sci. 1992;75:1979–1986. [PubMed: 1500591]
- Muller LD, Schaffer LV, Ham LC, Owens MJ. Cafeteria style free-choice mineral feeder for lactating dairy cows. J. Dairy Sci. 1977;60:1574–1582.
- Mullis LA, Spears JW, McCraw RL. Effects of breed (Angus vs Simmental) and copper and zinc source on mineral status of steers fed high dietary iron. J. Anim. Sci. 2003;81:318–322. [PubMed: 12597403]
- Mulryan G, Mason J. Assessment of liver copper status in cattle from plasma copper and plasma copper enzymes. Ann. Rech. Vet. 1992;23:233–238. [PubMed: 1416727]
- Murray CW, Egan SK, Kim H, Beru N, Bolger PM. US Food and Drug Administration's Total Diet Study: Dietary intake of perchlorate and iodine. J. Expo. Sci. Environ. Epidemiol. 2008;18(6):571–580. [PubMed: 18167505]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Nutrient Requirements of Beef Cattle. 8th rev. Washington, DC: The National Academies Press; 2016.
- Ndiweni N, Finch JM. Effects of in vitro supplementation of bovine mammary gland macrophages and peripheral blood lymphocytes with alpha-tocopherol and sodium seleniate: Implications for udder defences. Vet. Immunol. Immunopathol. 1995;47:111–121. [PubMed: 8533288]
- Neathery MW, Rachmat S, Miller WJ, Gentry RP, Blackmon DM. Effect of chemical form of orally administered 65Zn on absorption and metabolism in cattle. Exp. Biol. Med. 1972;139:953–956. [PubMed: 5023784]
- Neathery MW, Blackmon DM, Miller WJ, Heinmiller S, McGuire S, Tarabula JM, Gentry RP, Allen JC. Chloride deficiency in Holstein calves from a low chloride diet and removal of abomasal contents. J. Dairy Sci. 1981;64:2220–2233. [PubMed: 7199542]
- Nellans HN. Contributions of cellular and paracellular pathways to transepithelial intestinal calcium transport. In: Bronner F, Peterlik M, editors. Cellular Calcium and Phosphate Transport in Health and Disease. New York: Liss; 1988. pp. 269–276. [PubMed: 3347619]
- Nelson AB, MacVicar RW Jr, Meiske JC. Effect of a high salt intake on the digestibility of ration constituents and on nitrogen, sodium, and chloride retention by steers and wethers. J. Anim. Sci. 1955;14:825–830.
- Nelson TS, Ferrara LW, Storer NL. Phytate phosphorus content of feed ingredients derived from plants. Poultry Sci. 1968;47:1372–1374. [PubMed: 5749596]
- Nichols CA, Bremer VR, Watson AK, Buckner CD, Harding JL, Smith DR, Erickson GE, Klopfenstein T. Meta-analysis of the effect of dietary sulfur on feedlot health. Nebraska Beef Cattle Rep. 2012;680:82–84.
- Nietner T, Pfister M, Brakowiecka-Sassy B, Glomb MA, Fauhl-Hassek C. Screening for sulfate in distillers dried grains and solubles by FT–IR spectroscopy. J. Agric. Food Chem. 2015;63(2):476–484. [PubMed: 25529246]
- NRC (National Research Council). Nutrient Requirements of Dairy Cattle. 4th rev. Washington, DC: National Academy Press; 1971.
- NRC. Nutrient Requirements of Dairy Cattle. 5th rev. Washington, DC: National Academy Press; 1978.
- NRC. Selenium in Nutrition. 2nd rev. Washington, DC: National Academy of Sciences; 1983.
- NRC. Nutrient Requirements of Dairy Cattle. 6th rev. Washington, DC: National Academy Press; 1989.
- NRC. The Role of Chromium in Animal Nutrition. Washington, DC: National Academy Press; 1997.
- NRC. Nutrient Requirements of Dairy Cattle. 7th rev. Washington, DC: National Academy Press; 2001.
- NRC. Mineral Tolerance of Animals. Washington, DC: The National Academies Press; 2005.
- NRC. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: The National Academies Press; 2006.
- O'Dell GD, Miller WJ, King WA, Ellers JC, Jurecek H. Effect of nickel supplementation on production and composition of milk. J. Dairy Sci. 1970;53:1545–1548. [PubMed: 5530305]
- Odongo NE, McKnight D, Koekkoek A, Fisher JW, Sharpe P, Kebreab E, France J, McBride BW. Long-term effects of feeding diets without mineral phosphorus supplementation on the performance and phosphorus excretion in high-yielding dairy cows. Can. J. Anim. Sci. 2007;87:639–646.
- Oestreicher P, Cousins RJ. Copper and zinc absorption in the rat: Mechanism of mutual antagonism. J. Nutr. 1985;115:159–166. [PubMed: 3968585]
- Ortman K, Pehrson B. Selenite and selenium yeast as feed supplements for dairy cows. J. Vet. Med. Ser. A. 1997;44(6):373–380. [PubMed: 9342929]
- Ortman K, Pehrson B. Effect of selenate as a feed supplement in dairy cows in comparison to selenite and selenium yeast. J. Anim. Sci. 1999;77(12):3365–3370. [PubMed: 10641885]
- Ortman K, Andersson R, Holst H. The influence of supplements of selenite, selenate, and selenium yeast on the selenium status of dairy heifers. Acta Vet. Scand. 1999;40:23–34. [PMC free article: PMC8043236] [PubMed: 10418193]
- O'Toole D, Raisbeck MF. Pathology of experimentally induced chronic selenoisis (alkali disease) in yearling cattle. J. Vet. Diagn. Invest. 1995;7:364–373. [PubMed: 7578453]
- Ott EA, Smith WH, Harrington RB, Beeson WM. Zinc toxicity in ruminants: II. Effect of high levels of dietary zinc on gains, feed consumption and feed efficiency of beef cattle. J. Anim. Sci. 1966a;25:419–423.
- Ott EA, Smith WH, Harrington RB, Parker HE, Beeson WM. Zinc toxicity in ruminants: IV. Physiological changes in tissues of beef cattle. J. Anim. Sci. 1966b;25:432–438.
- Panev A, Hauptmanova K, Pavlata L, Pechova A, Filipek J, Dvorak R. Effect of supplementation of various selenium forms and doses on selected parameters of ruminal fluid and blood in sheep. Czech J. Anim. Sci. 2013;58:37–46.
- Paquay R, Lomba F, Lousse A, Bienfet V. Statistical research on the fate of dietary mineral elements in dry and lactating cows: V. Potassium. J. Agric. Res. 1969a;73:445–452.
- Paquay R, Lomba F, Lousse A, Bienfet V. Statistical research on the fate of dietary mineral elements in dry and lactating cows: IV. Chloride. J. Agric. Sci. 1969b;73:223–229.
- Parkins JJ, Hemingway RG, Lawson DC, Ritchie NS. The effectiveness of copper oxide powder as a component of a sustained-release multi-trace element and vitamin rumen bolus system for cattle. Br. Vet. J. 1994;150:547–553. [PubMed: 7850444]
- Paterson JE, MacPherson A. The influence of a low cobalt intake on the neutrophil function and severity of Ostertagia infection in cattle. Br. Vet. J. 1990;146:519–530. [PubMed: 2271909]
- Paulikova I, Kovac G, Bires J, Paulik S, Seidel H, Nagy O. Iodine toxicity in ruminants. Vet. Med. Czech. 2002;47:343–350.
- Pavlata AP. Chromium as an essential nutrient: A review. Vet. Med. 2007;52:1–18.
- Pechova A, Podhorsky A, Lokajova E, Pavlata L, Illek J. Metabolic effects of chromium supplementation in dairy cows in the peripartal period. Acta Vet. Brno. 2002;71:9–18.
- Pechova A, Pavlata L, Dvorak R, Lokajova E. Contents of Zn, Cu, Mn, and Se in milk in relation to their concentrations in blood, milk yield, and stage of lactation in dairy cows. Acta Vet. Brno. 2008;77:523–531.
- Peeler HT. Biological availability of nutrients in feeds: Availability of major mineral ions. J. Anim. Sci. 1972;35:695–712. [PubMed: 4560285]
- Perdomo JT, Shirley RL, Chicco CF. Availability of nutrient minerals in four tropical forages fed freshly chopped to sheep. J. Anim. Sci. 1977;45(5):1114–1119.
- Perry TW, Beeson WM, Smith WH, Mohler MT. Value of zinc supplementation of natural rations for fattening beef cattle. J. Anim. Sci. 1968;27:1674–1677. [PubMed: 5637656]
- Pfeffer E, Thompson A, Armstrong D. Studies on intestinal digestion in sheep: 3. Net movement of certain inorganic elements in the digestive tract on rations containing different proportions of hay and rolled barley. Br. J. Nutr. 1970;24:197–204. [PubMed: 5424257]
- Phipps RH, Grandison AS, Jones AK, Juniper DT, Ramos-Morales E, Bertin G. Selenium supplementation of lactating dairy cows: Effects on milk production and total selenium content and speciation in blood, milk, and cheese. Animal. 2008;2:1610–1618. [PubMed: 22444012]
- Podoll KL, Bernard JB, Ullrey DE, DeBar SR, Ku PK, Magee WT. Dietary selenate versus selenite for cattle, sheep, and horses. J. Anim. Sci. 1992;70:1965–1970. [PubMed: 1321804]
- Pogge DJ, Drewnoski ME, Hansen SL. High dietary sulfur decreases the retention of copper, manganese, and zinc in steers. J. Anim. Sci. 2014;92:2182–2191. [PubMed: 24663179]
- Pollock JM, McNair J, Kennedy S, Kennedy DG, Walsh DM, Goodall EA, Crockard AD. Effects of dietary vitamin E and selenium on in vitro cellular immune responses in cattle. Res. Vet. Sci. 1994;56:100–107. [PubMed: 8146439]
- Pond WG, Wallace MH. Effect of gestation-lactation diet calcium and zinc levels and of parenteral vitamins A, D, and E during gestation on ewe body weight and on lamb weight and survival. J. Anim. Sci. 1986;63:1019–1025. [PubMed: 3771384]
- Poole DBR, Connolly JF. Some observations on the use of the cobalt heavy pellet in sheep. Irish J. Agric. Res. 1967;6:281–284.
- Pradhan K, Hemken RW. Potassium depletion in lactating dairy cows. J. Dairy Sci. 1968;51:1377–1381. [PubMed: 5691776]
- Puggaard L, Kristensen NB, Sehested J. Effect of decreasing dietary phosphorus supply on net recycling of inorganic phosphate in lactating dairy cows. J. Anim. Sci. 2011;94:1420–1429. [PubMed: 21338807]
- Puschner B, Young-Ku C, Tegzes JH, Thurmond MC. Influence of age, sex and production class on liver zinc concentration in calves. J. Vet. Diagn. Invest. 2004;16:278–282. [PubMed: 15305737]
- Qi K, Owens FN, Lu CD. Effects of sulfur deficiency on performance of fiber-producing sheep and goats: A review. Small Ruminant Res. 1994;14:115–126.
- Qu Y, Fadden AN, Traber MG, Bobe G. Potential risk indicators of retained placenta and other diseases in multiparous cows. J. Dairy Sci. 2014;97:4151–4165. [PubMed: 24792789]
- Rabiansky PA, McDowell LR, Velasquez Pereira J, Wilkinson NS, Percival SS, Martin FG, Bates DB, Johnson AB, Batra TR, Salgado Madriz E. Evaluating copper lysine and copper sulfate sources for heifers. J. Dairy Sci. 1999;82:2642–2650. [PubMed: 10629812]
- Rahnema S, Wu Z, Ohajuruka OA, Weiss WP, Palmquist DL. Site of mineral absorption in lactating cows fed high-fat diets. J. Anim. Sci. 1994;72:229–235. [PubMed: 8138494]
- Rajagopalan KV. Molybdenum: An essential trace element in human nutrition. Annu. Rev. Nutr. 1988;8:401–427. [PubMed: 3060171]
- Ram L, Schonewille JT, Martens H, van't Klooster AT, Beynen AC. Magnesium absorption by wethers fed potassium bicarbonate in combination with different dietary magnesium concentrations. J. Dairy Sci. 1998;81:2485–2492. [PubMed: 9785240]
- Ramberg CF Jr. Kinetic analysis of calcium metabolism in the cow. Fed. Proc. 1974;33:183–187. [PubMed: 4811891]
- Randall WE, Hemken RW, Bull LS, Douglas LW. Effect of dietary sodium and potassium on udder edema in Holstein heifers. J. Dairy Sci. 1974;57:472–475. [PubMed: 4835388]
- Ray PP, Shang C, Maguire RO, Knowlton KF. Quantifying phytate in dairy digesta and feces: Alkaline extraction and high-performance ion chromatography. J. Dairy Sci. 2012;95:3248–3258. [PubMed: 22612959]
- Reid M, O'Donovan M, Elliott CT, Bailey JS, Watson CJ, Lalor STJ, Corrigan B, Fenelon MA, Lewis E. The effect of dietary crude protein and phosphorus on grass-fed dairy cow production, nutrient status, and milk heat stability. J. Dairy Sci. 2015;98:517–531. [PubMed: 25465549]
- Reinhardt TA, Conrad HR. Mode of action of pharmacological doses of cholecalciferol during parturient hypocalcemia in dairy cows. J. Nutr. 1980;110:1589–1596. [PubMed: 6249900]
- Reinhardt TA, Horst RL, Goff JP. Calcium, phosphorus, and magnesium homeostasis in ruminants. Vet. Clin. North Am. 1988;4:331–350. [PubMed: 3061612]
- Reinhardt TA, Lippolis JD, McCluskey BJ, Goff JP, Horst RL. Prevalence of subclinical hypocalcemia in dairy herds. Vet. J. 2011;188:122–124. [PubMed: 20434377]
- Reis LS, Chiacchio SB, Pardo PE, Oba E, Giuffrida R, Frazatti-Gallina NM. Selenium supplementation enhances weight gain in cattle. Arch. Zootec. 2008;57:271–274.
- Renkema JA, Senshu T, Gaillard BDE, Brouwer E. Regulation of sodium excretion and retention by the intestine in cows. Nature. 1962;195:389–390. [PubMed: 14491472]
- Richards CJ, Blalock HM, Jacques KA, Loveday HD. Efficacy of feeding selenium-enriched yeast to finishing beef cattle. Prof. Anim. Sci. 2011;27:1–8.
- Richter EL, Drewnoski ME, Hansen SL. Effects of increased dietary sulfur on beef steer mineral status, performance, and meat fatty acid composition. J. Anim. Sci. 2012;90:3945–3953. [PubMed: 22665674]
- Roberts T, Chapinal N, LeBlanc SJ, Kelton DF, Dubuc J, Duffield TF. Metabolic parameters in transition cows as indicators for early-lactation culling risk. J. Dairy Sci. 2012;95:3057–3063. [PubMed: 22612941]
- Robinson PH, Beaucaire T, Meyer D. Mineral levels in bulk milk of California dairy cows. 2002. [July 11, 2012]. http:
//animalscience .ucdavis.edu/faculty /robinson/Articles/FullText /PDF/Web200211.pdf . - Rodehutscord M, Heuvers H, Pfeffer E. Effect of organic matter digestibility on obligatory faecal phosphorus loss in lactating goats, determined from balance data. Anim. Sci. 2000;70:561–568.
- Rojas MA, Dyer IA, Cassatt WA. Manganese deficiency in the bovine. J. Anim. Sci. 1965;24:664–667. [PubMed: 14313723]
- Rojas LX, McDowell LR, Cousins RJ, Martin FG, Wilkinson NS, Johnson AB, Velasquez JB. Relative bioavailability of two organic and two inorganic zinc sources fed to sheep. J. Anim. Sci. 1995;73:1202–1207. [PubMed: 7628965]
- Rojas LX, McDowell LR, Martin FG, Wilkinson NS, Johnson AB, Njeru CA. Relative bioavailability of zinc methionine and two inorganic zinc sources fed to cattle. J. Trace Elements Med. Biol. 1996;10:205–209. [PubMed: 9021670]
- Ross JG, Spears JW, Garlich JD. Dietary electrolyte balance effects on performance and metabolic characteristics in finishing steers. J. Anim. Sci. 1994a;72:1600–1608. [PubMed: 8071186]
- Ross JG, Spears JW, Garlich JD. Dietary electrolyte balance effects on performance and metabolic characteristics in growing steers. J. Anim. Sci. 1994b;72:1842–1848. [PubMed: 7928764]
- Rude RK, Gruber HE. Magnesium deficiency and osteoporosis: Animal and human observations. J. Nutr. Biochem. 2004;15:710–716. [PubMed: 15607643]
- Sadri H, Khorvash M, Bruckmaier RM, Samie AH, Ghorbani GR, Rahmani HR. Chromium supplementation and substitution of barley grain with corn: Effects on performance and lactation in periparturient dairy cows. J. Dairy Sci. 2009;92:5411–5418. [PubMed: 19841203]
- Sahota O, Mundey MK, San P, Godber IM, Hosking DJ. Vitamin D insufficiency and the blunted PTH response in established osteoporosis: The role of magnesium deficiency. Osteoporos. Int. 2006;17:1013–1021. [PubMed: 16596461]
- Salman S, Khol-Parisini A, Schafft H, Lahrssen-Wiederholt M, Hulan HW, Dinse D, Zentek J. The role of dietary selenium in bovine mammary gland health and immune function. Anim. Health Res. Rev. 2009;10:21–34. [PubMed: 19195425]
- Sanchez WK, McGuire MA, Beede DK. Macromineral nutrition by heat stress interactions in dairy cattle: Review and original research. J. Dairy Sci. 1994a;77:2051–2079. [PubMed: 7929965]
- Sanchez WK, Beede DK, DeLorenzo MA. Macromineral element interrelationships and lactational performance: Empirical models from a large data set. J. Dairy Sci. 1994b;77:3096–3001. [PubMed: 7836598]
- Sanchez WK, Beede DK, Cornell JA. Interactions of sodium, potassium, and chloride on lactation, acid-base status, and mineral concentrations. J. Dairy Sci. 1994c;77:1661–1675. [PubMed: 8083426]
- Sanchez WK, Beede DK, Cornell JA. Dietary mixtures of sodium bicarbonate, sodium chloride, and potassium chloride: Effects on lactational performance, acid-base status, and mineral metabolism of Holstein cows. J. Dairy Sci. 1997;80:1207–1216. [PubMed: 9201593]
- Sandoval M, Henry PR, Littell RC, Cousins RJ, Ammerman CB. Estimation of the relative bioavailability of zinc from inorganic zinc sources for sheep. Anim. Feed Sci. Technol. 1997;66:223–235.
- Sansom BF, Symonds HW, Vagg MJ. The absorption of dietary manganese by dairy cows. Res. Vet. Sci. 1978;24:366–369. [PubMed: 674849]
- Sasser LB, Ward GM, Johnson JE. Variations in potassium concentration of cow's milk. J. Dairy Sci. 1966;49(7):893–895. [PubMed: 5967710]
- Saxena KK, K RanjhanS. A note on the effect of cobalt and copper supplementation on in vitro cellulose digestion by nylon-bag technique in Hariana calves. Indian J. Anim. Sci. 1978;48:833–835.
- Scaletti RW, Trammell DS, Smith BA, Harmon RJ. Role of dietary copper in enhancing resistance to Escherichia coli mastitis. J. Dairy Sci. 2003;86:1240–1249. [PubMed: 12741549]
- Schneider PL, Beede DK, Wilcox CJ, Collier RJ. Influence of dietary sodium and potassium bicarbonate and total potassium on heat-stressed dairy cows. J. Dairy Sci. 1984;67:2546–2553. [PubMed: 6097604]
- Schneider PL, Beede DK, Wilcox CJ. Responses of lactating cows to dietary sodium source and quantity and potassium quantity during heat stress. J. Dairy Sci. 1986;69:99–110. [PubMed: 3009576]
- Schnell SA, Ohtsuka H, Kakinuma S, Yoshikawa Y, Watanabe K, Orino K. Iron and ferritin levels in the serum and milk of bovine leukemia virus-infected dairy cows. Front. Vet. Sci. 2015;2:12. [PMC free article: PMC4672180] [PubMed: 26664941]
- Schonewille JT, van't Klooster AT, Beynen AC. High phosphorus intake depresses apparent magnesium absorption in pregnant heifers. J. Anim. Physiol. Anim. Nutr. 1994;71:15–21.
- Schonewille JT, Ram L, van't Klooster AT, Wouterse H, Beynen AC. Native corn starch versus either cellulose or glucose in the diet and the effects on apparent magnesium absorption in goats. J. Dairy Sci. 1997;80:1738–1743. [PubMed: 9276814]
- Schonewille JT, van't Klooster AT, Wouterse H, Beynen AC. Effects of intrinsic potassium in artificially dried grass and supplemental potassium bicarbonate on apparent magnesium absorption in dry cows. J. Dairy Sci. 1999;82:1824–1830. [PubMed: 10480109]
- Schonewille JT, van't Klooster AT, Cone JW, Kalsbeek-van der Valk HJ, Wouterse H, Beynen AC. Neither native nor popped cornmeal in the ration of dry cows affects magnesium absorption. Livestock Prod. Sci. 2000a;63:17–26.
- Schonewille JT, van't Klooster AT, Wouterse H, Beynen AC. Time courses of plasma magnesium concentrations and urinary magnesium excretion in cows subjected to acute changes in potassium intake. Vet. Q. 2000b;22:136–140. [PubMed: 10952442]
- Schonewille JT, Everts H, Jittakhot S, Beynen AC. Quantitative prediction of magnesium absorption in dairy cows. J. Dairy Sci. 2008;91:271–278. [PubMed: 18096949]
- Schwartz WA, Kirchgessner M. Experimental zinc deficiency in lactating dairy cows. Vet. Med. Rev. 1975;1:19–23.
- Schwarz FJ, Kirchgessner M. Copper and zinc contents in milk and plasma of cows after high nutritional copper supplements. Z. Lebensm. Unters. Forsch. 1978;166:5–8. [PubMed: 626035]
- Schwarz FJ, Kirchgessner M, Stangl GL. Cobalt requirement of beef cattle—feed intake and growth at different levels of cobalt supply. J. Anim. Physiol. Anim. Nutr. 2000;83:121–131.
- Scott D, McLean AF, Buchan W. The effect of variation in phosphorus uptake on net intestinal phosphorus absorption, salivary phosphorus secretion and pathway of excretion in sheep fed roughage diets. Q. J. Exp. Physiol. 1984;69:439–452. [PubMed: 6089254]
- Shen H, Qin H, Guo J. Cooperation of metallothionein and zinc transporters for regulating zinc homeostasis in human intestinal Caco-2 cells. Nutr. Res. 2008;28:406–413. [PubMed: 19083439]
- Siciliano-Jones JL, Socha MT, Tomlinson DJ, DeFrain JM. Effect of trace mineral source on lactation performance, claw integrity, and fertility of dairy cattle. J. Dairy Sci. 2008;91:1985–1995. [PubMed: 18420629]
- Sielman ES, Sweeney RW, Whitlock RH, Reams RY. Hypokalemia syndrome in dairy cows: 10 cases (1992–1996). J. Am. Vet. Med. Assoc. 1997;210:240–243. [PubMed: 9018360]
- Silanikove N, Maltz E, Halevi A, Shinder D. Metabolism of water, sodium, potassium, and chlorine by high yielding dairy cows at the onset of lactation. J. Dairy Sci. 1997;80:949–956. [PubMed: 9178136]
- Singh A, Kumar M, Kumar V, Roy D, Kushwaha R, Vaswani S, Kumar A. Effects of nickel supplementation on antioxidant status, immune characteristics, and energy and lipid metabolism in growing cattle. Biol. Trace Element Res. 2019;190:65–75. [PubMed: 30238420]
- Smart ME, Cymbaluk NF, Christensen DA. A review of copper status of cattle in Canada and recommendations for supplementation. Can. Vet. J. 1992;33:163–170. [PMC free article: PMC1481200] [PubMed: 17423962]
- Smith KL, Conrad HR, Amiet BA, Todhunter DA. Incidence of environmental mastitis as influenced by vitamin E and selenium. Kieler Milchwirtshaftliche Forsch. 1985;37:482–486.
- Smith KL, Waldron MR, Drackley JK, Socha MT, Overton TR. Performance of dairy cows as affected by prepartum dietary carbohydrate source and supplementation with chromium throughout the transition period. J. Dairy Sci. 2005;88:255–263. [PubMed: 15591388]
- Smith KL, Waldron MR, Ruzzi LC, Drackley JK, Socha MT, Overton TR. Metabolism of dairy cows as affected by prepartum dietary carbohydrate source and supplementation with chromium throughout the periparturient period. J. Dairy Sci. 2008;91:2011–2020. [PubMed: 18420631]
- Smith NE, Collar LS, Bath DL, Dunkley WL, Franke AA. Digestibility and effects of whole cottonseed fed to lactating cows. J. Dairy Sci. 1981;64:2209–2215. [PubMed: 6895903]
- Smith RM. Cobalt. Mertz W, editor. San Diego, CA: Academic Press; Trace Elements in Human Health and Disease. 1987;1:143–184.
- Smith RM. Cobalt. In: O'Dell B, Sunde R, editors. Handbook of Nutritionally Essential Mineral Elements. New York: Marcel Dekker; 1997. pp. 357–387.
- Smith RM, Marston HR. Production, absorption, distribution, and excretion of vitamin B12 in sheep. Br. J. Nutr. 1970;24:857–877. [PubMed: 5484730]
- Smith SE, Lengemann FW, Reid JT. Block vs. loose salt consumption by dairy cattle. J. Dairy Sci. 1953;36:762–765.
- Soares JH. Calcium bioavailability. In: Ammerman CB, Baker DH, Lewis AJ, editors. Bioavailability of Nutrients for Animals. New York: Academic Press; 1995a. pp. 95–113.
- Soares JH. Phosphorus bioavailability. In: Ammerman CB, Baker DH, Lewis AJ, editors. Bioavailability of Nutrients for Animals. New York: Academic Press; 1995b. pp. 257–294.
- Somers GF. The affinity of onion cell walls for calcium ions. Am. J. Botany. 1983;60:987–990.
- Son E-W, Lee S-R, Choi H-S, Koo H-J, Huh J-E, Kim M-H, Pyo S. Effects of supplementation with higher levels of manganese and magnesium on immune function. Arch. Pharm. Res. 2007;30:743–749. [PubMed: 17679553]
- Sonner Z, Wilder E, Heikenfeld J, Kasting G, Beyette F, Swaile D, Sherman F, Joyce J, Hagen J, Kelley-Loughnane N, Naik R. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics. 2015;9(3):031301. [PMC free article: PMC4433483] [PubMed: 26045728]
- Sordillo LM. Selenium-dependent regulation of oxidative stress and immunity in periparturient dairy cattle. Vet. Med. Int. 2013;2013:8. [PMC free article: PMC3557619] [PubMed: 23401850]
- Sorensen P. Use of Radioisotopes in Animal Biology and Medical Sciences. New York: Academic Press; 1962. Studies of thyroid function in cattle and pigs; p. P.
- Spears JW. Nickel as a “newer trace element” in the nutrition of domestic animals. J. Anim. Sci. 1984;59:823–835. [PubMed: 6386782]
- Spears JW. Ionophores and nutrient digestion and absorption in ruminants. J. Nutr. 1990;120:632–638. [PubMed: 2191094]
- Spears JW, Smith CJ, Hatfield EE. Rumen bacterial urease requirement for nickel. J. Dairy Sci. 1977;60:1073–1076. [PubMed: 881474]
- Spears JW, Burns JC, Hatch PA. Sulfur fertilization of cool season grasses and effect on utilization of minerals, nitrogen, and fiber by steers. J. Dairy Sci. 1985;68:347–355.
- Spears JW, Schricker BR, Burns JC. Influence of lysocellin and monensin on mineral metabolism of steers fed forage-based diets. J. Anim. Sci. 1989;67:2140. [PubMed: 2793627]
- Spears JW, Kegley EB, Mullis LA. Bioavailability of copper from tribasic copper chloride and copper sulfate in growing cattle. Anim. Feed Sci. Tech. 2004;116:1–13.
- Spears JW, Whisnant CS, Huntington GB, Lloyd KE, Fry RS, Krafka K, Lamptey A, Hyda J. Chromium propionate enhances insulin sensitivity in growing cattle. J. Dairy Sci. 2012;95:2037–2045. [PubMed: 22459849]
- Spears JW, Lloyd KE, Krafka K. Chromium concentrations in ruminant feed ingredients. J. Dairy Sci. 2017;100(5):3584–3590. [PubMed: 28237600]
- Speikers H, Brintrup R, Balmelli M, Pfeffer E. Influence of dry matter intake on faecal phosphorus losses in dairy cows fed rations low in phosphorus. J. Anim. Physiol. Anim. Nutr. 1993;69:37–43.
- Spek JW, Bannink A, Gort G, Hendriks WH, Dijkstra J. Effect of sodium chloride intake on urine volume, urinary urea excretion, and milk urea concentration in lactating dairy cattle. J. Dairy Sci. 2012;95:7288–7298. [PubMed: 23063155]
- Sperber I, Hyden S. Transport of chloride through the ruminal mucosa. Nature. 1952;169:587–593. [PubMed: 14929246]
- Stabel JR, Nonnecke BJ, Reinhardt TA. Effect of in vitro selenium repletion on bovine lymphocyte proliferation. Nutr. Res. 1990;10:1053–1059.
- Standish JF, Ammerman CB, Palmer AZ, Simpson CF. Influence of dietary iron and phosphorus on performance, tissue mineral composition and mineral absorption in steers. J. Anim. Sci. 1971;33:171–178. [PubMed: 5571083]
- Stangl GI, Schwarz FJ, Muller H, Kirchgessner M. Evaluation of the cobalt requirement of beef cattle based on vitamin B12, folate, homocysteine and methylmalonic acid. Br. J. Nutr. 2000;84:645–653. [PubMed: 11177177]
- Steevens BJ, Bush LJ, Stout JD, Williams EI. Effects of varying amounts of calcium and phosphorus in rations for dairy cows. J. Dairy Sci. 1971;54:655–661. [PubMed: 5105953]
- Steffen DJ, Carlson MP, Casper HH. Copper toxicosis in suckling beef calves associated with improper administration of copper oxide boluses. J. Vet. Diagn. Invest. 1997;9:443–446. [PubMed: 9376443]
- Stewart PA. Independent and dependent variables of acid base control. Respir. Physiol. 1978;33:9–26. [PubMed: 27857]
- Stewart PA. How to Understand Acid-Base: A Quantitative Acid-Base Primer for Biology and Medicine. New York: Elsevier North Holland; 1981.
- Stewart PA. Modern quantitative acid–base chemistry. Can. J. Physiol. Pharmacol. 1983;61(12):1444–1461. [PubMed: 6423247]
- Storry JE. Studies on calcium and magnesium in the alimentary tract of sheep: II. The effect of reducing the acidity of abomasal digesta in vitro on the distribution of calcium and magnesium. J. Agric. Sci. 1961;57:103–105.
- Storry JE, Rook JAF. The magnesium nutrition of the dairy cow in relation to the development of hypomagnesaemia in the grazing animal. J. Sci. Food Agric. 1962;13:621–627.
- Stuart SM, Ketelsen SM, Weaver CM, Erdman JW. Bioavailability of zinc to rats as affected by protein source and previous dietary intake. J. Nutr. 1986;116:1423–1431. [PubMed: 3760999]
- Subiyatno A, Mowat DN, Yang WZ. Metabolite and hormonal responses to glucose or propionate infusions in periparturient dairy cows supplemented with chromium. J. Dairy Sci. 1996;79:1436–1445. [PubMed: 8880468]
- Sumner JM, Valdez F, McNamara JP. Effects of chromium propionate on response to an intravenous glucose tolerance test in growing Holstein heifers. J. Dairy Sci. 2007;90:3467–3474. [PubMed: 17582130]
- Suttle NF. Effects of age and weaning on the apparent availability of dietary copper to young lambs. J. Agric. Sci. 1975;84:255–261. [PubMed: 4760779]
- Suttle NF. Copper, iron, manganese and zinc concentrations in the carcasses of lambs and calves and the relationship to trace elements required for growth. Br. J. Nutr. 1979;42:89–96. [PubMed: 486397]
- Suttle NF. The interactions between copper, molybdenum and sulphur in ruminant nutrition. Annu. Rev. Nutr. 1991;11:121–140. [PubMed: 1892697]
- Suttle NF, Abrahams P, Thornton I. The role of a soil × dietary sulphur interaction in the impairment of copper absorption by ingested soil in sheep. J. Agric. Sci. 1984;103:81–86.
- Swanson CA, Zimmermann MB, Skeaff S, Pearce EN, Dwyer JT, Trumbo PR, Zehaluk C, Andrews KW, Carriquiry A, Caldwell KL, Egan SK, Long SE, Bailey R, Sullivan KM, Holden JM, Betz JM, Phinney KW, Brooks SPJ, Johnson CL, Haggans CJ. Summary of an NIH workshop to identify research needs to improve the monitoring of iodine status in the United States and to inform the DRI. J. Nutr. 2012;142(6):1175s–1185s. [PMC free article: PMC3738225] [PubMed: 22551802]
- Takatsuki K, Hanley D, Sherwood L. Effects of magnesium ion on parathyroid hormone secretion in vitro. Calcified Tissue Int. 1980;32:201–206. [PubMed: 6775789]
- Taylor MS, Knowlton KF, McGilliard ML, Swecker WS, Ferguson JD, Wu Z, Hanigan MD. Dietary calcium has little effect on mineral balance and bone mineral metabolism through twenty weeks of lactation in Holstein cows. J. Dairy Sci. 2009;92:223–237. [PubMed: 19109282]
- Tebbe AW, Wyatt DJ, Weiss WP. Effects of magnesium source and monensin on nutrient digestibility and mineral balance in lactating dairy cows. J. Dairy Sci. 2018;101:1152–1163. [PubMed: 29248228]
- Templeton DM, Liu Y. Genetic regulation of cell function in response to iron overload or chelation. Biochim. Biophys. Acta. 2003;1619(2):113–124. [PubMed: 12527106]
- Thompson VA, Fadel JG, Sainz RD. Meta-analysis to predict sweating and respiration rates for Bos indicus, Bos taurus, and their crossbreds. J. Anim. Sci. 2011;89(12):3973–3982. [PubMed: 21821814]
- Tiffany ME, Spears JW. Differential responses to dietary cobalt in finishing steers fed corn- versus barely-based diets. J. Anim. Sci. 2005;83:2580–2589. [PubMed: 16230655]
- Tiffany ME, Spears JW, Xi L, Horton J. Influence of dietary cobalt source and concentration on performance, vitamin B12 status, and ruminal and plasma metabolites in growing and finishing steers. J. Anim. Sci. 2003;81:3151–3159. [PubMed: 14677871]
- Tillman AD, Brethour JR. Dicalcium phosphate and phosphoric acid as phosphorus sources for beef cattle. J. Anim. Sci. 1958;17:100–103.
- Tran CD, Butler RN, Philcox JC, Rofe AM, Howarth GS, Coyle P. Regional distribution of metallothionein and zinc in the mouse gut. Biol. Trace Element Res. 1998;63:239–251. [PubMed: 9840820]
- Tucker WB, Harrison GA, Hemken RW. Influence of dietary cation-anion balance on milk, blood, urine, and rumen fluid in lactating dairy cattle. J. Dairy Sci. 1988;71:346–354. [PubMed: 3379168]
- Tucker WB, Jackson JA, Hopkins DM, Hogue JF. Influence of dietary sodium bicarbonate on the potassium metabolism of growing calves. J. Dairy Sci. 1991;74:2296–2302. [PubMed: 1654349]
- Underwood EJ. The Mineral Nutrition of Livestock. 2nd ed. Slough, UK: Commonwealth Agricultural Bureaux; 1981.
- Underwood EJ, Suttle NF. The Mineral Nutrition of Livestock. 3rd ed. Wallingford, UK: CABI Publishing; 1999.
- Vagg MJ. Assessment of trace element metabolism in farm animals. Proc. R. Soc. Med. 1976;69:13–14. [PMC free article: PMC1864342] [PubMed: 822424]
- Valk H, Sebek LBJ. Influence of long-term feeding of limited amounts of phosphorus on dry matter intake, milk production, and body weight of dairy cows. J. Dairy Sci. 1999;82:2157–2163. [PubMed: 10531601]
- Valk H, Sebek LBJ, Beynen AC. Influence of phosphorus intake on excretion and blood plasma and saliva concentrations of phosphorus in dairy cows. J. Dairy Sci. 2002;85:2642–2649. [PubMed: 12416818]
- van Bruwaene R, Gerber GB, Kirchmann R, Colard J, Van Kerkom J. Metabolism of 51Cr, 54Mn, 59Fe and 60Co in lactating dairy cows. Health Phys. 1984;46:1069–1082. [PubMed: 6539319]
- van Hulzen KJE, Sprong RC, van der Meer R, van Arendonk JAM. Genetic and nongenetic variation in concentration of selenium, calcium, potassium, zinc, magnesium, and phosphorus in milk of Dutch Holstein-Friesian cows. J. Dairy Sci. 2009;92:5754–5759. [PubMed: 19841235]
- van Leeuwen JM. Keukenzout in de Rundveevoeding. Versl. Landbouwkd. Onderz. 737. Instituut voor Veevoedingsonderzoek “Hoorn.”; 1970. Physiological aspects of the supplementation of sodium chloride in rations with low and normal sodium contents.
- van Ravenswaay RO, Henry PR, Ammerman CB, Littell RC. Comparison of methods to determine relative bioavailability of magnesium in magnesium oxides for ruminants. J. Dairy Sci. 1989;72:2968–2980. [PubMed: 2625487]
- van Ravenswaay RO, Henry PR, Ammerman CB. Effects of time and dietary iron on tissue iron concentration as an estimate of relative bioavailability of supplemental iron sources for ruminants. Anim. Feed Sci. Technol. 2001;90:185–198.
- van Ryssen JBJ, van Malsen PSM, Hartmann F. Contribution of dietary sulphur to the interaction between selenium and copper in sheep. J. Agric. Sci. 1998;130:107–114.
- van't Klooster AT. Adaptation of calcium absorption from the small intestine of dairy cows to changes in the dietary calcium intake and at the onset of lactation. Z. Tierphysiol. Tierernahr. Futtermittelkd. 1976;37(4):169–182. [PubMed: 1034384]
- Vargas-Rodriguez CF, Yuan K, Titgemeyer EC, Mamedova LK, Griswold KE, Bradford BJ. Effects of supplemental chromium propionate and rumen-protected amino acids on productivity, diet digestibility, and energy balance of peak-lactation dairy cattle. J. Dairy Sci. 2014;97:3815–3821. [PubMed: 24731641]
- Vincent JB. The biochemistry of chromium. J. Nutr. 2000;130:715–718. [PubMed: 10736319]
- Vinson JA, Hsiao K. Comparative effect of various forms of chromium on serum glucose: An assay for biologically active chromium. Nutr. Reports Internat. 1985;32:1–7.
- Visek WJ, Monroe RA, Swanson EW, Comar CL. Calcium metabolism in dairy cows as studied with Ca45. J. Dairy Sci. 1953;36(4):373–384.
- Visentin G, Penasa M, Gottardo P, Cassandro M, De Marchi M. Predictive ability of mid-infrared spectroscopy for major mineral composition and coagulation traits of bovine milk by using the uninformative variable selection algorithm. J. Dairy Sci. 2016;99:8137–8145. [PubMed: 27522421]
- Walker GP, Dunshea FR, Heard JW, Stockdale CR, Doyle PT. Output of selenium in milk, urine, and feces is proportional to selenium intake in dairy cows fed a total mixed ration supplemented with selenium yeast. J. Dairy Sci. 2010;93:4644–4650. [PubMed: 20854998]
- Ward GM. Oral potassium chloride fatal to a cow. J. Am. Vet. Med. Assoc. 1966a;148:543–544. [PubMed: 5917436]
- Ward GM. Potassium metabolism of domestic ruminants: A review. J. Dairy Sci. 1966b;49:268–276. [PubMed: 5335468]
- Ward G, Dobson RC, Dunham JR. Influences of calcium and phosphorus intakes, vitamin D supplement, and lactation on calcium and phosphorus balances. J. Dairy Sci. 1972;55(6):768–776. [PubMed: 5032207]
- Ward JD, Spears JW. Comparison of copper lysine and copper sulfate as copper sources for ruminants using in vitro methods. J. Dairy Sci. 1993;76:2994–2998. [PubMed: 8227625]
- Wasserman RH. Intestinal absorption of calcium and phosphorus. Fed. Proc. 1981;40:68–72. [PubMed: 7192650]
- Wasserman RH, Taylor AN. Gastrointestinal absorption of calcium and phosphorus. Parathyroid Gland GD Aubach, editor. Washington, DC: American Physiological Society; Handbook of Physiology. Sect. 7 Endocrinology. 1976;VII:137–155.
- Wedekind KJ, Titgemeyer EC, Twardock AR, Baker DH. Phosphorus, but not calcium, affects manganese absorption and turnover in chicks. J. Nutr. 1991;121:1776–1786. [PubMed: 1941186]
- Weil AB, Tucker WB, Hemken RW. Potassium requirements of calves. J. Dairy Sci. 1988;71:1868–1872. [PubMed: 3410994]
- Weiss WP. Selenium nutrition of dairy cows: Comparing responses to organic and inorganic selenium forms. In: Lyons TP, Jacques KA, editors. Proceedings of Nutritional Biotechnology in the Feed and Food Industries. Lexington, KY: Alltech; 2003. pp. 333–343.
- Weiss WP. Macromineral digestion by lactating dairy cows: Factors affecting digestibility of magnesium. J. Dairy Sci. 2004;87:2167–2171. [PubMed: 15328230]
- Weiss WP, Hogan JS. Effect of selenium source on selenium status, neutrophil function, and response to intramammary endotoxin challenge of dairy cows. J. Dairy Sci. 2005;88:4366–4374. [PubMed: 16291628]
- Weiss WP, Socha MT. Dietary manganese for dry and lactating Holstein cows. J. Dairy Sci. 2005;88:2517–2523. [PubMed: 15956314]
- Weiss WP, Spears JW. Vitamin and trace mineral effects on immune function of ruminants. In: Sejrsen K, Hvelplund T, Nielsen MO, editors. 10th International Symposium on Ruminant Physiology. Wageningen, The Netherlands: Wageningen Academic; 2005. pp. 473–496.
- Weiss WP, St-Pierre NR. A method to quantify changes in supply of metabolizable methionine to dairy cows using concentrations of selenium in milk. J. Dairy Sci. 2009;92:2835–2842. [PubMed: 19448017]
- Weiss WP, Hogan JS, Smith KL, Hoblet KH. Relationships among selenium, vitamin E, and mammary gland health in commercial dairy herds. J. Dairy Sci. 1990;73:381–390. [PubMed: 2329203]
- Weiss WP, Pinos-Rodríguez JM, Socha MT. Effects of feeding supplemental organic iron to late gestation and early lactation dairy cows. J. Dairy Sci. 2010;93(5):2153–2160. [PubMed: 20412930]
- Weiss WP, Wyatt DJ, Kleinschmit DH, Socha MT. Effect of including canola meal and supplemental iodine in diets of dairy cows on short-term changes in iodine concentrations in milk. J. Dairy Sci. 2015a;98:4841–4849. [PubMed: 25958290]
- Weiss WP, Steinberg W, Azem E, Reinhardt TA. Effect of feeding 25-hydroxylvitamin D3 with a negative cation-anion difference diet on calcium and vitamin d status of periparturient cows and their calves. J. Dairy Sci. 2015b;98:5588–5600. [PubMed: 26051311]
- West JW, Coppock CE, Milam KZ, Nave DH, LaBore JM, Rowe LD Jr. Potassium carbonate as a potassium source and dietary buffer for lactating Holstein cows during hot weather. J. Dairy Sci. 1987;70:309–320. [PubMed: 3571637]
- West JW, Haydon KD, Mullinix BG, Sandifer TG. Dietary cation-anion balance and cation source effects on production and acid-base status of heat-stressed cows. J. Dairy Sci. 1992;75:2776–2786. [PubMed: 1430483]
- Whanger PD. Selenocompounds in plants and animals and their biological significance. J. Am. Coll. Nutr. 2002;21:223–232. [PubMed: 12074249]
- Whelan BR, Peter DW, Barrow NJ, Young P, Buscall DJ, Cox VC. Proceedings of the XVI International Grasslands Congress. Nice, France: Association Française pour la Production Fourragère; 1989. The effect of selenium fertilizer on the selenium status of sheep grazing annual pasture in south-west Western Australia; p. 761.
- Wichtel JJ, Craigie AL, Varela-Alvarez H, Williamson NB. The effect of intra-ruminal selenium pellets on growth rate, lactation, and reproductive efficiency in dairy cattle. N. Z. Vet. J. 1994;42:205–210. [PubMed: 16031784]
- Wildman CD, West JW, Bernard JK. Effects of dietary cation-anion difference and potassium to sodium ratio on lactating dairy cows in hot weather. J. Dairy Sci. 2007;90:970–977. [PubMed: 17235174]
- Wilkens MR, Oberheide I, Schröder B, Azem E, Steinberg W, Breves G. Influence of the combination of 25-hydroxyvitamin d3 and a diet negative in cation-anion difference on peripartal calcium homeostasis of dairy cows. J. Dairy Sci. 2012;95:151–164. [PubMed: 22192194]
- Williams DL. Purdue University, West Lafayette, IN; 1973. Biological value of vanadium for rats, chicken, and sheep PhD diss.
- Wittenberg KM, Boila RJ, Shariff MA. Comparison of copper sulfate and copper proteinate as copper sources for copper-depleted steers fed high molybdenum diets. Can. J. Anim. Sci. 1990;70:895–908.
- Wohlt JE, Ritter DE, Evans JL. Calcium sources for milk production in Holstein cows via changes in dry matter intake, mineral utilization, and mineral source buffering potential. J. Dairy Sci. 1986;69(11):2815–2824. [PubMed: 3805457]
- Wollenberg P, Rummel W. Dependence of intestinal iron absorption on the valency state of iron. MNaunyn Schmiedebergs Arch. Pharmacol. 1987;336:578–582. [PubMed: 3125486]
- Wright CL, Spears JW, Engle TE, Armstrong TA. Effect of dietary copper level and high sulfate water on copper metabolism and growth in cattle. Roussel AM, Anderson RA, Favrier AE, editors. Boston, MA: Springer US; Trace Elements in Man and Animals. 2000;10:759–762.
- Wu Z, Satter LD. Milk production and reproductive performance of dairy cows fed two concentrations of phosphorus for two years. J. Dairy Sci. 2000;83:1052–1063. [PubMed: 10821580]
- Wu Z, Satter LD, Sojo R. Milk production, reproductive performance, and fecal excretion of phosphorus by dairy cows fed three amounts of phosphorus. J. Dairy Sci. 2000;83:1028–1041. [PubMed: 10821578]
- Wu Z, Tallam SK, Ishler VA, Archibald DD. Utilization of phosphorus in lactating cows fed varying amounts of phosphorus and forage. J. Dairy Sci. 2003;86:3300–3308. [PubMed: 14594248]
- Xin Z, Tucker WB, Hemken RW. Effect of reactivity rate and particle size of magnesium oxide on magnesium availability, acid-base balance, mineral metabolism, and milking performance of dairy cows. J. Dairy Sci. 1989;72:462–470. [PubMed: 2703568]
- Xin Z, Waterman DF, Hemken RW, Harmon RJ, Jackson JA. Effects of copper sources and dietary cation-anion balance on copper availability and acid-base status in dairy calves. J. Dairy Sci. 1991;74:3167–3173. [PubMed: 1779066]
- Yamamoto A, Wada O, Suzuki H. Purification and properties of biologically active chromium complex from bovine colostrum. J. Nutr. 1988;118:39–45. [PubMed: 3275760]
- Young R. Cobalt in Biology and Biochemistry. London, UK: Academic Press; 1979.
- Yu Y, Lu L, Luo XG, Liu B. Kinetics of zinc absorption by in situ ligated intestinal loops of broilers involved in zinc transporters. Poultry Sci. 2008;87:1146–1155. [PubMed: 18493004]
- Yuan K, Vargas-Rodriguez CF, Mamedova LK, Muckey MB, Vaughn MA, Burnett DD, Gonzalez JM, Titgemeyer EC, Griswold KE, Bradford BJ. Effects of supplemental chromium propionate and rumen-protected amino acids on nutrient metabolism, neutrophil activation, and adipocyte size in dairy cows during peak lactation. J. Dairy Sci. 2014;97:3822–3831. [PubMed: 24731640]
- Zarqami A, Ganjkhanlou M, Zali A, Rezayazdi K, Jolazadeh AR. Effects of vanadium supplementation on performance, some plasma metabolites and glucose metabolism in Mahabadi goat kids. J. Anim. Physiol. Anim. Nutr. 2018;102:e972–e977. [PubMed: 29120071]
- Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G, Haile DJ, Vogel W, Weiss G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology. 2001;120:1412–1419. [PubMed: 11313311]
- Minerals - Nutrient Requirements of Dairy CattleMinerals - Nutrient Requirements of Dairy Cattle
Your browsing activity is empty.
Activity recording is turned off.
See more...