NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Menini A, editor. The Neurobiology of Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2010.
1.1. INTRODUCTION
Over 30 years ago, Nobel laureate Sydney Brenner recognized that an intellectually straightforward strategy to delineate the basic principles in neurobiology is to utilize a model organism with a nervous system that is simple enough to lend itself to anatomical, cellular, genetic, and molecular analysis, yet be complex enough that lessons learned in that organism would give us insight into general principles of neural function. The humble organism he chose, the nematode Caenorhabditis elegans, is now one of the most thoroughly characterized metazoans, particularly in terms of its nervous system. One of Brenner’s motivations in adapting C. elegans as a model organism was to understand the totality of the molecular and cellular basis for the control of animal behavior (Brener 1988). In this chapter, we review what is arguably the best-studied aspect of C. elegans behavior: response to chemical stimuli. The C. elegans neurobiology literature can be intimidating for the uninitiated; we attempt to limit the use of “worm jargon” in this review. For a more C. elegans-centric review, we refer you to other excellent sources (Bargmann 2006).
1.1.1. Caenorhabditis elegans Basics
C. elegans are hermaphroditic, free-living nematodes. The adult hermaphrodite is ~1 mm in length. Each hermaphrodite produces oocytes and sperm, can internally self-fertilize, and has several hundred self-progeny that reach reproductive maturity in 3–5 days, depending on cultivation temperature. Hermaphrodites cannot cross-fertilize, but they can mate with males, resulting in numerous cross-progeny. In normal populations, C. elegans males appear at a 0.1–0.2% frequency (Figure 1.1). The ability to set up genetic crosses, relatively large brood sizes, and rapid generation time greatly facilitates forward genetic analysis in C. elegans. Much of what is known about olfaction in C. elegans is based on classical genetic studies of mutant strains defective in chemosensory response.

FIGURE 1.1
Schematic of the C. elegans life cycle. Adult hermaphrodite C. elegans typically produce 100–200 self-fertilized eggs. Under normal conditions, larvae go through four larval stages, called L1–L4 (not shown); the time it takes to reach (more...)
In the laboratory, C. elegans is reared in agar dishes seeded with Escherichia coli bacteria, which serve as a food source for the animals. On flat agar surfaces, C. elegans lies on its side and, when unperturbed, spends most of its time crawling forward with dorsal–ventral sinusoidal body bends at a rate of about 0.5 mm/s, punctuated by occasional spontaneous reversals in locomotion direction. C. elegans can initiate turns via omega bends, where the anterior end of the animal bends toward the posterior end and forms the shape of the Greek letter Ω, or by altering the amplitude of body bends (Gray et al. 2005). This mode of locomotion on flat agar surfaces is probably very artificial. Under conditions designed to simulate soil (presumably similar to the natural environment of C. elegans), the locomotory pattern of C. elegans changes to a more efficient hybrid mode between crawling and swimming, which is up to 10 fold faster than locomotion on surfaces (Park et al. 2008; Lockery et al. 2008).
1.1.2. Chemosensory Neuroanatomy
1.1.2.1. The Sensory Nervous System of Caenorhabditis elegans
C. elegans follows an almost invariant developmental pattern. Each adult hermaphrodite has precisely 959 somatic cells, of which almost one-third (302) are neurons. Each neuron is designated with a unique name, typically consisting of three or four letters (e.g., AWA, AWC). The synaptic connectivity of all 302 neurons has been mapped out using serial electron microscopy reconstruction and is remarkably reproducible between animals (Chen et al. 2006; White et al. 1986), thus making C. elegans the only metazoan with a completely characterized neuroanatomy down to the synaptic level. Among neurons, 16 pairs of anatomically bilaterally symmetric neurons (i.e., 32 neurons or ~10% of the nervous system) have been confirmed or inferred to be chemosensory based on functional studies or anatomy, as described below. These chemosensory neurons respond to a wide variety of soluble and volatile odorants. In a survey of volatile organic compounds, C. elegans exhibited either attraction or repulsion to 50 out of 120 compounds tested (Bargmann et al. 1993). C. elegans chemosensory neurons synapse directly or indirectly onto a set of command interneurons (named AVA, AVB, AVD, AVE, and PVC) that control forward or backward locomotion through synapses with motor neurons that control body wall muscles (Chalfie et al. 1985; Vonstetina et al. 2005) (Figure 1.2). Additional interneurons relevant to chemosensory behavior are discussed in Section 1.4.
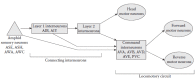
FIGURE 1.2
Simplified wiring diagram of the C. elegans chemosensory nervous system. Most amphid sensory neurons synapse to the locomotory circuit via multiple layers of connecting interneurons. However, some sensory neurons (e.g., ASH) synapse directly onto the (more...)
C. elegans has four types of visible chemosensory organs: the amphid, phasmid, inner labial, and outer labial organs (Figure 1.3). Each consists of two support cells, called sheath and socket cells, which form a pore through which sensory neuron endings are exposed to the external milieu (White et al. 1986; Ward et al. 1975). The pores are bilaterally or quadrilaterally symmetric and contain a poorly characterized substance probably reminiscent of mucosal secretions in vertebrates. The two amphid pores are located at the tip of the head and play a critical role in response to attractive chemical stimuli. Each contains the sensory endings of 11 chemosensory neurons and one thermosensory neuron (AFD). The ciliated sensory endings of these bilaterally symmetric chemosensory neurons are located in the amphid pore (ADL, ADF, ASE, ASG, ASH, ASI, ASJ, and ASK) or embedded in the sheath cell (AWA, AWB, and AWC neurons; also called wing neurons). The phasmid pores are structurally similar to the amphids, but are smaller, located behind the anus near the tail, and contain the sensory endings of the PHA and PHB neurons (Hall and Russell 1991; White et al. 1986; Ward et al. 1975). These neurons have been implicated in chemosensory avoidance. The fourfold symmetric inner and outer labial pores are located in the labia surrounding the mouth of C. elegans. Based on anatomical evidence, neurons associated with these organs (IL1, IL2, OLL, OLQ) probably play a role in sensory response. Laser ablation of the inner labial IL2 neurons has not resulted in any apparent defects in response to chemosensory cues thus far (Bargmann et al. 1993), and the outer labial OLQ neurons are required for mechanosensory response to nose touch (Kaplan and Horvitz 1993). The role of the other labial neurons remains unclear.
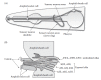
FIGURE 1.3
The structure of the amphid sensilla. (A) The soma of amphid sensory neurons are arranged around the pharynx, which is the feeding organ of C. elegans. The axons synapse with interneurons in a structure called the nerve ring. The dendrites extend anteriorly. (more...)
Sensory neuron ciliated endings are the probable sites of olfactory reception in C. elegans. Candidate seven transmembrane domain chemosensory receptors and other proteins implicated in the initial steps of olfactory response, localize to the sensory endings. For example, ODR-10, which is required specifically for response to diacetyl, localizes to the tip of the AWA ciliated endings (Sengupta et al. 1996). Early cellular ablation studies with a laser microbeam suggest that specific amphid sensory neurons are required for response to either attractive or repulsive stimuli, but not both (Bargmann and Avery 1995; Bargmann et al. 1993). More recent genetic studies suggest that altering neural activity in sensory neurons can alter odor preference (Tsunozaki et al. 2008; also see below).
1.1.2.2. Functions of Specific Chemosensory Neurons
The role of specific C. elegans neurons in behavior has classically been defined using cellular ablation with a laser microbeam (Bargmann and Avery 1995). Briefly, an early larval stage animal is anesthetized and neurons are identified under high power differential interference contrast (DIC) microscopy. A nitrogen pulsed dye laser is focused through the objective of the microscope to heat and kill targeted cell nuclei. Animals are transferred to a Petri dish to recover and develop into adulthood, upon which they can be tested for behavioral responses to odorants. This powerful approach has allowed the matching of specific odorants to specific chemosensory neurons.
Assignment of neurons to specific behavioral responses by laser ablation is augmented by two other strategies: cellular rescue studies and optical imaging. In the first strategy, a gene normally expressed in sensory neurons that is required for response to a stimulus is identified. Heterologous promoters are used to drive cDNA expression in specific subsets of neurons in mutant animals. If cDNA expression rescues the behavioral defect in mutant animals, then the gene is required in those sensory neurons and those neurons are important for behavioral response. The second strategy relies on the optical detection of stimulus-induced activity in neurons of the live animals (see Section 1.1.3.3). Immediate evoked responses in sensory neurons suggest they play a role in behavioral response. Although none of these approaches are definitive in isolation, in combination they have allowed C. elegans researchers to define distinct roles for specific neurons.
In general, the ASE neurons detect soluble attractants, whereas the AWA and AWC neurons detect volatile attractants (Bargmann et al. 1993). The ASH, ADL, and AWB neurons detect volatile repellants (Chao et al. 2004; Troemel et al. 1995). The ASH neurons also detect soluble repellants, including heavy metals such as Cu2+ and Cd2+ (Sambongi et al. 1999), SDS, and quinine (Hilliard et al. 2004, 2005). Other amphid neurons play minor roles in either chemotaxis or avoidance (Bargmann and Horvitz 1991; Sambongi et al. 1999; Hilliard et al. 2002). The phasmid sensory neurons PHA and PHB appear to detect repulsive stimuli and coordinate avoidance responses by antagonizing ASH sensory neurons in the amphid (Hilliard et al. 2002). Recently, a set of sensory neurons, including URX, AQR, and PQR, have been shown to be involved in aerotaxis, or attraction to oxygen (Chang et al. 2006; Gray et al. 2004; Rogers et al. 2006). In addition, the BAG sensory neurons are involved in avoidance of CO2 (Hallem and Sternberg 2008) (Table 1.1). A few amphid neurons (ASI, ADF, ASJ, and ASG) play clear roles in dauer formation and maintenance, but make only minor contributions to behavioral chemosensory response (dauer is an alternative third larval stage, which is adopted under harsh conditions and starvation, is long-lived and resistant to environmental stress; see Figure 1.1, and reviewed in Fielenbach and Antebi 2008). IL2 labial neurons may also impact dauer recovery (Ouellet et al. 2008).
TABLE 1.1
Chemosensory Neurons in C. elegans.
What determines if a particular chemosensory neuron drives attractive or repulsive behavior? Normally, AWA neurons drive response to the attractive odorant diacetyl (used as artificial butter odor in popcorn). When AWA neurons are killed with a laser microbeam, C. elegans are no longer attracted to diacetyl. AWB neurons, on the other hand, normally drive avoidance responses to the volatile repellant 2-nonanone. When AWB neurons are killed, C. elegans no longer avoid 2-nonanone. The G-protein-coupled receptor (GPCR), ODR-10, is the olfactory receptor protein for diacetyl; ODR-10 is normally expressed in AWA neurons, and animals lacking ODR-10 are not attracted to diacetyl (see Section 1.2.1). When ODR-10 is heterologously expressed in AWB neurons, transgenic animals are repelled by diacetyl (Troemel et al. 1997). This suggests that in some cases, sensory neurons are developmentally hard-wired for attractive or repulsive behaviors and their synaptic targets determine the behavioral response.
A more recent study has shown that this strict labeled line paradigm may be less rigid than previously thought. AWC neurons (more specifically, AWCON neurons; see Section 1.1.2.3), which normally mediate attraction to the volatile chemical butanone, mediate avoidance to butanone in a gcy-28 mutant background (Tsunozaki et al. 2008). gcy-28 codes for a receptor-like guanylyl cyclase that may act through the diacyl glycerol kinase DGK-1 and the protein kinase C TTX-4/PKC-1. This switch in behavioral encoding may result in a change in navigational strategy (see Section 1.4) rather than a change in neuronal wiring, as gcy-28 function in adults is sufficient for normal butanone attraction.
1.1.2.3. Asymmetrical Function in Anatomically Symmetric Pairs
Anatomically, amphid sensory neurons form bilaterally symmetric pairs. For example, ASEL and ASER are the left and right neurons in the ASE neuron pair, respectively. While most left-right pairs seem to be functionally identical, at least two pairs of sensory neurons, the ASE and AWC neuron pairs, are asymmetric in terms of their function.
The first asymmetry was discovered in the ASE neurons. The guanylyl cyclase gene, gcy-5, is exclusively expressed in ASER, whereas the related genes, gcy-6 and gcy-7, are only expressed in ASEL (Yu et al. 1997). Indeed, the ASEL and ASER neurons are also functionally distinct. ASEL neurons preferentially detect Na+ ions, whereas the ASER neurons detect K+ and Cl– ions (Pierce-Shimomura et al. 2001). A well-characterized regulatory cascade controls this difference in gene expression. Specification of the ASER cell fate, which appears to be the default state, requires the homeodomain protein CHE-1 (Uchida et al. 2003). The zinc finger transcription factors, LSY-2 (Johnston and Hobert 2005) and DIE-1 (Chang et al. 2004), are specifically expressed in ASEL and are required for the cell-specific expression of the microRNA (miRNA) lsy-6 in ASEL. Another miRNA, mi-273, functions in ASER to repress DIE-1 expression (Chang et al. 2004). The lsy-6 miRNA represses expression of the Nkx-type homeodomain protein COG-1 (Johnston and Hobert 2003). COG-1 acts together with UNC-37/Groucho to repress expression of ASEL-specific markers, such as gcy-7 and lim-6 (Chang et al. 2003). LIM-6, which is a homeodomain transcription factor, in turn represses expression of ASER-specific markers such as gcy-5. It is not clear what directly regulates ASEL-specific expression of LSY-2 and DIE-1, but ASE left-right asymmetry is specified early in embryogenesis in a LIN-12 Notch-dependent pathway that acts through the T-box transcription factors TBX-37 and TBX-38 (Poole and Hobert 2006). A substantial number of mutants defective in ASE left-right asymmetry have recently been isolated in a large-scale genetic screen (Sarin et al. 2007), indicating a wealth of knowledge waiting to be discovered.
AWC neurons also show left-right asymmetry. However, unlike the ASE neurons, wherein the left-right cell fate is developmentally hard-wired, an AWC neuron can randomly adapt one of two fates, with the other AWC neuron in the pair developing the other fate. AWC cell fate decisions are marked by expression of str-2, which codes for a putative olfactory receptor (see below): one AWC neuron expresses str-2 (the “str-2 on” or AWCON fate) and the other does not (the “str-2 off” or AWCOFF fate) (Troemel et al. 1999). The AWCON neuron detects the volatile attractant butanone, whereas the AWCOFF neuron detects the volatile attractant 2,3-pentanedione; both AWC neurons detect another attractant, benzaldehyde (Wes and Bargmann 2001). Proper AWC leftright specification requires synaptic contact between the neurites of the left-right AWC neurons (Troemel et al. 1999). The voltage-gated Ca2+ channel subunits UNC-2 and UNC-36 are required for proper left-right specification. Influx of Ca2+ probably activates the Ca2+ modulated protein kinase UNC-43, which then activates NSY-1/ASK1, a MAP kinase–kinase–kinase (Sagasti et al. 2001). A MAP kinase-signaling cascade is likely to inhibit str-2 expression, thereby promoting the AWCOFF cell fate. NSY-4 and NSY-5 appear to function in parallel to induce the AWCON fate. NSY-4 is a claudin superfamily protein related to the γ-subunits of voltage-gated Ca2+ channels (VanHoven et al. 2006). NSY-5/INX-19 is an innexin protein that forms gap junction channels. NSY-5 appears to act cell autonomously in AWC neurons to promote the AWCON fate during development, but it also appears to function in other neurons (ASH and AFD) as well (Chuang et al. 2007).
1.1.3. Chemosensory Assays in Caenorhabditis elegans
There are many variations of C. elegans chemosensory assays that have been employed by researchers, but they can be grouped into a few basic techniques (Figure 1.4).

FIGURE 1.4
C. elegans chemosensory assays. (A) The chemotaxis assay. Animals are placed on a large Petri dish, on which a spot of a volatile attractant and a dilutant have been placed on opposite sides of the plate (ethanol is usually used as a dilutant because (more...)
1.1.3.1. Chemotaxis Assays (Attractants)
Quantitative measurement of attraction to chemicals is reported as a chemotaxis index (CI), which reflects the response of a population of animals (Bargmann et al. 1993). A large, round Petri dish containing normal C. elegans culture media is used (NGM agar); no bacterial lawn (i.e., food) is present. For volatile attractants, the chemical of interest (usually diluted in ethanol) is pipetted onto a spot on one side of the dish; a drop of the dilutant is added to the opposite side as a control. An anesthetic (usually sodium azide) is also added at each spot. Animals are placed between the two drops for the assay, and those that reach one of the spots are anesthetized and thus immobilized. After ~1 h, the number of animals that have accumulated at each spot is determined. The CI is then calculated as:

Thus, the closer the CI is to 1.0, the stronger the attraction to the odorant; a CI close to zero means that the odorant is neutral to animals; and a negative CI suggests that animals are repelled by the odorant.
Originally, the CI was actually designed for soluble attractants. Agar plugs containing high concentrations of an attractant are placed snugly into holes in an assay plate and gradients are allowed to develop overnight via passive dilution (Bargmann and Horvitz 1991); assays are otherwise similar. In a variation of this assay, a round Petri dish is divided into four quadrants (Wicks et al. 2000). Two nonadjacent quadrants are filled with NGM containing the soluble attractant, whereas the remaining quadrants lack the attractant. Animals are allowed to disperse from the center of the dish during the assay. The CI is then calculated as above.
1.1.3.2. Avoidance Assays for Repellants
A variation of the chemotaxis assay can be used to measures active avoidance of volatile compounds (Troemel et al. 1997). However, more recent studies on volatile chemical repellants (Chao et al. 2004; Ferkey et al. 2007; Fukuto et al. 2004; Wragg et al. 2007) utilize the more rapid “smellon-a-stick” assay (Troemel et al. 1995). A thin paintbrush hair taped to a Pasteur pipette is dipped into a volatile odorant (e.g., octanol or nonanone) and placed in front of an animal that is crawling forward (the hair is not allowed to contact the animal). A wild type C. elegans rapidly initiates backward locomotion, usually within seconds. Avoidance is reported as average time required to initiate a reversal. It is likely that the circuitry and genetic requirements for these two assays (populations vs individual animals) may not be absolutely identical. The advantage of the individual assay over the plate assay is that it measures an immediate response and that individual animals are easier to assay.
For soluble repellants, a plate-based assay can be used wherein a barrier of the repellant is “painted” on the dish to form an enclosed border. Animals are placed inside the border and the avoidance index is measured as the number of C. elegans that are retained in the border divided by the total number of animals (Wicks et al. 2000). An interesting variation of this assay tested the navigational abilities of C. elegans. A Cu2+ maze was painted onto the agar surface, revealing navigational defects in animals lacking NMR-1, a C. elegans homolog of an NMDA-type glutamate receptor subunit (Brockie et al. 2001). Another assay used for soluble repellants is the drop assay (Hilliard et al. 2002, 2004). In this assay, a capillary micropipette is used place a drop of repellant on the tail of a forward-moving animal. Capillary action causes the repellant to move to the animal’s nose, and a reversal is initiated. The behavior is then scored similar to the smell-on-a-stick assay. The role of the phasmid neurons in chemosensory response was revealed using this assay (Hilliard et al. 2002).
1.1.3.3. Neuroimaging Approaches
Recently, techniques have been developed for directly imaging neural activity in C. elegans neurons upon mechanical or chemical stimulation. In C. elegans (as well as the parasitic nematode Ascaris suum), Ca2+ is thought to be the major cation that carries depolarizing currents (Davis and Stretton 1989; Goodman et al. 1998). Imaging neural activity takes advantage of the genetically encoded Ca2+ indicator, cameleon (Miyawaki et al. 1997). Cameleon consists of CFP (the cyan variant of GFP) fused to YFP (the yellow variant of GFP), linked by calmodulin and the M13 calmodulin-binding domain. When Ca2+ is present at sufficient levels, calmodulin wraps around the M13 domain, thereby closely juxtaposing CFP and YFP. When cameleon is exposed to CFP excitation wavelengths, this conformational change causes fluorescence resonance energy transfer (FRET); YFP excitation only occurs via nearby photonic energy release from CFP emission. Both CFP and YFP signal are acquired in real-time using a beam splitter, and Ca2+ flux is measured as a change in CFP/YFP ratio. Cameleon can be expressed in specific neurons using well-characterized promoters with defined expression patterns. Due the small size of C. elegans neurons, most cameleon measurements are made with animals that have been immobilized in some way. Early Ca2+ imaging experiments utilized veterinarian’s glue (Kerr et al. 2000); subsequent advances in the field now utilize microfluidic chambers that either immobilize animals using a custom microfabricated elastomer trap (Chronis et al. 2007) or that allow free movement in an environment that mimics soil and tracks freely moving animals at high magnification using computer-controlled stages (Lockery et al. 2008). Similar types of in vivo Ca2+ flux measurements can be performed in C. elegans (e.g., Tsunozaki et al. 2008; Chalasani et al. 2007) using G-CaMP, a nonratiometric Ca2+ indicator based on a circularly permutated GFP (Nakai et al. 2001). Ca2+ imaging is typically performed on cell bodies, but in some cases, imaging of the neurites can also be performed (Chalasani et al. 2007; Clark et al. 2006).
1.2. SIGNAL TRANSDUCTION
The literature on C. elegans chemosensory signal transduction is extensive, and the nomenclature can be confusing. Table 1.2 lists C. elegans protein/gene names and a concise description of their function and vertebrate counterparts, where applicable; and Figure 1.5 summarizes the relevant signaling pathways.
TABLE 1.2
list of Proteins and genes Involved in C. elegans chemosensation.
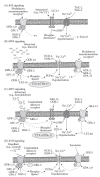
FIGURE 1.5
Signal transduction pathways of major C. elegans chemosensory neurons. Protein names followed by a question mark indicate that the position of that protein in the pathway is ambiguous. Dotted lines between signaling components indicates that there are (more...)
1.2.1. Seven Transmembrane Domain Olfactory Receptors
The C. elegans genome contains a dizzying array of genes that encode seven transmembrane serpentine receptors; most of these are presumed to encode olfactory receptors that probably couple to heterotrimeric G-proteins. Based on phylogenetic analyses, these receptors can be roughly classified into four superfamilies: str, sra, srg, and srw. Collectively, family members compromise ~1300 receptor genes and ~400 pseudogenes (Robertson and Thomas 2006). Expression analysis using promoter GFP fusion reporters of a representative subset of these genes suggests that these receptor genes are expressed in chemosensory neurons (Troemel et al. 1995). Neuron-specific gene expression profiling also indicated expression of a subset of putative olfactory receptors in AWB chemosensory neurons (Colosimo et al. 2004), which is consistent with a chemosensory function for these receptors. Interestingly, there is very little functional information on these odorant receptors. ODR-10, a member of the str superfamily, was identified in a classical genetic screen for animals defective for chemotaxis toward the volatile attractant diacetyl, and remains the only functionally defined C. elegans olfactory receptor with a defined ligand (Sengupta et al. 1996) (Figure 1.5B). It is noteworthy that after the seminal work of Buck and Axel (1991) in discovering olfactory receptor gene families in mammalian olfactory neurons, ODR-10 was the first seven transmembrane receptor in any organism to be functionally characterized as an olfactory receptor. SRA-13, a member of the sra superfamily, acts antagonistically with C. elegans Ras/MAPK signaling to negatively regulate chemotaxis to diacetyl and another attractant, isoamyl alcohol. However, it is unknown if SRA-13 codes for a bona fide chemoreceptor or has some kind of constitutive regulatory function (Battu et al. 2003). At least one genome-wide RNA interference (RNAi) screen unrelated to chemotaxis identified several putative chemoreceptor genes that are involved in fat metabolism (Ashrafi et al. 2003), suggesting that these receptor proteins may have diverse functions that are currently unexplored.
In contrast with the large number of olfactory receptor genes, C. elegans has a limited repertoire of chemosensory neurons. Thus, multiple receptors are expressed in each sensory neuron. This sort of anatomical limitation can be thought of as a more extreme example of the organization of olfactory receptors in Drosophila, where some olfactory neurons express two to three different receptors (reviewed in Fiala 2007; see also Chapter 2). This is in contrast to the organization of the vertebrate olfactory system, wherein a single olfactory receptor gene is expressed per sensory neuron (Chess et al. 1994), and odor perception is interpreted when a combination of different olfactory receptors (and therefore different sensory neurons) are activated in response to a single odorant (Malnic et al. 1999, see also Chapter 7).
The mechanism of olfactory receptor trafficking and insertion into the membrane is poorly understood (Bush and Hall 2008). Olfactory receptor proteins are actively trafficked to the sensory cilia in a process that depends on unc-101, which codes for a clathrin adapter protein. There also appear to be trafficking pathways for olfactory receptors that act semiredundantly with unc-101 (Dwyer et al. 2001). Olfactory receptors (and GPCRs, in general) do not target the plasma membrane efficiently when expressed in heterologous cells. ODR-4 is a novel protein that is required for proper localization of the olfactory receptor ODR-10 to the sensory cilia in C. elegans (Dwyer et al. 1998). When ODR-4 is expressed in mammalian cell lines, it facilitates proper trafficking of at least one rat olfactory receptor (Gimelbrant et al. 2001). ODR-4 is apparently conserved in vertebrates (Lehman et al. 2005), although no functional studies on this protein have been done other than in C. elegans. odr-8 is another C. elegans gene required for proper trafficking of ODR-10, but it has not yet been cloned (Dwyer et al. 1998).
GPCR kinases (GRKs) impact neuron response in C. elegans differently than expected, based on vertebrate studies. In vertebrates, olfactory signal transduction is negatively regulated by phosphorylation of olfactory receptors by GRKs and β-arrestin (Dawson et al. 1993). Odorant stimulation of wild-type olfactory epithelia in mice leads to rapid desensitization of cAMP formation, but this desensitization is absent in GRK-3 knockout mice (Peppel et al. 1997). In C. elegans, loss of function of the GRK homolog grk-2 leads to loss of behavioral response to attractive and repulsive odorants. The loss of behavioral response could not be attributed to a loss of desensitization; rather, loss of grk-2 function presumably leads to decreased G-protein signaling, likely via a feedback mechanism. Loss of function in the C. elegans β-arrestin gene arr-1 does not overtly affect chemotaxis (Fukuto et al. 2004). However, loss of function in arr-1 affects adaptation and recovery from adaptation to odorants (Palmitessa et al. 2005). These results suggest that GRK signaling (specifically GRK-2) in C. elegans might positively regulate chemosensation in a fashion that is distinct from GRK regulation of odorant receptors in mammals (Figure 1.5B through D), but that C. elegans ARR-1 functions more analogously to mammalian arrestin proteins. The C. elegans genome contains two GRK homologs, and GRK-1 has not yet been characterized. One possibility is that GRK-1, and not GRK-2, acts in conjunction with ARR-1 to regulate adaptation of chemosensation. Alternatively, GRK-2 may play more than one role in chemosensory neurons.
1.2.2. Heterotrimeric G-Proteins
C. elegans olfactory receptors probably couple to heterotrimeric G-proteins. The C. elegans genome contains 21 genes encoding heterotrimeric Gα subunits (Jansen et al. 1999). gsa-1, goa-1, egl-30, and gpa-12 code for homologs of mammalian Gs, Gi/o, Gq, and G12/13 α subunits, respectively. These conserved genes are expressed in a variety of cell types including neurons. (Note that gpa-12 expression is somewhat more limited.) GOA-1 and EGL-30 are required for olfactory adaptation (see below), but not olfaction itself (Matsuki et al. 2006). There is no obvious C. elegans homolog for Golf, the Gα subunit used for signal transduction in mammalian olfactory neurons. The remaining C. elegans Gα-coding genes (the gpa genes and odr-3) are Gi-like but unique to C. elegans, and 14 of these are expressed almost exclusively in chemosensory neurons. Immunohistochemistry showed that some Gα subunits are expressed in sensory cilia (Lans et al. 2004; Roayaie et al. 1998), suggesting that they might play a direct role in chemosensation. The first identified noncanonical Gi-like Gα subunit, ODR-3, was identified in a genetic screen for mutants defective for chemotaxis toward benzaldehyde, an attractive odorant detected by the AWC sensory neurons (Roayaie et al. 1998; Bargmann et al. 1993). The different Gα subunits have distinct and complex contributions to olfactory signal transduction in specific neurons. For instance, odr-3 loss-of-function animals are strongly defective for chemotaxis to the attractants diacetyl, pyrazine (detected by AWA neurons), and isoamyl alcohol (detected by AWC neurons) (Figure 1.5). However, detection of butanone (also detected by AWC neurons) requires the redundant function of GPA-2 and ODR-3 (Roayaie et al. 1998) (Figure 1.5C). ODR-3 and GPA-3 both function in ASH sensory neurons to facilitate avoidance responses (Figure 1.5D). odr-3 mutant animals are defective for response to the chemical repellant octanol, but not quinine (Fukuto et al. 2004; Hilliard et al. 2004). gpa-3 mutant animals are weakly defective for response to octanol (M.Y. Chao and A.C. Hart; unpublished observations) and quinine, but odr-3;gpa-3 double mutants are strongly defective for quinine response (Hilliard et al. 2004). One interpretation of these data is that some receptors may couple only to specific Gα subunits, whereas other receptors may be more promiscuous. Other GPA proteins may have regulatory functions. For instance, GPA-11 modulates the activity of ASH neurons, depending on the presence or absence of food (Chao et al. 2004) (Figure 1.5D). GPA-2, GPA-5, and GPA-13 also have regulatory roles in AWA and AWC neurons (Lans et al. 2004) (Figure 1.5B and C). One possibility is that these GPA proteins may couple to biogenic amine neurotransmitter receptors, which may have modulatory rather than excitatory activity. Serotonin, dopamine, octopamine, and tyramine have all been shown to modulate C. elegans chemosensory response (Chao et al. 2004; Ferkey et al. 2007; Wragg et al. 2007) (see Section 1.3.2).
C. elegans has two Gβ (GPB-1 and GPB-2) and two Gγ (GPC-1 and GPC-2) subunits (Jansen et al. 1999). GPB-1, GPB-2, and GPC-2 are widely expressed in many tissues, while GPC-1 expression is restricted to a limited set of sensory neurons. Due to the limited number of Gβ and Gγ proteins, the various Gα proteins probably share these Gβ and Gγ proteins in limited combinations to form heterotrimers. GPB-1 is required for ASH neuron-mediated avoidance responses (Esposito et al. 2007). GPB-2 and GPC-1, while not directly required for chemosensation, are required for adaptation to NaCl, a type of chemosensory learning (Matsuki et al. 2006; Jansen et al. 2002) (Figure 1.5A). This suggests that GPC-2 may be the Gγ subunit that participates in general signal transduction in sensory neurons, although GPC-2 has not yet been functionally characterized in sensory neurons.
Regulators of G-protein signaling (RGS) proteins negatively regulate G-protein signaling by promoting GTP hydrolysis (Hollinger and Hepler 2002; Ross and Wilkie 2000). They have been shown to regulate chemosensory signaling in at least two cases. First, EAT-16 negatively regulates chemosensory signaling in AWA sensory neurons (Figure 1.5B). Loss-of-function mutations in eat-16 suppress chemosensory behavioral defects in grk-2 mutants. However, only defects for which the normal behavioral response is mediated by AWA neurons are suppressed; grk-2 behavioral defects that are caused by impaired function in AWC neurons are not suppressed (Fukuto et al. 2004). Second, RGS-3 negatively regulates signaling in several chemosensory neurons, including AWC and ASH neurons (Ferkey et al. 2007) (Figure 1.5C and D). Interestingly, loss-of-function mutations in rgs-3 resulted in defects in behavioral responses to strong stimuli but not weak stimuli, suggesting that one role of RGS signaling is gain control. Excessive signaling in sensory neurons caused by loss of rgs-3 does not lead to a corresponding increase in behavioral output (Ferkey et al. 2007), suggesting that signal gain must fall within an optimal range for normal behavioral response.
1.2.3. Downstream Effectors of Chemosensory Signaling
Different sensory neurons in C. elegans are specialized for specific sensory modalities. In terms of chemosensation, the major players can be classified into five groups: ASE neurons, which detect soluble attractants; AWA and AWC, which detect volatile attractants; ASH, ADL, and AWB, which detect volatile (and some soluble) repellants; ASI, ADF, and ASJ, which are involved in dauer formation and play minor roles in soluble attractants (dauer formation is a developmental phenomenon and is not explored further herein); and the sensory neurons involved in detection of O2 and CO2. Each class utilizes different signaling mechanisms, although there is some overlap in the signaling molecules involved (Figure 1.5). Thus, our discussion will be organized on the basis of sensory modalities.
1.2.3.1. Soluble Attractants: Chemotaxis toward NaCl and ASE Neurons
C. elegans is attracted to a variety of soluble chemicals, including Na+, Cl–, biotin, cAMP, lysine, and serotonin (Bargmann and Horvitz 1991). The best-studied soluble attractant for C. elegans is NaCl. Low concentrations of NaCl (0.1–200 mM) attract C. elegans, and this is mostly mediated by ASE neurons, with minor contributions from ADF, ASG, and ASI neurons (Bargmann and Horvitz 1991). High concentrations of NaCl (>200 mM) actually repel C. elegans, but this is probably due to a general osmotic avoidance mechanism involving the ASH neurons rather than a chemosensory avoidance response (Hukema et al. 2006).
The receptor(s) that mediate NaCl attraction are unknown. At least some seven transmembrane receptors of the putative chemoreceptor superfamily are expressed in ASE neurons, suggesting that detection of some soluble attractants is mediated by G-protein signaling (Etchberger et al. 2007). However, detection of NaCl may not involve G-protein signaling. When the entire complement of mutant strains lacking Gα subunits expressed in ASE neurons were tested, none were defective for chemotaxis toward NaCl (Hukema et al. 2006). G-protein signaling does have important cell autonomous and noncell autonomous roles in behavioral plasticity related to chemotaxis to NaCl (Hukema et al. 2006; Jansen et al. 2002).
NaCl attraction requires cGMP signaling. tax-2 and tax-4, which code for subunits of a cGMPgated channel, are required for attraction to NaCl (Coburn and Bargmann 1996; Komatsu et al. 1996). Vertebrate cyclic nucleotide-gated channels involved in sensory transduction, such as those in rod cells and olfactory neurons, function as heterotetramers (reviewed in Pifferi et al. 2006; see also Chapter 8), and based on in vitro evidence, TAX-2 and TAX-4 probably multimerize to form a heteromeric active channel (Komatsu et al. 1999). The source of cGMP is unknown. The receptor-like transmembrane guanylyl cyclases ODR-1 (L’Etoile and Bargmann 2000) and DAF-11 (Birnby et al. 2000) are required for NaCl attraction. However, neither ODR-1 nor DAF-11 are expressed in ASE neurons, suggesting that they act non-cell autonomously in NaCl attraction, and thus, are not the direct source of cGMP for TAX-2/TAX-4. The C. elegans genome encodes 34 guanylyl cyclases, 24 of which are transmembrane receptor-like proteins; of the transmembrane guanylyl cyclases, nine are expressed in ASE neurons, with some showing left-right asymmetry in their expression patterns (Ortiz et al. 2006). Since the left and right ASE neurons detect Na+ and Cl–, respectively, one intriguing possibility is that transmembrane guanylyl cyclases function as Na+ and Cl– receptors that mediate ASE NaCl attraction; currently, there is no functional evidence for this as of this writing. The C. elegans calcineurin protein TAX-6 is also required in sensory neurons (probably in ASE neurons) for chemotaxis toward NaCl, suggesting that Ca2+ signaling is required (Kuhara et al. 2002). However, where TAX-6 functions in the signaling pathway is unknown (Figure 1.5A).
A recent study suggests that AWC neurons, which are typically assigned the function of sensing volatile odorants (see below), also sense NH4+ ions in soluble form (Frøkjaer-Jensen et al. 2008). Ammonium acetate is an interesting odorant that is sensed both as a soluble and volatile compound, using a distributed combination of exposed (ASE and others) and nonexposed (AWA and/or AWC) ciliated sensory neurons (Frøkjaer-Jensen et al. 2008).
1.2.3.2. Volatile Attractants: Chemotaxis Mediated by AWA and AWC Neurons
Attraction to volatile odorants is primarily mediated by the AWA and AWC sensory neurons, and is most likely a part of a general foraging strategy for C. elegans to locate bacteria and other food sources. In contrast to soluble attractants such as NaCl, wherein diffusion is limited by the presence of water, volatile odorants that diffuse through the air represent long-distance attractive cues. C. elegans responds to a variety of volatile organic compounds, including alcohols, ketones, aldehydes, esters, amines, sulfhydryls, acids, aromatic, and heterocyclic compounds (Bargmann et al. 1993). Using laser ablation, a subset of these odorants has been assigned as being detected by either AWA or AWC (Table 1.1). Signal transduction in AWA and AWC neurons both probably involve G-protein-coupled olfactory receptors, but there are important differences in downstream mechanisms.
In the AWA sensory neurons, olfactory receptors such as the diacetyl receptor ODR-10 probably couple to the Gi-like Gα protein, ODR-3 (Roayaie et al. 1998). Activation of ODR-3 is likely to lead to the metabolic release of polyunsaturated fatty acids (PUFAs) from membrane phospholipids via an unidentified phospholipase activity (Kahn-Kirby et al. 2004). Mutants defective in poly unsaturated fatty acid (PUFA) biosynthesis (fat mutants) (Watts and Browse 2002) are defective for chemotaxis toward diacetyl and pyrazine (odorants sensed by AWA neurons), but not benzaldehyde (an odorant sensed by AWC neurons) (Kahn-Kirby et al. 2004). Gas chromatograph analysis of the fatty acid content in the fat mutants, together with behavioral analysis, suggested that arachidonic acid (AA) and eicopentaenoic acid (EPA) are the PUFAs most important for AWA neuron activity. AA and EPA are likely to directly activate the TRPV channels OSM-9 and OCR-2 (Colbert et al. 1997; Tobin et al. 2002) (Figure 1.5B).
In contrast, the major signal transduction pathway utilized by the AWC sensory neurons appears to be through the DAF-11/ODR-1 guanylyl cyclases and the TAX-2/TAX-4 cGMP-gated channels. daf-11 and odr-1 mutants are defective for AWC-mediated chemosensory responses (L’Etoile and Bargmann 2000; Vowels and Thomas 1994; Birnby et al. 2000). Unlike the ASE neurons, DAF-11 and ODR-1 are expressed in the AWC neurons, and are thus, most likely to be the cell autonomous source of cGMP (L’Etoile and Bargmann 2000). AWC neurons are probably glutamatergic (Chalasani et al. 2007) (see Section 1.4).
Several other signaling molecules have been implicated in AWC signaling, although their placement in the signaling pathway is unclear. Interestingly, the C. elegans Ras homolog, LET-60, is required for AWC-mediated chemotaxis, and probably acts downstream of TAX-2/TAX-4 (Hirotsu et al. 2000). The putative olfactory receptor, SRA-13, negatively regulates LET-60 Ras signaling via the Gα protein GPA-5 (Battu et al. 2003). The Ca2+-independent protein kinase C (nPKC) TTX-4 is also required for AWC (and AWA) mediated behaviors, and the related nPKC TPA-1 acts semiredundantly with TTX-4 (Okochi et al. 2005). The cGMP-dependent kinase EGL-4 is required for olfactory adaptation in AWC neurons, but not for general olfaction (L’Etoile et al. 2002) (Figure 1.5C). Interestingly, the EGL-4 homolog in the related nematode, Pristionchus pacificus, is required for olfaction of insect pheromones (Hong et al. 2008a) (see Section 1.5.2).
1.2.3.3. Chemical Repellants
Repellants are detected by at least three pairs of amphid neurons, ASH, ADL, and AWB, with minor contributions by other neurons. The AWB neurons require ODR-1 function for response to the repellant nonanone (L’Etoile and Bargmann 2000) and are involved in serrawettin avoidance (Pradel et al. 2007) (see 1.5.1) but otherwise are poorly characterized. The ASH neurons have been intensely studied. These polymodal neurons detect mechanical (Kaplan and Horvitz 1993) as well as soluble and volatile chemical stimuli. Here, we focus on the chemosensory role of ASH neurons. Laser ablation studies have shown that volatile repellents, such as the long-chain alcohol 1-octanol, are sensed by ASH neurons (ADL and AWB neurons also contribute to the response to volatile repellants; see below). ASH neurons also respond to soluble repellents, such as quinine, SDS, Cu2+, H+, and others (Dusenbery 1974; Sambongi et al. 1999; Hilliard et al. 2002, 2004). Using an analogy to bipolar cells in the vertebrate retinal nervous system (Yang 2004), ASH neurons function as both ON-sensing and OFF-sensing cells Figure 1.6). Tonic increases in Ca2+ levels are detected in ASH neurons when ASH-activating stimuli are presented (Hilliard et al. 2005; Chronis et al. 2007), but transient Ca2+ spikes are also detected when those stimuli are removed (Chronis et al. 2007).
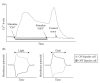
FIGURE 1.6
Ca2+ currents in ASH neurons after stimulation. (A) ASH neurons display a tonic increase in Ca2+ levels after stimulation with chemical repellants, such as quinine or Cu2+. They also show a transient spike in Ca2+ levels after the stimulus is removed. (more...)
The ASH neurons synapse directly onto the command interneurons, unlike the AWA and AWC neurons that connect to the command interneurons through multiple layers of other interneurons (see Figure 1.2). This neuroanatomy reflects a difference in aversive vs attractive behavior; aversive responses are relatively rapid and occur on the order of several seconds, whereas attractive responses occur over a longer time span (30–60 min; see Figure 1.4). ASH signaling shares several components with AWA neurons. Odorants presumably are detected via GPCRs coupled to ODR-3, with GPA-3 playing a minor role. Similar to AWA neurons, activation of G-protein signaling presumably leads to the release of PUFAs from the plasma membrane, leading to activation of the ODR-9/OCR-2 TRPV channels (Kahn-Kirby et al. 2004) (Figure 1.5D). In addition to ODR-9, the L-type voltage-gated Ca2+ channel, EGL-19, is also required for a transient increase in somatic Ca2+ levels, as measured by the Ca2+ indicator protein cameleon (Hilliard et al. 2005). The ASH neurons are probably glutamatergic (Lee et al. 1999), and chemosensory response requires the postsynaptic glutamate receptor GLR-1 (and probably others) expressed in the command interneurons (Chao et al. 2004; Mellem et al. 2002).
The ability of ASH neurons to respond to different sensory modalities requires the expression of modality-specific genes. For instance, OSM-10 is a novel cytoplasmic protein expressed in ASH neurons. Animals lacking OSM-10 fail to respond to osmotic stimuli, but still respond to volatile and soluble repellants (Hart et al. 1999; Hilliard et al. 2005). Similarly, the novel WD-40 repeat protein QUI-1 (also expressed in ASH neurons) is required for avoidance of soluble repellant quinine (Hilliard et al. 2004) and the volatile repellant octanol (Fukuto 2004), but not for osmotic avoidance. The polymodal nature of ASH neurons appears to be an evolutionarily conserved trait among nematodes (Srinivasan et al. 2008).
1.2.3.4. Oxygen and Carbon Dioxide
In soil or on bacterial lawns in laboratories, oxygen levels can vary widely over short distances. Physiologically, C. elegans can adapt to a wide range of oxygen concentrations. Metabolic rates remain relatively constant between 4 and 21 kPa of O2 (Van Voorhies and Ward 2000). At low (hypoxic) oxygen concentrations (0.25–1 kPa), C. elegans survive via a HIF-1-dependent hypoxic response pathway (Jiang et al. 2001). Alternatively, under anoxic conditions (<0.001 kPa), animals survive by entering a HIF-1 independent state of suspended animation (Nystul et al. 2003).
C. elegans respond behaviorally to changes in oxygen concentration. This behavior is termed aerotaxis. When animals are presented with a gradient of oxygen concentrations in specially designed chambers, they distribute between 4 and 12 kPa (Gray et al. 2004). C. elegans also expresses its preference for O2 concentration under standard laboratory culture conditions. Agar plates are typically seeded with a 100–200 μL drop of E. coli bacteria grown in liquid culture. As the liquid dries, the E. coli form a lawn that is slightly thicker at the edges. C. elegans of the laboratory wild-type reference strain (the N2 strain) typically disperse throughout the bacterial lawn to feed (solitary feeding), whereas certain wild isolates of C. elegans (e.g., the CB4856 strain from Hawaii; Wicks et al. 2001) tend to clump together, particularly at the edge of the bacterial lawn (social feeding). There is a measurably lower oxygen concentration in the thick edges of the bacterial lawn than the thinner regions in the middle of the lawn (Gray et al. 2004). The difference in oxygen preferences between different wild isolates is due to a single amino acid polymorphism in NPR-1, a G-protein-coupled FMRF amide-like neuropeptide receptor (de Bono and Bargmann 1998), and is discussed below.
Oxygen is sensed by the body cavity sensory neurons AQR, PQR, and/or URX, with contributions from the SDQ, ALN, and/or PLN neurons (Gray et al. 2004). Unlike the amphid neurons, AQR, PQR, and URX are exposed to the pseudocoelomic body fluids of the animal instead of the outside environment. Presumably, these neurons sense dissolved oxygen in the body cavity fluid. The O2 sensor proteins are the soluble heme-binding guanylyl cyclases (sGCs) GCY-35 and GCY-36 that bind O2, and are unlike previously characterized soluble guanylyl cyclases that bind NO (Gray et al. 2004; Cheung et al. 2005; Chang et al. 2006). cGMP produced by these sGCs act via the TAX-2/TAX-4 cGMP-gated channels to activate the oxygen-sensing neurons (Chang et al. 2006). Aerotaxis toward the optimal O2 concentration also requires the TRPV channels ODR-9/OCR-2 acting in the nociceptive ASH and serotonergic ADF neurons (Chang et al. 2006; Rogers et al. 2006). Thus, aerotaxis appears to be mediated by a distributed network of neurons with different sensory modalities.
The NPR-1 neuropeptide receptor is expressed in AQR, PQR, URX, and other neurons (Coates and De Bono 2002), and is activated by the FMRF amide-like neuropeptides FLP-18 and FLP-21 (Rogers et al. 2003). Activation of NPR-1 probably antagonizes the activity of TAX-2/TAX-4 in AQR, PQR, and URX neurons (Coates and De Bono 2002). Thus, O2 levels sensed by the soluble guanylyl cyclases, GCY-35 and GCY-36, are likely to function as the primary signal to regulate social feeding, with other modes of sensory input modulating cGMP signaling via neuropeptides and NPR-1.
C. elegans also detects CO2. In contrast to O2 sensing, wherein C. elegans migrate to an optimal O2 concentration, C. elegans detects and actively avoids CO2 concentrations greater than 0.5–1.0 pKa (Bretscher et al. 2008; Hallem and Sternberg 2008). This avoidance behavior can be measured using a concentration gradient similar to that used in assays for aerotaxis for O2, or in acute puffs of CO2 administered through a syringe. Atmospheric CO2 levels (which is in the range of 300–400 ppm) are very low compared to atmospheric O2, but the natural microenvironment of C. elegans (bacteria-rich soil) can have very high local CO2 concentrations (up to 10 kPa) (Sposito 2008). Thus, the detection threshold for CO2 is well above atmospheric levels. This suggests that CO2 avoidance is used by C. elegans to avoid potentially inhospitable environments in the soil. CO2 avoidance requires the BAG sensory neurons (and probably other neurons), but does not require the AQR, PQR, and URX neurons required for aerotaxis toward O2 (Bretscher et al. 2008). Some downstream signaling components of O2 and CO2 sensing are similar; both pathways require TAX-4 and are modulated by NPR-1. However, CO2 detection does not require the same sGCs as those used for O2 detection (Hallem and Sternberg 2008). CO2 detection also requires the RGS protein RGS-3 and Ca2+ signaling via TAX-6/CNB-1 calcineurin (Hallem and Sternberg 2008).
1.3. MODULATORY PATHWAYS FOR CHEMOSENSORY BEHAVIORS
C. elegans modulates its behavioral responses to chemosensory stimuli, presumably based on a summation of different sources of sensory information. Modulation by the presence or absence of food is perhaps the best-studied phenomenon. Signaling via several modulatory neurotransmitters has been linked to changes in food availability. Among these, the best studied is the role of serotonin.
1.3.1. Food and Serotonin
1.3.1.1. Relationship Between Food and Serotonin Signaling
Food is an important modulator of many C. elegans behaviors, including locomotion (Sawin et al. 2000), pharyngeal pumping (Avery and Horvitz 1990), male mating (Loer and Kenyon 1993), egg laying (Hajdu-Cronin et al. 1999; Horvitz et al. 1982), and chemosensory response (see below). The standard laboratory food source for C. elegans is E. coli bacteria. Ironically, although the effect of food on C. elegans behavior has been well characterized, the precise chemical and/or physical cues by which C. elegans detect E. coli remain unclear. A mechanical component is probable, as animals will slow when entering a bacterial lawn or a viscous substance (Sawin et al. 2000). Genetic evidence suggests that the presence of food increases overall levels of the modulatory neurotransmitter serotonin (Avery and Horvitz 1990; Colbert and Bargmann 1997; Sze et al. 2000). At least for locomotion and chemosensation, serotonin probably acts humorally rather than synaptically, as laser ablation of single pairs of serotonergic neurons has little effect on locomotory behavior and exogenous serotonin can compensate for genetic deficiencies in serotonin production (Sawin et al. 2000; Chao et al. 2004).
1.3.1.2. Serotonin and Modulation of Chemosensation
C. elegans exhibits adaptation to attractive chemosensory stimuli. Briefly, if C. elegans are pre-exposed to benzaldehyde (an attractive odorant sensed by AWC neurons), their acute response to benzaldehyde is diminished compared to naive C. elegans that were not pre-exposed. When C. elegans are first deprived of food, adaptation to benzaldehyde is enhanced. Exogenous serotonin restores normal adaptation response, consistent with serotonin as the endogenous signal mediating olfactory adaptation (Colbert and Bargmann 1997).
Serotonin also has effects on the neural circuitry that mediates the avoidance response to the volatile chemical repellant, octanol. This effect is twofold. First, serotonin acts directly on the ASH sensory neurons to modulate response to octanol (Chao et al. 2004; Hilliard et al. 2005). Exogenous serotonin can directly potentiate Ca2+ influx in ASH neurons (Hilliard et al. 2005). Serotonin probably modulates this response via at least three serotonin receptors: the GPCR SER-5, which is expressed in ASH neurons (Harris and Kommuniecki, Personal Communication); the serotonin-gated chloride channel MOD-1 (Ranganathan et al. 2000), which potentially functions in AIY and/or AIB interneurons; and the GPCR SER-1, which potentially acts in RIA interneurons (Harris et al. 2009). The Gα protein, GPA-11, which is also expressed in ASH neurons, is also required for serotonin modulation (Chao et al. 2004), although SER-5 may not directly couple to GPA-11 (G. Harris and R. Komuniecki, personal communication). Second, serotonin alters the neural circuitry used to detect octanol. When serotonin levels are high, C. elegans primarily utilize ASH neurons to sense octanol; when serotonin levels are decreased (caused by mild starvation, for instance), the ADL and AWB neurons are also recruited to sense octanol (Chao et al. 2004). In this second pathway, it is unclear whether serotonin acts presynaptically in sensory neurons or postsynaptically in interneurons (or elsewhere). Recruitment of ADL and AWB does not require GPA-11, but does require the glutamate receptor GLR-1, which is expressed in the command interneurons immediately postsynaptic to the sensory neurons. This suggests that serotonin may act on the command interneurons, but further studies are needed to clarify the mechanism of this switch in circuitry. Since the life cycle of C. elegans is so short and the developmental program of the organism is essentially invariant, plasticity at the level of neural circuitry may be preferred over de novo synaptogenesis. Clearly, this plasticity is exerted at multiple levels of the neural circuit, and reflects the complexity of the modulatory input into a very simple behavioral response.
1.3.1.3. Food and Modulation of CO2 Avoidance
Food also modulates C. elegans avoidance of CO2, although signaling pathways other than those used for serotonin may be involved. Avoidance of CO2 is partially suppressed by starvation, which activates the DAF-2 insulin and DAF-7 TGFβ signaling pathways (Bretscher et al. 2008; Hallem and Sternberg 2008). These signaling pathways are involved in nutritional signaling during dauer formation (see Fielenbach and Antebi 2008 for review). The nuclear hormone receptor NHR-49, which is involved in transcriptional regulation of fat metabolism (Van Gilst et al. 2005), is also required for CO2 avoidance (Hallem and Sternberg 2008), although it is unclear whether starvation changes NHR-49 expression. Finally, C. elegans modulates CO2 avoidance by integrating sensory information on food and O2 levels. During hypoxia, low levels of O2 prevent activation of the O2-dependent prolyl hydroxylase, EGL-9, thereby preventing the proteolytic degradation of the transcription factor HIF-1 (Epstein et al. 2001; Jiang et al. 2001). egl-9 mutants do not avoid CO2 (in fact, they are attracted to CO2), but this defect is suppressed by loss of function in hif-1. Furthermore, wild-type animals that have been exposed to hypoxic conditions also stop avoiding CO2. When C. elegans are presented with a choice between being attracted to O2 or repelled by CO2, their decision is modulated by the presence or absence of food, as well as their genotype. In strains with an NPR-1 neuropeptide receptor with the 215V polymorphism (which is found in solitary feeding strains such as the laboratory wild-type reference strain N2), CO2 avoidance prevails over O2 attraction. In contrast, in strains with the 215F polymorphism (such as the social feeding CB4856 Hawaiian isolate), the presence of food causes O2 response to dominate and the absence of food causes the CO2 response to dominate (Bretscher et al. 2008).
1.3.2. Other Modulatory Neurotransmitters
The catecholamines dopamine, tyramine, and octopamine also play roles in modulation of response to octanol, although the details are less clear. Dopamine seems to dampen signaling in the ASH neuron response to octanol. rgs-3 mutants are defective for response to high concentrations of octanol, due to excessive signaling in ASH neurons (see above; Ferkey et al. 2007). This behavioral defect can be suppressed by decreasing the function of cat-2, which encodes the C. elegans homolog of tyrosine hydroxylase and is essential for dopamine biosynthesis (Lints and Emmons 1999). Other investigators have found that while serotonin potentiates response to octanol, exogenous dopamine suppresses this serotonin-dependent potentiation, consistent with a role for dopamine in dampening signaling (Wragg et al. 2007). Tyramine and octopamine negatively modulate octanol response as well. Octopamine probably acts directly on ASH neurons via the octopamine receptor coded by designated gene F14D12.6, whereas tyramine probably acts indirectly on the octanol response circuit via the receptor TYRA-3, which is expressed in the dopaminergic ADE/CEP neurons (Wragg et al. 2007).
1.3.3. Temperature
C. elegans is able to detect and respond to changes in temperature (reviewed in Mori et al. 2007), and behavioral response toward soluble attractants is modulated by temperature, but the mechanism of this modulation is unclear. Temperature is sensed by the AFD neurons (Mori 1999). In response to step changes in temperature, Ca2+ influx increases transiently in AFD neurons (Kimura et al. 2004; Ramot et al. 2008). C. elegans are attracted to NaCl sources most effectively at temperatures ±5°C of the temperature at which they are raised (Dusenbery et al. 1978). Na+ and Cl– ions are sensed by the functionally asymmetric ASEL and ASER sensory neurons, respectively. One study has suggested that ASEL and ASER are modulated by temperature differently (Adachi et al. 2008). Interestingly, AWC-mediated attraction to isoamyl alcohol does not seem to be affected by temperature (Adachi et al. 2008).
1.4. HOW DOCAENORHABDITIS ELEGANS NAVIGATE?
C. elegans transitions between three general states of locomotion (Gray et al. 2005; Wakabayashi 2004; Zhao et al. 2003). These states are best understood in the context of foraging behavior (i.e., food searching). In the presence of food, C. elegans moves forward slowly, and turns or reversals are very frequent. These reversals are generally short in duration, which results in lower turn angles. This behavior is termed dwelling, and allows animals to stay in the vicinity of food. Initially, when animals are removed from food, the frequency of reversals remains high, but turn angles increase, resulting in larger direction changes. Furthermore, their rate of locomotion increases tenfold. This results in a rapid scan of the local environment for food in a behavior termed area-restricted search (Hills et al. 2004). When animals are deprived of food for longer periods of time, reversal rates decrease whereas speed remains high; this results in dispersal, which presumably allows animals to move away from an exhausted food source.
How is chemotaxis impacted by these changes in feeding and locomotion status? Taxis toward a point source of any attractant can be essentially thought of as an exercise in how gradients are interpreted by a sensory system: in other words, how an organism understands its spatial orientation relative to its environment. Detailed motion-tracking and behavioral analysis suggests that C. elegans navigates gradients using a strategy called a biased random walk for salt chemotaxis (Pierce-Shimomura et al. 1999; Ryu and Samuel 2002; Zariwala et al. 2003). As animals increase forward movement and decrease turns, they tend to move away from a previous location, and as they decrease forward movement and increase turns, they tend to stay in the same general location. Changes in locomotory patterns are controlled by sensory input via chemosensory neurons (Gray et al. 2005; Wakabayashi 2004).
The activity of the AWC-mediated chemotaxis toward volatile attractants has been characterized at the level of the neural circuit in detail (Chalasani et al. 2007). Using the analogy of retinal bipolar cells again (Yang 2004) (see Figure 1.6), AWC neurons function as OFF-sensing neurons; that is, AWC neurons are tonically active in the absence of odorant, are suppressed in the presence of odorant, and are stimulated by odorant removal. AWC neurons form glutamatergic synapses onto the AIB and AIY neurons. AIB neurons are also OFF-sensing neurons; they receive excitatory glutamatergic input via the AMPA/kainate-like receptor GLR-1. Activation of AIB neurons by odorant removal results in more turning, whereas inactivation of AIB by the presence of odorant results in less turning. In contrast, the AIY neurons are ON-sensing neurons. They receive inhibitory glutamatergic input via the chloride-gated glutamate channel GLC-3 (Horoszok et al. 2001). Inhibition of AIY neurons by odorant removal results in more turning, whereas activation of AIY by the presence of odorant results in less turning. The net result is longer runs (fewer turns) toward a point source of odorant when animals are moving up a concentration gradient of odorant and shorter runs (more turns) when animals are moving down a concentration gradient (Figure 1.7).
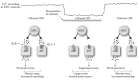
FIGURE 1.7
C. elegans navigation up a concentration gradient toward a point source of attractant. AWC and AIB neurons are OFF-sensing neurons and are active in the absence of odorant; AIY neurons are ON-sensing neurons and are active in the presence of odorant. (more...)
It is interesting to note the many parallels between AWC-AIB-AIY synapses and photoreceptor-bipolar OFF-bipolar ON synapses. Sensory neurons in both systems use GPCR signaling involving Giα-like proteins, receptor-like transmembrane guanylyl cyclases, and cGMP-gated channels. Both systems have neurons that do not spike with action potentials, but use tonic-graded neurotransmitter release, which is well suited for detecting graded stimuli such as a gradient of odorant emanating from a point source. Additionally, both systems use glutamate as both an excitatory and inhibitory neurotransmitter (although mammals utilize inhibitory metabotropic glutamate receptors and nematodes use hyperpolarizing chloride-gated channels). It will be interesting to determine how other sensory modalities integrate into chemotaxis. For instance, salt-sensing and temperature-sensing neurons also synapse onto AIB and AIY interneurons (White et al. 1986; Chen et al. 2006), and these two types of stimuli can modulate chemotaxis.
The asymmetrical salt-sensing ASEL and ASER neurons are also organized in an ON–OFF manner (Suzuki et al. 2008). ASEL neurons, which function as ON-sensing neurons, sense Na+ and exhibit a transient Ca2+ increase when an increase in Na+ levels is detected. ASER neurons, on the other hand, function as OFF-sensing neurons; they sense Cl– and exhibit a transient Ca2+ increase when a decrease in Cl– levels is detected. Activation of ASEL increases forward movement, whereas activation of ASER increases turning. Thus, navigation toward salt uses the same general principle as navigation toward point sources of volatile odorants. It is unclear whether the difference between tonic activation of AWC neurons and transient activation of ASE neurons is physiologically relevant, as different handling techniques were used to immobilize C. elegans for imaging in these two studies (Chalasani et al. 2007; Suzuki et al. 2008).
1.5. WHAT DO CAENORHABDITIS ELEGANS SMELL?
C. elegans has traditionally been used as a model organism in the laboratory. Hence, little is known about its natural history and ecology (Kiontke 2006). As C. elegans becomes more familiar to researchers outside the original C. elegans community, some interesting studies have emerged on how C. elegans olfaction might play a role in the greater world outside a Petri dish and an incubator.
1.5.1. Olfactory Responses in Foraging
An innate behavior of Caenorhabditis nematodes is to search for food. Although little is known about what it eats in the wild, it seems reasonable to assume that a major source of food is various forms of soil bacteria. The olfactory system of C. elegans obviously plays a role in finding food and there is innate preference for certain types of bacterial odors. For instance, C. elegans is strongly attracted to the pathogenic soil bacteria Serratia marcesens (Zhang et al. 2005), despite the fact that S. marcesens is actually toxic to C. elegans. This incongruity suggests that the interaction of C. elegans and bacteria may be as complex as other prey/predator interactions.
The chemical cues produced by bacteria that are innately attractive to C. elegans are not well studied. One early study suggested that ammonium ions, which are attractive to C. elegans as both soluble and volatile odorants (Frøkjaer-Jensen et al. 2008), are produced by some attractive bacterial species, such as E. coli and the pseudomonad species Pseudomonas aeruginosa and P. fluorescens (Andrew and Nicholas 1976). Another candidate class of chemical attractants is acylated homoserine lactones (AHSLs), signaling molecules secreted by Gram-negative bacteria that utilize quorum-sensing (QS) systems. QS is a signaling mechanism employed by bacteria wherein molecules called autoinducers (such as AHSLs) are expressed at low levels by bacterial cells. As cell density increases, autoinducer concentration increases, which leads to a positive feedback loop and increased autoinducer expression. The autoinducer then activates signaling pathways, leading to physiological changes in bacteria (reviewed in Von Bodman et al. 2008). Purified AHSLs are weak chemoattractants for C. elegans (Beale et al. 2006). This makes sense, as the presence of AHSLs would indicate a potentially abundant food source. Chemotaxis toward other identified QS molecules, such as quinolones and boronated furanones, has not been tested.
There are also molecules made by bacteria that are inherently repulsive to C. elegans. Certain strains of S. marcescens produce cyclic lipopentapeptides called serrawettins. Serrawettins are biosurfactants that are essential for a type of bacterial behavior called swarming motility (Matsuyama et al. 1992). When lawns of E. coli bacteria mixed with the serrawettins W2 or W3 are spotted onto Petri dishes, naive C. elegans animals avoid E. coli. A mutant S. marcescens strain that does not produce serrawettin W2 is not repulsive to C. elegans, even though the bacteria remain pathogenic for C. elegans. The related serrawettin W1 does not alter the animal’s preference for E. coli. This suggests that avoidance of specific serrawettins is a specific chemosensory response and not a response to general changes in surface tension caused by a biosurfactant or general pathogenicity of the bacteria. This avoidance response is mediated by the AWB neurons (which are known to detect volatile repellants) via cGMP signaling through the TAX-2/TAX-4 cGMP-gated channels. The context of chemical presentation is also important. In contrast to the Petri dish-based population assay described above, serrawettins W1, W2, and W3 induce acute avoidance responses when they are directly applied to the animal in a liquid drop. Surfactin, a biosurfactant produced by Bacillus subtilis, repels C. elegans in the plate assay, but is inactive in the drop assay (Pradel et al. 2007). Collectively, these results suggest that while some responses to bacterial metabolites are innate, others are highly context-dependent and may involve factors such as the immediate chemical environment and feeding state.
C. elegans also learns to avoid toxic bacteria after pre-exposure, a process that requires serotonin (Zhang et al. 2005). There is some plasticity in this “learned behavior.” As mentioned above, acylated homoserine lactone (ASHL) autoinducer compounds are weak attractants for naive C. elegans, but when they are paired with the pathogenic Pseudomonas strain, PAO1, AHSLs become weakly repulsive (Beale et al. 2006). Thus, other sensory stimuli (e.g., gustatory stimuli and/or innate immune responses) can influence the response of C. elegans to bacterial-specific odorants.
1.5.2. Olfactory Interactions with Other Organisms
Nematodes represent an extremely diverse phylum, with individual species occupying distinct ecological niches (Kiontke and Sudhaus 2006). Thus, there are probably species-specific evolutionary adaptations to olfactory behavior that facilitate particular lifestyles. One remarkable example of this is in the olfactory behavior of genus Pristionchus nematodes, of which one species, P. pacificus, has now been sequenced (Dieterich et al. 2008). Pristionchus was previously grouped into Diplogastridae (Blaxter et al. 1998), but more recently in Rhabditidae (Sommer 2006; Kiontke and Fitch 2005). Pristionchus nematodes have recently been found to adapt a necromenic lifestyle. Unlike true parasites that feed off live hosts, necromenic Pristionchus nematodes feed off the corpses of their host insects (Pristionchus also feeds on bacteria, similar to C. elegans). P. maupasi is one species from this genus that feeds off the corpses of adult cockchafer beetles (Melolontha spp.) (Hong et al. 2008a). Cockchafers spend three years in pupal and larval stages, and then metamorphose into a short-lived, three-week adult stage, which is the preferred host for P. maupasi. In chemotaxis assays, P. maupasi is strongly attracted to cuticle washes from adult, but not larval cockchafers. There are at least two classes of chemical components from the cuticle washes that are chemoattractants. First is the cockchafer sex pheromone phenol, which attracts P. maupasi but not related Pristionchus species. Second are plant defense volatiles, including compounds such as green leaf alcohol ((Z)-3-hexen-1-ol, or GLA) and linalool. These are chemicals whose production is upregulated by plants when they are grazed upon by herbivorous insects, and their presence strongly synergizes the chemoattraction to phenol in the nematodes. Only the adult cockchafers feed upon these plants and they release a small amount of plant defense volatiles that they ingest. Since only the adult cockchafers feed on plants, and since P. maupasi prefer adult cockchafers, the sex pheromone phenol acts as a species-specific cue, whereas the plant defense volatiles act as a temporal cue to indicate adulthood of the host insect (Figure 1.8).
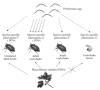
FIGURE 1.8
Host species and lifestage specificity in Pristionchus nematodes. The nematodes are attracted to two different attractants: beetle species-specific pheromones and plant defense volatiles (PDVs). This second attractant serves as a temporal cue for the (more...)
Parasite-host specific interactions between different Pristionchus species and insects probably involve other species-specific odorants, as different species show different chemoattractive profiles for odorants typically associated with their natural habitats and insect hosts (Hong and Sommer 2006). In P. pacificus, the homolog of C. elegans cGMP-activated protein kinase (PKG) EGL-4 may be required for attraction to the pheromone ETDA (Hong et al. 2008b). Natural variation in the P. pacificus egl-4 locus may account for natural variation in attraction to ETDA in wild P. pacificus strains isolated from different parts of the world (Hong et al. 2008b), similar to how natural variation in NPR-1 accounts for variation in feeding behavior in C. elegans (de Bono and Bargmann 1998) (Figure 1.8).
From the reverse perspective, other organisms may exploit the chemosensory behavior of nematodes. For instance, the roots of legume plants such as Cajanus cajan release soluble flavonoids that attract nitrogen-fixing symbiotic rhizobacteria to their root nodules (Pandya et al. 1999). However, since soluble cues can only act at a short distance (a few millimeters), they are probably insufficient to attract rhizobacteria to root nodules from greater distances. C. elegans are attracted to the legume Medicago truncatula, whereas they are indifferent to Arabidopsis, a nonlegume plant. This attraction is mediated by the volatile attractant, dimethyl sulfide, which is released by M. truncatula (but not Arabidopsis) and can presumably act at longer distances. C. elegans that have been grazing on S. melioti can populate the root nodules of aseptically grown legumes with the rhizobacteria under laboratory conditions (Horiuchi et al. 2005). While it is unknown whether C. elegans and M. truncatula occupy similar ecological niches in nature, it is known that agriculturally cultivated legumes initially lacking symbiotic rhizobacteria eventually acquire rhizobacteria over time (Purchase and Nutman 1957). These experiments suggest that soil nematodes might play a role in enriching and dispersing microbial diversity within the rhizosphere.
1.6. CONCLUDING REMARKS
Understanding a nervous system, even the relatively small C. elegans nervous system, is no small task. Clearly, the analysis of the nervous system of C. elegans is allowing researchers to identify the basic mechanisms involved in chemosensation and related behaviors. Forward genetic approaches like those used in C. elegans excel at identifying previously unsuspected mechanisms and pathways. We can expect the invertebrate communities to continue to lead the field of neuroscience in defining the basic mechanisms of cellular and molecular behavioral neuroscience. However, completely understanding the cellular and molecular basis of behavior will require the neuroscience community to integrate information obtained from disparate sources and experimental approaches. This becomes more difficult as we learn more, in part because of the large amount of information even the C. elegans community has generated. A major challenge for the next decade will not just be continuing to define the basic principles of neuronal function, but also consolidating the data into useful models and databases.
ACKNOWLEDGMENTS
We thank Gareth Harris and Rick Komuniecki for sharing unpublished data, Paul Orwin, John Skillman, and Dave Polcyn for useful discussions, Morris Maduro and Dan Bumbarger for critical reading of the manuscript, and Zeynep Altun, Jon Law, and Horla Varlan for sharing images. This work was supported in part by the National Institute of Drug Abuse (M.Y.C.) and the National Institute of General Medical Sciences (A.C.H.). All images © Michael Chao except where noted; images are released under the Creative Commons Attribution-Share Alike 3.0 License (see http://creativecommons.org/licenses/by-sa/3.0/us/ for details). Color versions of images are available upon request.
REFERENCES
- Adachi R., Wakabayashi T., Oda N., Shingai R. Modulation of Caenorhabditis elegans chemotaxis by cultivation and assay temperatures. Neurosci Res. 2008;60:300–306. [PubMed: 18192049]
- Andrew P.A., Nicholas W. Effect of bacteria on dispersal of Caenorhabditis elegans (Rhabditidae). Nematologica. 1976;22:451–61.
- Ashrafi K., Chang F.Y., Watts J.L., Fraser A., Kamath R., Ahringer J., Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–72. [PubMed: 12529643]
- Avery L., Horvitz H.R. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool. 1990;253:263–70. [PubMed: 2181052]
- Bargmann C.I., Avery L. Laser killing of cells in Caenorhabditis elegans. Methods Cell Biol. 1995;48:225–50. [PMC free article: PMC4442485] [PubMed: 8531727]
- Bargmann C.I. Chemosensation in C. elegans. In: The C. elegans Research Community, editor. WormBook. 2006. doi/10.1895/wormbook.1.123.1, http://www
.wormbook.org. - Bargmann C.I., Hartwieg E., Horvitz H.R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–27. [PubMed: 8348618]
- Bargmann C.I., Horvitz H.R. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–42. [PubMed: 1660283]
- Battu G., Hoier E.F., Hajnal A. The C. elegans G-protein-coupled receptor SRA-13 inhibits RAS/MAPK signalling during olfaction and vulval development. Development. 2003;130:2567–77. [PubMed: 12736202]
- Beale E., Li G., Tan M.W., Rumbaugh K.P. Caenorhabditis elegans senses bacterial autoinducers Appl Environ Microbiol. 2006;72:5135–37. [PMC free article: PMC1489312] [PubMed: 16820523]
- Birnby D.A., Link E.M., Vowels J.J., Tian H., Colacurcio P.L., Thomas J.H. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics. 2000;155:85–104. [PMC free article: PMC1461074] [PubMed: 10790386]
- Blaxter M.L., De Ley P., Garey J.R., Liu L.X., Scheldeman P., Vierstraete A., Vanfleteren J.R., Mackey L.Y., Dorris M., Frisse L.M., Vida J.T., Thomas W.K. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. [PubMed: 9510248]
- Brenner S. Forward. In: Wood W.B., editor. The Nematode Caenorhabditis Elegans. Cold Spring Harbor Laboratory Press; Cold Spring Harbor NY: 1988. pp. x–xi.
- Bretscher A.J., Busch K.E., DeBono M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:8044–49. [PMC free article: PMC2410288] [PubMed: 18524954]
- Brockie P.J., Mellem J.E., Hills T., Madsen D.M., Maricq A.V. The C. elegans glutamate receptor subunit NMR-1 is required for slow NMDA-activated currents that regulate reversal frequency during locomotion. Neuron. 2001;31:617–30. [PubMed: 11545720]
- Buck L., Axel R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell. 1991;65:175–87. [PubMed: 1840504]
- Bush C.F., Hall R.A. Olfactory receptor trafficking to the plasma membrane. Cell Mol Life Sci. 2008;65:2289–95. [PubMed: 18373063]
- Chalasani S.H., Chronis N., Tsunozaki M., Gray J.M., Ramot D., Goodman M.B., Bargmann C. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450:63–70. [PubMed: 17972877]
- Chalfie M., Sulston J.E., White J.G., Southgate E., Thomson J.N., Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–64. [PMC free article: PMC6565008] [PubMed: 3981252]
- Chang A.J., Chronis N., Karow D.S., Marletta M.A., Bargmann C. A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol. 2006;4:e274. [PMC free article: PMC1540710] [PubMed: 16903785]
- Chang S., Johnston R.J., Frøkjaer-Jensen C., Lockery S., Hobert O. MicroRNAs act sequentially and asymmetrically to control chemosensory laterality in the nematode. Nature. 2004;430:785–89. [PubMed: 15306811]
- Chang S., Johnston R.J., Hobert O. A transcriptional regulatory cascade that controls left/right asymmetry in chemosensory neurons of C. elegans. Genes Dev. 2003;17:2123–37. [PMC free article: PMC196454] [PubMed: 12952888]
- Chao M., Komatsu H., Fukuto H.S., Dionne H., Hart A. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci USA. 2004;101:15512–17. [PMC free article: PMC524441] [PubMed: 15492222]
- Chen B.L., Hall D.H., Chklovskii D.B. Wiring optimization can relate neuronal structure and function. Proc Natl Acad Sci USA. 2006;103:4723–28. [PMC free article: PMC1550972] [PubMed: 16537428]
- Chess A., Simon I., Cedar H., Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78:823–34. [PubMed: 8087849]
- Cheung B.H., Cohen M., Rogers C., Albayram O., De Bono M. Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr Biol. 2005;15:905–17. [PubMed: 15916947]
- Chronis N., Zimmer M., Bargmann C. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Methods. 2007;4:727–31. [PubMed: 17704783]
- Chuang C.F., VanHoven M.K., Fetter R.D., Verselis V.K., Bargmann C. An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans. Cell. 2007;129:787–99. [PubMed: 17512411]
- Clark D.A., Biron D., Sengupta P., Samuel A.D. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J Neurosci. 2006;26:7444–51. [PMC free article: PMC6674189] [PubMed: 16837592]
- Coates J.C., De Bono M. Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature. 2002;419:925–29. [PubMed: 12410311]
- Coburn C.M., Bargmann C.I. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. [PubMed: 8893026]
- Colbert H.A., Bargmann C.I. Environmental signals modulate olfactory acuity, discrimination, and memory in Caenorhabditis elegans. Learn Mem. 1997;4:179–91. [PubMed: 10456062]
- Colbert H.A., Smith T.L., Bargmann C.I. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–69. [PMC free article: PMC6573730] [PubMed: 9334401]
- Colosimo M.E., Brown A., Mukhopadhyay S., Gabel C., Lanjuin A.E., Samuel A.D., Sengupta P. Identification of thermosensory and olfactory neuron-specific genes via expression profiling of single neuron types. Curr Biol. 2004;14:2245–51. [PubMed: 15620651]
- Davis R.E., Stretton A.O. Passive membrane properties of motorneurons and their role in long-distance signaling in the nematode Ascaris. J Neurosci. 1989;9:403–14. [PMC free article: PMC6569814] [PubMed: 2918369]
- Dawson T.M., Arriza J.L., Jaworsky D.E., Borisy F.F., Attramadal H., Lefkowitz R.J., Ronnett G.V. Beta-adrenergic receptor kinase-2 and beta-arrestin-2 as mediators of odorant-induced desensitization. Science. 1993;259:825–29. [PubMed: 8381559]
- de Bono M., Bargmann C.I. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–89. [PubMed: 9741632]
- Dieterich C., Clifton S., Schuster L., Chinwalla A., Delehaunty K., Dinkelacker I., Fulton L., Fulton R., Godfrey J., Minx P., Mitreva M., Roeseler W., Tian H., Witte H., Yang S., Wilson R., Sommer R.J. The Pristionchus pacificus genome provides a unique perspective on nematode lifestyle and parasitism. Nat Genet. 2008;40:1193–98. [PMC free article: PMC3816844] [PubMed: 18806794]
- Dusenbery D., Anderson G., Anderson E. Thermal acclimation more extensive for behavioral parameters than for oxygen consumption in the nematode Caenorhabditis elegans. J Exp Zool. 1978;206:191–97.
- Dusenbery D.B. Analysis of chemotaxis in the nematode Caenorhabditis elegans by countercurrent separation. J Exp Zool. 1974;188:41–47. [PubMed: 4822548]
- Dwyer N.D., Adler C.E., Crump J.G., L’Etoile N.D., Bargmann C.I. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31:277–87. [PubMed: 11502258]
- Dwyer N.D., Troemel E.R., Sengupta P., Bargmann C.I. Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell. 1998;93:455–66. [PubMed: 9590179]
- Epstein A.C., Gleadle J.M., McNeill L.A., Hewitson K.S., O’Rourke J., Mole D.R., Mukherji M., Metzen E., Wilson M.I., Dhanda A., Tian Y.M., Masson N., Hamilton D.L., Jaakkola P., Barstead R., Hodgkin J., Maxwell P.H., Pugh C.W., Schofield C.J., Ratcliffe P.J. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation Cell. 2001;107:43–54. [PubMed: 11595184]
- Esposito G., Di Schiavi E., Bergamasco C., Bazzicalupo P. Efficient and cell specific knock-down of gene function in targeted C. elegans neurons. Gene. 2007;395:170–76. [PubMed: 17459615]
- Etchberger J., Lorch A., Sleumer M., Zapf R., Jones S., Marra M., Holt R., Moerman D., Hobert O. The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev. 2007;21:1653–74. [PMC free article: PMC1899474] [PubMed: 17606643]
- Ferkey D., Hyde R., Haspel G., Dionne H., Hess H., Suzuki H., Schafer W., Koelle M., Hart A. C. elegans G protein regulator RGS-3 controls sensitivity to sensory stimuli Neuron. 2007;53:39–52. [PMC free article: PMC1855255] [PubMed: 17196529]
- Fiala A. Olfaction and olfactory learning in Drosophila Recent progress. Curr Opin Neurobiol. 2007;17:720–26. [PubMed: 18242976]
- Fielenbach N., Antebi A. C. elegans dauer formation and the molecular basis of plasticity Genes Dev. 2008;22:2149–65. [PMC free article: PMC2735354] [PubMed: 18708575]
- FrøkjaerJensen C., Ailion M., Lockery S.R. Ammonium-acetate is sensed by gustatory and olfactory neurons in Caenorhabditis elegans. PLoS ONE. 2008;3:e2467. [PMC free article: PMC2413426] [PubMed: 18560547]
- Fukuto H.S. Genetic analysis of chemosensation in C. elegans: The role of G protein-coupled receptor kinase. PhD Thesis. Harvard University. 2004
- Fukuto H.S., Ferkey D.M., Apicella A., Lans H., Sharmeen T., Chen W., Lefkowitz R.J., Jansen G., Schafer W., Hart A. G protein-coupled receptor kinase function is essential for chemosensation in C. elegans. Neuron. 2004;42:581–93. [PubMed: 15157420]
- Gimelbrant A.A., Haley S.L., McClintock T.S. Olfactory receptor trafficking involves conserved regulatory steps. J Biol Chem. 2001;276:7285–90. [PubMed: 11060288]
- Goodman M.B., Hall D.H., Avery L., Lockery S.R. Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron. 1998;20:763–72. [PMC free article: PMC4444786] [PubMed: 9581767]
- Gray J.M., Hill J.J., Bargmann C. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2005;102:3184–91. [PMC free article: PMC546636] [PubMed: 15689400]
- Gray J.M., Karow D.S., Lu H., Chang A.J., Chang J.S., Ellis R.E., Marletta M.A., Bargmann C. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–22. [PubMed: 15220933]
- Hajdu-Cronin Y.M., Chen W.J., Patikoglou G., Koelle M.R., Sternberg P.W. Antagonism between G(o)alpha and G(q)alpha in Caenorhabditis elegans: The RGS protein EAT-16 is necessary for G(o) alpha signaling and regulates G(q)alpha activity. Genes Dev. 1999;13:1780–93. [PMC free article: PMC316886] [PubMed: 10421631]
- Hall D.H., Altun Z.F. C. elegans Atlas. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY; 2008.
- Hall D.H., Russell R.L. The posterior nervous system of the nematode Caenorhabditis elegans: Serial reconstruction of identified neurons and complete pattern of synaptic interactions. J Neurosci. 1991;11:1–22. [PMC free article: PMC6575198] [PubMed: 1986064]
- Hallem E.A., Sternberg P.W. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:8038–43. [PMC free article: PMC2430355] [PubMed: 18524955]
- Hart A.C., Kass J., Shapiro J.E., Kaplan J.M. Distinct signaling pathways mediate touch and osmosensory responses in a polymodal sensory neuron. J Neurosci. 1999;19:1952–58. [PMC free article: PMC6782580] [PubMed: 10066248]
- Hilliard M., Apicella A., Kerr R., Suzuki H., Bazzicalupo P., Schafer W. In vivo imaging of C. elegans ASH neurons: Cellular response and adaptation to chemical repellents. EMBO J. 2005;24:63–72. [PMC free article: PMC544906] [PubMed: 15577941]
- Hilliard M., Bargmann C., Bazzicalupo P. C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail Curr Biol. 2002;12:730–34. [PubMed: 12007416]
- Hilliard M., Bergamasco C., Arbucci S., Plasterk R.H., Bazzicalupo P. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO J. 2004;23:1101–11. [PMC free article: PMC380969] [PubMed: 14988722]
- Hills T.P., Brockie J., Maricq A.V. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci. 2004;24:1217–25. [PMC free article: PMC6793574] [PubMed: 14762140]
- Hirotsu T., Saeki S., Yamamoto M., Iino Y. The Ras-MAPK pathway is important for olfaction in Caenorhabditis elegans. Nature. 2000;404:289–93. [PubMed: 10749212]
- Hollinger S., Hepler J.R. Cellular regulation of RGS proteins: Modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–59. [PubMed: 12223533]
- Hong R.L., Sommer R.J. Chemoattraction in Pristionchus nematodes and implications for insect recognition. Curr Biol. 2006;16:2359–65. [PubMed: 17141618]
- Hong R.L., Svatos A., Herrmann M., Sommer R.J. Species-specific recognition of beetle cues by the nematode Pristionchus maupasi. Evol Dev. 2008a;10:273–79. [PubMed: 18460089]
- Hong R.L., Witte H., Sommer R.J. Natural variation in Pristionchus pacificus insect pheromone attraction involves the protein kinase EGL-4. Proc Natl Acad Sci USA. 2008b;105:7779–84. [PMC free article: PMC2409422] [PubMed: 18509055]
- Horiuchi J., Prithiviraj B., Bais H., Kimball B., Vivanco J. Soil nematodes mediate positive interactions between legume plants and rhizobium bacteria. Planta. 2005;222:848–57. [PubMed: 16025342]
- Horoszok L., Raymond V., Sattelle D.B., Wolstenholme A.J. GLC-3 A novel fipronil and BIDN-sensitive, but picrotoxinin-insensitive, L-glutamate-gated chloride channel subunit from Caenorhabditis elegans. Br J Pharmacol. 2001;132:1247–54. [PMC free article: PMC1572670] [PubMed: 11250875]
- Horvitz H.R., Chalfie M., Trent C., Sulston J.E., Evans P.D. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–14. [PubMed: 6805073]
- Hukema R., Rademakers S., Dekkers M., Burghoorn J., Jansen G. Antagonistic sensory cues generate gustatory plasticity in Caenorhabditis elegans. EMBO J. 2006;25:312–22. [PMC free article: PMC1383522] [PubMed: 16407969]
- Jansen G., Thijssen K.L., Werner P., van der Horst M., Hazendonk E., Plasterk R.H. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet. 1999;21:414–19. [PubMed: 10192394]
- Jansen G., Weinkove D., Plasterk R.H. The G-protein gamma subunit GPC-1 of the nematode C. elegans is involved in taste adaptation. EMBO J. 2002;21:986–94. [PMC free article: PMC125897] [PubMed: 11867526]
- Jiang H., Guo R., Powell-Coffman J.A. The Caenorhabditis elegans hif-1 gene encodes a bHLH- PAS protein that is required for adaptation to hypoxia. Proc Natl Acad Sci USA. 2001;98:7916–21. [PMC free article: PMC35443] [PubMed: 11427734]
- Johnston R.J., Hobert O. A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature. 2003;426:845–49. [PubMed: 14685240]
- Johnston R.J., Hobert O. A novel C. elegans zinc finger transcription factor, lsy-2, required for the cell type-specific expression of the lsy-6 microRNA. Development. 2005;132:5451–60. [PubMed: 16291785]
- Kahn-Kirby A.H., Dantzker J.L., Apicella A., Schafer W., Browse J., Bargmann C., Watts J.L. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell. 2004;119:889–900. [PubMed: 15607983]
- Kaplan J.M., Horvitz H.R. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1993;90:2227–31. [PMC free article: PMC46059] [PubMed: 8460126]
- Kerr R., Lev-Ram V., Baird G., Vincent P., Tsien R.Y., Schafer W.R. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron. 2000;26:583–94. [PubMed: 10896155]
- Kimura K.D., Miyawaki A., Matsumoto K., Mori I. The C. elegans thermosensory neuron AFD responds to warming. Curr Biol. 2004;14:1291–95. [PubMed: 15268861]
- Kiontke K., Sudhaus W. Ecology of Caenorhabditis species. In: The C. elegans Research Community, editor. WormBook. 2006. doi/10.1895/wormbook.1.37.1, http://www
.wormbook.org. [PMC free article: PMC4780885] [PubMed: 18050464] - Kiontke K., Fitch D.H.A. The Phylogenetic relationships of Caenorhabditis and other rhabditids. In: The C. elegans Research Community, editor. WormBook. 2005. doi/10.1895/wormbook.1.11.1, http://www
.wormbook.org. [PMC free article: PMC4781183] [PubMed: 18050394] - Komatsu H., Jin Y.H., L’Etoile N., Mori I., Bargmann C.I., Akaike N., Ohshima Y. Functional reconstitution of a heteromeric cyclic nucleotide-gated channel of Caenorhabditis elegans in cultured cells. Brain Res. 1999;821:160–68. [PubMed: 10064800]
- Komatsu H., Mori I., Rhee J.S., Akaike N., Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–18. [PubMed: 8893027]
- Kuhara A., Inada H., Katsura I., Mori I. Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron. 2002;33:751–63. [PubMed: 11879652]
- L’Etoile N.D., Bargmann C.I. Olfaction and odor discrimination are mediated by the C. elegans guanylyl cyclase ODR-1. Neuron. 2000;25:575–86. [PubMed: 10774726]
- L’Etoile N.D., Coburn C.M., Eastham J., Kistler A., Gallegos G., Bargmann C. The cyclic GMP-dependent protein kinase EGL-4 regulates olfactory adaptation in C. elegans. Neuron. 2002;36:1079–89. [PubMed: 12495623]
- Lans H., Rademakers S., Jansen G. A network of stimulatory and inhibitory Galpha-subunits regulates olfaction in Caenorhabditis elegans. Genetics. 2004;167:1677–87. [PMC free article: PMC1470997] [PubMed: 15342507]
- Lee R.Y., Sawin E.R., Chalfie M., Horvitz H.R., Avery L. EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J Neurosci. 1999;19:159–67. [PMC free article: PMC3759158] [PubMed: 9870947]
- Lehman C.W., Lee J.D., Komives C.F. Ubiquitously expressed GPCR membrane-trafficking orthologs. Genomics. 2005;85:386–91. [PubMed: 15718105]
- Lints R., Emmons S.W. Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFbeta family signaling pathway and a Hox gene. Development. 1999;126:5819–31. [PubMed: 10572056]
- Lockery S.R., Lawton K.J., Doll C., Faumont S., Coulthard S.M., Thiele T.R., Chronis N., McCormick K.E., Goodman M.B., Pruitt B.L. Artificial dirt: Microfluidic substrates for nematode neurobiology and behavior. J Neurophysiol. 2008;99:3136–43. [PMC free article: PMC2693186] [PubMed: 18337372]
- Loer C.M., Kenyon C.J. Serotonin-deficient mutants and male mating behavior in the nematode Caenorhabditis elegans. J Neurosci. 1993;13:5407–17. [PMC free article: PMC6576401] [PubMed: 8254383]
- Malnic B., Hirono J., Sato T., Buck L.B. Combinatorial receptor codes for odors. Cell. 1999;96:713–23. [PubMed: 10089886]
- Matsuki M., Kunitomo H., Iino Y. Goalpha regulates olfactory adaptation by antagonizing Gqalpha-DAG signaling in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2006;103:1112–17. [PMC free article: PMC1347976] [PubMed: 16418272]
- Matsuyama T., Kaneda K., Nakagawa Y., Isa K., Hara-Hotta H., Yano I. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J Bacteriol. 1992;174:1769–76. [PMC free article: PMC205777] [PubMed: 1548227]
- Mellem J.E., Brockie P.J., Zheng Y., Madsen D.M., Maricq A.V. Decoding of polymodal sensory stimuli by postsynaptic glutamate receptors in C. elegans. Neuron. 2002;36:933–44. [PubMed: 12467596]
- Miyawaki A., Llopis J., Heim R., McCaffery J.M., Adams J.A., Ikura M., Tsien R.Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–87. [PubMed: 9278050]
- Mori I. Genetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Annu RevGenet. 1999;33:399–422. [PubMed: 10690413]
- Mori I., Sasakura H., Kuhara A. Worm thermotaxis: A model system for analyzing thermosensation and neural plasticity. Curr Opin Neurobiol. 2007;17:712–19. [PubMed: 18242074]
- Nakai J., Ohkura M., Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–41. [PubMed: 11175727]
- Nystul T.G., Goldmark J.P., Padilla P.A., Roth M.B. Suspended animation in C. elegans requires the spindle checkpoint. Science. 2003;302:1038–41. [PubMed: 14605367]
- Okochi Y., Kimura K.D., Ohta A., Mori I. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J. 2005;24:2127–37. [PMC free article: PMC1150891] [PubMed: 15920475]
- Ortiz C.O., Etchberger J.F., Posy S.L., Frøkjaer-Jensen C., Lockery S., Honig B., Hobert O. Searching for neuronal left/right asymmetry: Genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics. 2006;173:131–49. [PMC free article: PMC1461427] [PubMed: 16547101]
- Ouellet J., Li S., Roy R. Notch signalling is required for both dauer maintenance and recovery in C. elegans. Development. 2008;135:2583–92. [PubMed: 18599512]
- Palmitessa A., Hess H.A., Bany I.A., Kim Y.M., Koelle M.R., Benovic J.L. Caenorhabditis elegans arrestin regulates neural G protein signaling and olfactory adaptation and recovery J Biol Chem. 2005;280:24649–62. [PubMed: 15878875]
- Pandya S., Iyer P., Gaitonde V., Parekh T., Desai A. Chemotaxis of Rhizobium sp.S2 toward Cajanus cajan root exudate and its major components. Curr Microbiol. 1999;38:205–9. [PubMed: 10069855]
- Park S., Hwang H., Nam S.W., Martinez F., Austin R.H., Ryu W.S. Enhanced Caenorhabditis elegans locomotion in a structured microfluidic environment. PLoS ONE. 2008;3:e2550. [PMC free article: PMC2430527] [PubMed: 18575618]
- Peppel K., Boekhoff I., McDonald P., Breer H., Caron M.G., Lefkowitz R.J. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J Biol Chem. 1997;272:25425–28. [PubMed: 9325250]
- Perkins L.A., Hedgecock E.M., Thomson J.N., Culotti J.G. Mutant sensory cilia in the nematodeCaenorhabditis elegans. Dev Biol. 1986;117:456–87. [PubMed: 2428682]
- Pierce-Shimomura J.T., Faumont S., Gaston M.R., Pearson B.J., Lockery S.R. The homeobox gene lim-6 is required for distinct chemosensory representations in C. elegans. Nature. 2001;410:694–98. [PubMed: 11287956]
- Pierce-Shimomura J.T., Morse T.M., Lockery S.R. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci. 1999;19:9557–69. [PMC free article: PMC6782915] [PubMed: 10531458]
- Pifferi S., Boccaccio A., Menini A. Cyclic nucleotide-gated ion channels in sensory transduction. FEBS Lett. 2006;580:2853–59. [PubMed: 16631748]
- Poole R.J., Hobert O. Early embryonic programming of neuronal left/right asymmetry in C. elegans. Curr Biol. 2006;16:2279–92. [PubMed: 17141609]
- Pradel E., Zhang Y., Pujol N., Matsuyama T., Bargmann C., Ewbank J.J. Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104:2295–2300. [PMC free article: PMC1892944] [PubMed: 17267603]
- Purchase H.F., Nutman P.S. Studies on physiology of nodule formation IV. The influence of bacteria numbers in the rhizosphere on nodule initiation. Ann Bot. 1957;21:439–54.
- Ramot D., Macinnis B., Goodman M.B. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat Neurosci. 2008;11:908–15. [PMC free article: PMC2587641] [PubMed: 18660808]
- Ranganathan R., Cannon S.C., Horvitz H.R. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature. 2000;408:470–75. [PubMed: 11100728]
- Roayaie K., Crump J.G., Sagasti A., Bargmann C.I. The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron. 1998;20:55–67. [PubMed: 9459442]
- Robertson H.M., Thomas J.H. The putative chemoreceptor families of C. elegans, WormBook, ed. The C. elegans Research Community, WormBook. 2006 doi/10.1895/wormbook.1.66.1, http://www
.wormbook.org. [PMC free article: PMC4781013] [PubMed: 18050473] - Rogers C., Persson A., Cheung B., De Bono M. Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr Biol. 2006;16:649–59. [PubMed: 16581509]
- Rogers C., Reale V., Kim K., Chatwin H., Li C., Evans P., De Bono M. Inhibition of Caenorhabditis elegans social feeding by FMRFamide-related peptide activation of NPR-1. Nat Neurosci. 2003;6:1178–85. [PubMed: 14555955]
- Ross E.M., Wilkie T.M. GTPase-activating proteins for heterotrimeric G proteins: Regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. [PubMed: 10966476]
- Ryu W., Samuel A.D. Thermotaxis in Caenorhabditis elegans analyzed by measuring responses to defined thermal stimuli. J Neurosci. 2002;22:5727–33. [PMC free article: PMC6758190] [PubMed: 12097525]
- Sagasti A., Hisamoto N., Hyodo J., Tanaka-Hino M., Matsumoto K., Bargmann C.I. The CaMKII UNC-43 activates the MAPKKK NSY-1 to execute a lateral signaling decision required for asymmetric olfactory neuron fates. Cell. 2001;105:221–32. [PubMed: 11336672]
- Sambongi Y., Nagae T., Liu Y., Yoshimizu T., Takeda K., Wada Y., Futai M. Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Neuroreport. 1999;10:753–57. [PubMed: 10208543]
- Sarin S., O’Meara M.M., Flowers E.B., Antonio C., Poole R.J., Didiano D., Johnston R.J., Chang S., Narula S., Hobert O. Genetic screens for Caenorhabditis elegans mutants defective in left/right asymmetric neuronal fate specification. Genetics. 2007;176:2109–30. [PMC free article: PMC1950618] [PubMed: 17717195]
- Sawin E.R., Ranganathan R., Horvitz H.R. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–31. [PubMed: 10896158]
- Sengupta P., Chou J.H., Bargmann C.I. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl Cell. 1996;84:899–909. [PubMed: 8601313]
- Sommer R.J. Pristionchus pacificus. The C. elegans Research Community, editor. Worm Book. 2006 doi/10.1895/wormbook.1.102.1 http://www
.wormbook.org. [PMC free article: PMC4781627] [PubMed: 18050490] - Sposito G. The Chemistry of Soils. Oxford University Press; New York: 2008.
- Srinivasan J., Durak O., Sternberg P.W. Evolution of a polymodal sensory response network. BMC Biol. 2008;6:52. [PMC free article: PMC2636771] [PubMed: 19077305]
- Suzuki H., Thiele T.R., Faumont S., Ezcurra M., Lockery S.R., Schafer W. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature. 2008;454:114–17. [PMC free article: PMC2984562] [PubMed: 18596810]
- Sze J.Y., Victor M., Loer C., Shi Y., Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–64. [PubMed: 10676966]
- Tobin D., Madsen D., Kahn-Kirby A., Peckol E., Moulder G., Barstead R., Maricq A., Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–18. [PubMed: 12160748]
- Troemel E.R., Chou J.H., Dwyer N.D., Colbert H.A., Bargmann C.I. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell. 1995;83:207–18. [PubMed: 7585938]
- Troemel E.R., Kimmel B.E., Bargmann C.I. Reprogramming chemotaxis responses: Sensory neurons define olfactory preferences in C. elegans. Cell. 1997;91:161–69. [PubMed: 9346234]
- Troemel E.R., Sagasti A., Bargmann C.I. Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell. 1999;99:387–98. [PubMed: 10571181]
- Tsunozaki M., Chalasani S.H., Bargmann C. A behavioral switch: cGMP and PKC signaling in olfactory neurons reverses odor preference in C. elegans. Neuron. 2008;59:959–71. [PMC free article: PMC2586605] [PubMed: 18817734]
- Uchida O., Nakano H., Koga M., Ohshima Y. The C. elegans che-1 gene encodes a zinc finger transcription factor required for specification of the ASE chemosensory neurons. Development. 2003;130:1215–24. [PubMed: 12588839]
- Van Gilst M.R., Hadjivassiliou H., Jolly A., Yamamoto K.R. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005;3:e53. [PMC free article: PMC547972] [PubMed: 15719061]
- Van Voorhies W.A., Ward S. Broad oxygen tolerance in the nematode Caenorhabditis elegans. J Exp Biol. 2000;203:2467–78. [PubMed: 10903161]
- VanHoven M.K., Bauer Huang S.L., Albin S.D., Bargmann C. The claudin superfamily protein NSY-4 biases lateral signaling to generate left-right asymmetry in C. elegans olfactory neurons. Neuron. 2006;51:291–302. [PubMed: 16880124]
- Von Bodman S., Willey J., Diggle S. Cell-cell communication in bacteria: United we stand. J Bacteriol. 2008;190:4377–91. [PMC free article: PMC2446813] [PubMed: 18456806]
- Vonstetina S., Treinin M., Milleriii D. The motor circuit. Int Rev Neurobiol. 2005;69:125–67. [PubMed: 16492464]
- Vowels J.J., Thomas J.H. Multiple chemosensory defects in daf-11 and daf-21 mutants of Caenorhabditis elegans. Genetics. 1994;138:303–16. [PMC free article: PMC1206150] [PubMed: 7828815]
- Wakabayashi T. Neurons regulating the duration of forward locomotion in Caenorhabditis elegans. Neurosci Res. 2004;50:103–11. [PubMed: 15288503]
- Ward S., Thomson N., White J.G., Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–37. [PubMed: 1112927]
- Watts J.L., Browse J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:5854–59. [PMC free article: PMC122866] [PubMed: 11972048]
- Wes P.D., Bargmann C.I. C. elegans odour discrimination requires asymmetric diversity in olfactory neurons Nature. 2001;410:698–701. [PubMed: 11287957]
- White J., Southgate E., Thomson J.N., Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans Soc Lond. 1986;314:1–340. [PubMed: 22462104]
- Wicks S.R., de Vries C.J., van Luenen H.G., Plasterk R.H. CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Dev Biol. 2000;221:295–307. [PubMed: 10790327]
- Wicks S.R., Yeh R.T., Gish W.R., Waterston R.H., Plasterk R.H. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat Genet. 2001;28:160–64. [PubMed: 11381264]
- Wragg R., Hapiak V., Miller S., Harris G., Gray J., Komuniecki P., Komuniecki R. Tyramine and octopamine independently inhibit serotonin-stimulated aversive behaviors in Caenorhabditis elegans through two novel amine receptors. J Neurosci. 2007;27:13402–12. [PMC free article: PMC6673087] [PubMed: 18057198]
- Yang X.L. Characterization of receptors for glutamate and GABA in retinal neurons. Prog Neurobiol. 2004;73:127–50. [PubMed: 15201037]
- Yu S., Avery L., Baude E., Garbers D.L. Guanylyl cyclase expression in specific sensory neurons: A new family of chemosensory receptors. Proc Natl Acad Sci USA. 1997;94:3384–87. [PMC free article: PMC20379] [PubMed: 9096403]
- Zariwala H.A., Miller A.C., Faumont S., Lockery S.R. Step response analysis of thermotaxis in Caenorhabditis elegans. J Neurosci. 2003;23:4369–77. [PMC free article: PMC6741103] [PubMed: 12764126]
- Zhang Y., Lu H., Bargmann C. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–84. [PubMed: 16281027]
- Zhao B., Khare P., Feldman L., Dent J.A. Reversal frequency in Caenorhabditis elegans represents an integrated response to the state of the animal and its environment. J Neurosci. 2003;23:5319–28. [PMC free article: PMC6741178] [PubMed: 12832557]
- An overview of C. elegans biology.[Methods Mol Biol. 2006]An overview of C. elegans biology.Strange K. Methods Mol Biol. 2006; 351:1-11.
- Review C. elegans: a sensible model for sensory biology.[J Neurogenet. 2020]Review C. elegans: a sensible model for sensory biology.Iliff AJ, Xu XZS. J Neurogenet. 2020 Sep-Dec; 34(3-4):347-350. Epub 2020 Nov 16.
- Review A Transparent window into biology: A primer on Caenorhabditis elegans.[WormBook. 2015]Review A Transparent window into biology: A primer on Caenorhabditis elegans.Corsi AK, Wightman B, Chalfie M. WormBook. 2015 Jun 18; :1-31. Epub 2015 Jun 18.
- Of worms and men.[J Neurogenet. 2020]Of worms and men.White J. J Neurogenet. 2020 Sep-Dec; 34(3-4):255-258.
- Using C. elegans for aging research.[Invertebr Reprod Dev. 2015]Using C. elegans for aging research.Tissenbaum HA. Invertebr Reprod Dev. 2015 Jan 30; 59(sup1):59-63. Epub 2014 Dec 9.
- From Odors to Behaviors in Caenorhabditis elegans - The Neurobiology of Olfactio...From Odors to Behaviors in Caenorhabditis elegans - The Neurobiology of Olfaction
Your browsing activity is empty.
Activity recording is turned off.
See more...