NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Menini A, editor. The Neurobiology of Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2010.
2.1. INSECT OLFACTORY RECEPTORS
Most organisms rely on their olfactory system to detect and analyze olfactory cues in the environment, cues that are subsequently utilized in the context of behavior. Odorants are recognized by olfactory sensory neurons (OSNs), which are located in the olfactory epithelia of vertebrates or in the dendrites of olfactory sensory cells within the sensilla on the antennal surface of insects (Buck and Axel 1991; Carlson 2001; Chess et al. 1992; Vosshall et al. 2000). The OSNs express odorant receptors (ORs), which are related to seven transmembrane G-protein-coupled receptors (GPCR) and transduce odorant binding to cellular excitation (see also Chapter 7). The olfactory system of the genetic model organism, the fruit fly Drosophila melanogaster, has been the focus of numerous investigations. Drosophila has two pairs of olfactory organs, the antennae and the maxillary palps. Each antenna contains about 1200 OSNs housed in a total of 410 olfactory sensilla covering the antenna, while the maxillary palp has about 120 OSNs and 60 olfactory sensilla (Laissue and Vosshall 2007). The sensilla are of three morphological types: basiconic sensilla, trichoid sensilla, and coeloconic sensilla (Venkatesh and Singh 1984). Across insects, there is a beautiful diversity of sensillar morphologies, including pore plate sensilla, campaniform sensilla, sensilla ampullacea, and more (Kleineidam and Tautz 1996; Steinbrecht 1996). In recent studies, the OR gene family of D. melanogaster has been identified and shown to comprise 62 defned members (Table 2.1) (Clyne et al. 1999; Gao and Chess 1999; Vosshall et al. 1999). Several studies have been dedicated to characterize the molecular receptive ranges of identified ORs (Dobritsa et al. 2003; Hallem and Carlson 2006; Pelz et al. 2006).
TABLE 2.1
List of Sensilla, OR type, Innervated Glomerulus, and Best Ligand for Drosophila Melanogaster.
ORs are expressed following a conserved pattern in Drosophila as well as in mammals (see Chapter 7). Every OSN typically expresses only one type of OR (as well as the ubiquitous Or83b, see below). However, a given OSN can also coexpress up to three conventional ORs determining a specific molecular response profile along with the Or83 coreceptor (Table 2.1). OSNs expressing the same type of OR, converge to a single glomerulus in the antennal lobe (AL), which represents the first olfactory neuropil in the insect brain (see below). However, a few cases of 1:2 and 2:1 innervation ratios in Drosophila have also been described (Couto et al. 2005; Fishilevich and Vosshall 2005).
Interestingly, ORs in Drosophila possess no significant homology to other known GPCRs. However, the total length of the receptor proteins and the size of the internal and external loops are similar to most members of the GPCR protein family (Clyne et al. 1999). The identified genes of the OR family are highly divergent, even among drosophilid species. At least two receptors generate alternative splicing products (Clyne et al. 1999; Robertson et al. 2003).
While the functional organization of the olfactory system in vertebrates and insects shows clear similarities, the atypical heteromeric and topological design of the ORs in Drosophila appears to be insect-specific. Almost all OSNs express a chaperon receptor, called Or83b. Or83b is highly conserved in many insect species (Dahanukar et al. 2005), and it is also possible to functionally exchange Or83b with orthologous Or83b proteins from other insects (Hill et al. 2002; Jones et al. 2005; Krieger et al. 2003; Pitts et al. 2004).
Or83b has a membrane topology that differs from the related GPCR topology, with the N-terminus and the most conserved loops located in the cytoplasm (Benton et al. 2006). Furthermore, Or83b has a 70-amino acid long insert in the second intracellular loop, which might be responsible for the distinct function of Or83b vis-à-vis the remaining members of the insect OR family (Benton et al. 2006). In recent studies, it was shown that Or83b is a nonselective cation channel that interacts directly with the endogenous ligand-tuned OR (Sato et al. 2008; Wicher et al. 2008). In addition, Or83b is needed for the functional integration of receptor proteins in the dendritic part of the OSNs within the sensillum shaft (Larsson et al. 2004; Neuhaus et al. 2005per).
The possible role of the G-proteins expressed in OSNs remains unclear, and the involvement of G-proteins in the signal pathway is a controversial topic. While Sato et al. (2008) give evidence primarily for a direct ligand gating of the receptor complex, Wicher et al. (2008) show that the Or83b channel is also activated by internal cAMP or cGMP. The activation of the G-protein-coupled signal cascade appears considerably slower than the ionotropic response of the receptor complex. The G-protein-coupled signal cascade modulates the activation of the odorant through either cAMP and the subsequent opening of cyclic nucleotide-gated channels or through an IP3-mediated signal cascade (Krieger and Breer 1999). In OSNs of the maxillary palps, a phospholipase C could be substantiated as an important part of the IP3-signal pathway (Riesgo-Escovar et al. 1995). Even though molecular response profiles are known for many Drosophila receptors, the interaction of ORs with the component of the signal cascade is not yet well understood. An important task will be to localize both the binding sites of ORs with the odor ligand, and with the intracellular signaling components. Furthermore, it has to be solved which domains of the ORs and the Or83b channel interact with each other, and how this interaction leads to a functional cellular response.
Not all OSNs express ORs that are related to GPCRs. In Drosophila, a recent study has identified the receptors that are expressed in coeloconic sensilla. These receptors are related to the gene family encoding ionotropic glutamate receptors and were therefore named ionotropic receptors (IRs) (Benton et al. 2009). Their sequence shows that they do not belong to the hitherto described kainate, AMPA, or NMDA classes. IRs are expressed in a combinatorial fashion in OSNs housed in coeloconic sensilla that respond to many distinct odors, but do not express either insect ORs or gustatory receptors.
Another important player in the primary odor response consists of a family of odorant binding proteins (OBPs). OBPs are assumed to guide the odorous molecules through the sensillum lymph to allow interaction with a specific OR in the dendritic membrane of the OSNs, but more complex models of their action draw a multifunctional picture (Kaissling 2001). Evidence from various insect species indicates a complex interplay of several sensory molecules in the subsequent pheromone reception and transduction process (Vogt 2003; Vosshall 2008). While specialized pheromone binding proteins (PBPs) are supposed to shuttle hydrophobic pheromone molecules through the sensillum lymph toward specific receptors (Leal 2003; Xu et al. 2005), a so-called sensory neuron membrane protein is suggested to stabilize a functional receptor complex or dock PBPs to the receptor site (Vogt 2003; Jin et al. 2008). The former concept that a PBP just passively transports pheromones to sensory cells has been challenged by studies showing that PBPs specifically interact with pheromones and undergo distinct conformational changes (Mohl et al. 2002; Grosse-Wilde et al. 2006; Laughlin et al. 2008). Interestingly, the conformational change of a PBP has been found to be sufficient for neuronal activation, suggesting a direct interaction of a pheromone/PBP complex with the receptor (Laughlin et al. 2008). One identified member of the PBP family is the OBP, LUSH, which is expressed and secreted exclusively by non neuronal support cells in trichoid sensilla (Kim et al. 1998; Shanbhag et al. 2005). A study by Xu et al. (2005) shows that LUSH is required for activation of pheromone-sensitive OSNs in Drosophila. LUSH mutants lack the detection of the pheromone, cis-vaccenyl acetate, at the physiological as well as at the behavioral level. Unlike the situation in vertebrates, where OBPs are present in the olfactory mucosa and thus potentially in contact with all OSNs, the compartmentalization of OSNs into sensilla gives insects a much more specific control: OPBs are selectively present in particular sensilla, and are more likely to contribute to odor-specific response profles.
2.2. THE ANTENNAL LOBE (AL)
The first neuropil in the insect brain that processes olfactory information is the AL, a structure common to all insects, and secondarily lost in some anosmic species (Strausfeld and Hildebrand 1999). It is analogous in structure and function to the vertebrate olfactory bulb, but evolved independently (Strausfeld and Hildebrand 1999). However, unlike in mammals, insect neurons generally have their cell bodies outside the brain, and synaptic computation is accomplished entirely in the somaless neuropil. Similarly, while the mammalian bulb is structured in layers (glomerular layer, soma layer, etc.,) the insect AL is structured entirely in glomerular units, which are the interaction sites of OSNs, local neurons (LNs), projection neurons (PNs), and others (Figure 2.1A and B). Most synap-tic contacts are within olfactory glomeruli (Boeckh and Tolbert 1993; Gascuel and Masson 1991).
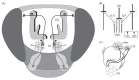
FIGURE 2.1
Olfactory system in the fruit fy, Drosophila melanogaster. (A) Schematic view of the fy head, with a cut-open brain. Olfactory sensory neurons (OSNs) are located on the antennae and project into the antennal lobe, where they interact with local neurons (more...)
Even though the structure and function of ALs appears close to universal, there is considerable diversity of AL organization across insects (Schachtner et al. 2005). Indeed, insects have an evolutionary history of over 400 million years, and most modern insect orders were already present 250 million years ago, allowing for considerable divergent evolution (Grimaldi and Engel 2004). Thus, glomerular arrangements, numbers, position of soma groups, neuron populations and more, differ across species. Several examples will be considered in the following section.
2.2.1. Sensory Neuron Axons
OSN numbers differ among species. Drosophila has ~1200 OSNs in both sexes (Stocker et al. 1990). Manduca have ~300,000 OSNs on each antenna (Oland and Tolbert 1988; Sanes and Hildebrand 1976b). Male honeybees (drones) have ~300,000 OSNs, while (female) worker bees have ~65,000 (Esslen and Kaissling 1976). Hemimetabolous insects increase the number of receptors with each nymphal instar (Chapman 2002 Ochieng et al. 1998); Schafer and Sanchez 1973). Adult cockroaches have ~150,000 OSNs (Ernst et al. 1977), locusts have between 50,000 (Ernst et al. 1977) and 105,000 (Anton et al. 2002) OSNs as adults.
OSN axons project to the AL via the antennal tract(s). In honeybees, there are four tracts, T1–T4 (Suzuki 1975). T1 innervates ~70 glomeruli, T2 ~7, T3 ~70, and T4 ~7 glomeruli (Arnold et al. 1983; Flanagan and Mercer 1989a; Galizia et al. 1999a). This segregation corresponds to distinct groups of PNs that leave the AL following different tracts (see below) (Abel et al. 2001; Kirschner et al. 2006). At this time, the functional signifcance of this segregation remains unknown. Mechanosensory and gustatory axons from the antenna also use the antennal tracts and bypass the AL toward the antennal mechanosensory and motor center in the dorsal lobe (Gewecke 1979; Kloppenburg et al. 1997; Mobbs 1982; Mobbs 1982). Efferent motor neurons from the dorsal lobe innervate the antennal muscles and use the antennal nerves to enter the antenna, together with axons from modulatory neurons, which most likely use biogenic amines as transmitters. The function of these modulatory neurons has not yet been elucidated directly, but an effect of biogenic amines on OSN responses has been shown (Pophof 2002).
2.2.2. Projection of Olfactory Sensory Neurons (OSNs) into the Antennal Lobe (AL)
Each OSN axon innervates a single ipsilateral glomerulus in the AL in most species. In flies, OSNs form an axonal commissure between the two Als, and individual axons innervate both homologous glomeruli (Stocker 1994; Stocker 1994). Each bilaterally innervated glomerulus receives equal input from both antennae (Vosshall et al. 2000). A few glomeruli in Drosophila are innervated unilaterally. These are the glomeruli V, VL1, VP1, as well as VP2 and VP3, which collect input from sensilla located on the Drosophila arista (Stocker 2001 Stocker et al. 1983). OSNs may innervate the entire glomerulus (e.g., those innervating T4 glomeruli in bees), or just the glomerular periphery (as the other bee glomeruli, or many fruit fly glomeruli). The innervation may follow the antennal topology: in bees, OSNs from the distal antennal segments innervate the outer layer of the glomerular cap, and more proximal OSNs innervate the central layers (Pareto 1972). Somatotopic projections are also known from Manduca (Christensen et al. 1995) and Periplaneta (Hösl 1990).
2.2.3. Targeting Mechanisms of Olfactory Sensory Neurons (OSNs)
How do receptor axons find the right glomerulus? The molecular mechanisms have been studied in Drosophila, where the relationship OR to innervated glomerulus is best known (Couto et al. 2005; Fishilevich and Vosshall 2005). Generally, each OSN expresses one (or sometimes a few) OR, and OSNs that express the same OR converge on a single glomerulus in each hemisphere (Vosshall et al. 2000). Examples of OSNs that express more than one OR include dOr33c and dOr85e, which are coexpressed in pb2a OSNs (palp basiconic type 2, neuron a), and where both contribute to these neurons’ odor responses (Goldman et al. 2005). dOr22a and dOr22b are coexpressed in ab3a neurons (antennal basiconic sensillum 3, neuron a), but no functional role for dOr22b has yet been found (Dobritsa et al. 2003). OSNs expressing dOR67d innervate the two glomeruli DA1 and VA6 (Fishilevich and Vosshall 2005).
While ORs are important for axon targeting to the glomerulus in mammalian OSNs (Feinstein et al. 2004; Feinstein and Mombaerts 2004; Wang et al. 1998), this is not the case in insects (Dobritsa et al. 2003). Several transcription factors are known to be required for correct OSN targeting in Drosophila (Rodrigues and Hummel 2008). These include the Src homology domain 2 (SH2)/SH3 adapter Dock (Ang et al. 2003), the serine/threonine kinase Pak (Ang et al. 2003), the cell surface proteins N-cadherin (Hummel and Zipursky 2004), the POU domain transcriptional factor Acj6 (Komiyama et al. 2004), and the immunoglobulin Dscam to be expressed in PNs or LNs (Hummel et al. 2003; Zhu et al. 2006). The transmembrane protein, semaphorin-1a, does not mediate large-scale target fnding, but it does mediate short-range precision and axon convergence into a single glomerulus (Lattemann et al. 2007). Probably, some sort of combinatorial mechanism of these factors is used for identity/target determination. For example, dOr22a targeting is independent of Dscam, but in other axons, Dscam mutation disrupts OSN targeting either partially or completely (Hummel et al. 2003). Acj6 is expressed in all OSNs in the antenna, but only in some in the maxillary palps. Axon-axon interaction is necessary for glomerular convergence (Komiyama et al. 2004), and glial cells are necessary too (see below) (Tolbert et al. 2004). The identity of the glomerulus itself, however, is not determined by OSNs, but rather by PNs that form “protoglomeruli” (Jefferis et al. 2004).
2.2.4. Antennal Lobe (AL) Glomeruli
Almost all animals, whether insects or mammals, have evolved olfactory glomeruli (Hildebrand and Shepherd 1997). Even though, on a small scale, the neighborhood relationship of glomeruli may follow a logical rule dictated by their molecular response profile (Sachse et al. 1999), this is not a general rule (Linster et al. 2005). Thus, glomeruli might reflect that there is no physical property common to all odors that can be mapped onto the two dimensions of the brain surfact. This differs from spatial position of visual stimuli, or frequency coding for sounds, cases in which topological arrangements are known (brain maps). However, not all sensory patterns that have physically defined dimensions are reflected with a corresponding topology in the brain. Colors, for example, derive from the spectral properties of photon wavelength, but rather than following this linear arrangement, all color-vision systems in animals create artificial dimensions using different color-sensitive photoreceptors and creating subsequent color-opponency channels (e.g., the green–red and the yellow–blue dimensions in humans). The glomerular organization in olfaction may reflect the multidimensionality of olfaction per se, or just reflect that many receptor types are necessary to cover all perceivable stimuli. Minimalistically, a glomerular organization may result from the need to have OSNs with the same response properties converging onto one target, thus glomeruli may be “just” the most parsimonious such organization.
Number, shape, and arrangement of glomeruli is a strongly species-specific property: adult Drosophila fruit fly has less than 50 glomeruli (Laissue et al. 1999; Stocker 1994), a moth (Manduca) has ~60 glomeruli (Rospars and Hildebrand 1992; Sanes and Hildebrand 1976c), a cockroach has ~125 glomeruli (Ernst et al. 1977), a worker honeybee ~160 (Flanagan and Mercer 1989a; Galizia et al. 1999a), and some ant species have over 200 (Rospars 1988) or even over 400 glomeruli (Zube et al. 2008). Glomeruli can be densely packed, as in Drosophila, or they can be arranged around a central area of the AL, the coarse neuropil. Individual glomeruli are not uniform: in bees the outer cap is the recipient of OSN input, while the core of each glomerulus is dominated by PNs. Some LNs branch in both the core and the cap of a glomerulus, others do not, or only in some glomeruli. Serotonergic neurons innervate only the cap, dopaminergic neurons only the core of glomeruli in bees. The functional relevance of this glomerular subdivision into cap and core is as yet unknown. Furthermore, in immunostainings for protein kinase C, small circular regions of dense staining are visible within honeybee glomeruli (Grünbaum and Müller 1998). Glomerular subcompartmentalizations have been shown for Drosophila (Laissue et al. 1999), and are also known from vertebrates (Kasowski et al. 1999). Thus, there is an inner life to a glomerulus that remains to be elucidated.
The arrangement of glomeruli in locusts differs from that in most other species (Ignell et al. 2001). Each OSN axon innervates not one, but many glomeruli, and PNs also innervate many of the approximately 1000 glomeruli (Anton and Hansson 1996; Ernst et al. 1977). Even though the AL of locusts differs from other insects, functional properties may be common. For example, application of the chloride channel blocker, picrotoxin, has similar effects on the temporal response structure in PNs in bees (Stopfer et al. 1997) and in locusts (MacLeod and Laurent 1996).
The best knowledge of synaptic connections between neurons in the AL comes from ultrastructural studies in cockroaches (Distler and Boeckh 1996, 1997; Distler et al. 1998b; Malun 1991a, 1991b) (see also Figure 2.1B). Synaptic contacts occur between all neuron types: OSNs synapse onto LNs and onto PNs, and their presynaptic terminals receive input from LNs. LNs synapse onto OSN terminals, onto other LNs, and onto PNs. PNs get input from OSNs and from LNs, and they synapse onto other cells in the AL. The only contact that has not yet been shown is from PNs onto OSNs. Synapses are often dyadic, i.e., one presynaptic element makes contact with two postsynaptic elements, or reciprocal, or even more complex.
One of the major strengths of insects in neuroscience research is the notion of identifiable neurons. Similarly, olfactory glomeruli are also identifiable, and can be mapped from one animal to the next, always within one species, and sometimes for closely related species also. The characteristic arrangement, size, and shape of individual glomeruli helps in identifying glomeruli, and three-dimensional atlases have now been created for a variety of species (Rospars 1988). Honeybee glomeruli are named according to the antennal tract that innervates them (T1–T4) and a number (i.e., T1–1, T1–2, or T3–33) (Flanagan and Mercer 1989a). An electronic atlas is available on the internet (Galizia et al. 1999a). Drosophila glomeruli are named with a letter or two, indicating the AL area, and a number, e.g., DM2 for dorso-medial-2 (Laissue et al. 1999; Stocker et al. 1990). Glomeruli that are identified later can be easily included into both nomenclatures (Couto et al. 2005; Kirschner et al. 2006). Atlases are also available for different moth species (Berg et al. 2002; Huetteroth and Schachtner 2005; Ignell et al. 2005; Masante-Roca et al. 2005; Rospars and Hildebrand 1992; Skiri et al. 2005).
2.2.5. Glomerular Odor Responses
Stimulation with an odor leads to characteristic spatiotemporal glomerular activity patterns (Figure 2.2). Optical imaging of intracellular calcium concentration has been performed in bees (Galizia and Menzel 2001), in flies (Fiala et al. 2002; Wang et al. 2003), in moths (Carlsson et al. 2002; Galizia et al. 2000b; Skiri et al. 2004), and in ants (Galizia et al. 1999b). The conceptional similarity of these results in species other than Drosophila suggests that, also in these species, OSNs expressing the same OR are likely to converge generally onto a single glomerulus (an assertion that is commonly assumed, but not shown). Additional support comes from the observation that in the honeybee, Apis mellifera, the number of OR genes is close to 160, matching the number of glomeruli (Robertson and Wanner 2006). However, in the beetle, Tribolium, there are fewer glomeruli than the number of 341 receptor genes—more research is needed to investigate whether the same processing logic is implemented differently, or whether the logic itself differs (Engsontia et al. 2008).

FIGURE 2.2
Examples for odor-evoked activity in the fruit fly. (A) (left) Responses to the odor isoamyl acetate in OSNs on the antenna (their dendritic compartments) and in the antennal lobe (their axonal compartments) are shown left. The calcium sensor was expressed (more...)
Some glomeruli are narrowly tuned (i.e., respond to a single or, at best, very few substances). Thus, activity in OSNs innervating this glomerules is sufficient information for an animal to behave assuming that the substance is in the environment. Such a specialized system would be able to code for only as many odors as there are glomeruli (which is approximately 43 in the adult Drosophila). Other glomeruli are broadly tuned, and create a combinatorial code. Here, the information carried by individual glomeruli is ambiguous, but by a comparison across glomeruli, odor information becomes precise. Many OSNs are broadly tuned at high concentration, but may be narrowly tuned at concentrations several orders of magnitude lower (Pelz et al. 2004; Røstelien et al. 2000; Stensmyr et al. 2003). In their natural environment, animals experience odorants over a large range of concentrations. Thus, the low-concentration mode may be useful in some situations; here, even though only a single receptor may be active, the lack of activity in the remaining glomeruli would signal the low-concentration mode. Thus, even the low-concentration coding scheme has a combinatorial logic.
Labeled lines are often mentioned in the context of sexual pheromones. However, in most cases, these are also combinatorial signals. Indeed, most pheromones consist of blends of several substances, which activate several glomeruli, and extraction of the correct pheromone-blend information is done by a combinatorial analysis of the respective glomerular activities (Christensen and Hildebrand 2002; Galizia et al. 2000b; Shirokova et al. 2005). Closely related species can thus use the same substances for their species-specific sex-pheromone signals, as long as they use them in a species-specific concentration ratio.
Most glomerular responses are temporally structured, and inherit this property from OSNs: some are inhibited by odors, some show rebound excitation at the end of the stimulus, some fre for a long time irrespective of stimulus duration, others only fre for a very short time, and stop even if the stimulus continues (de Bruyne et al. 2001). This is in addition to the intrinsic temporal complexity that airborne odors always have (Murlis et al. 1992; Vetter et al. 2006). As a consequence, odor-evoked activity patterns are already temporally complex at the input level of the olfactory system. These temporal patterns are further shaped by neural networks within the ALs (see Section 2.2.14). Possibly, these temporal structures are used for odor analysis.
2.2.6. Olfactory Sensory Neuron (OSN) Transmitters
The neurotransmitter used by OSNs is generally believed to be acetylcholine (ACh), which is the primary excitatory transmitter in the insect brain. The evidence is mostly based on the detection of the ACh-synthesizing enzyme, choline acetyltransferase (ChAT) (Bicker 1999b; Kreissl and Bicker 1989). ACh is present in Manduca OSNs (Sanes and Hildebrand 1976a; Stengl et al. 1990). Pressure application of ACh leads to activity (depolarization and hyperpolarization) in moth AL neurons, mediated by nicotinic receptors (Waldrop and Hildebrand 1989). In Drosophila, a ChAT/lacZ tranformant labels OSNs (Yasuyama et al. 1995), but immunoreactivity for ACh is low (Yasuyama and Salvaterra 1999). In all species studied so far, however, labeling was heterogeneous among glomeruli. Therefore, there might be another, as yet unknown, transmitter (or cotransmitter) in OSNs (Homberg et al. 1995; Homberg and Müller 1999; Kreissl and Bicker 1989), or glomeruli might differ in receptor and/or vesicle density.
An important neuronal messenger is nitric oxide (NO). The NO/cGMP system is found in insect’ ALs, but the cells involved may not always be the same. In moths, NO is released by OSNs (Gibson and Nighorn 2000), and is important for proper glomerular development in the AL (Gibson et al. 2001). In cockroaches and locusts, however, NO is not synthesized in LNs (Elphick et al. 1995; Ott and Elphick 2002; Seidel and Bicker 1997). The NO source is unknown for bees, but NO is involved in olfactory habituation, and blocking NO disrupts olfactory discrimination (Bicker 2001; Hosler et al. 2000; Müller and Hildebrandt 2002).
2.2.7. Local Neurons(LNs)
There are ~4000 LNs in bees (Witthöft 1967), ~360 LNs in moths (Manduca sexta) (Homberg et al. 1988), ~100 (GABAergic) LNs in Drosophila (Ng et al. 2002), and ~300 LNs both in cockroaches and locusts (Anton and Homberg 1999).
There are several different LN types in insects. Two classes are distinguished on morphological grounds: one type innervates most if not all glomeruli uniformly (homoLNs), the other innervates only a few (asymmetricLN) (Anton and Homberg 1999; Ernst and Boeckh 1983; Flanagan and Mercer 1989b; Matsumoto and Hildebrand 1981; Sun et al. 1993). Other “local” neurons include a population that innervates some dorsal glomeruli of the AL and areas of the dorsal lobe (Flanagan and Mercer 1989b). Bilateral neurons connecting both ALs have been found in bees (Arnold et al. 1985; Mobbs 1985) and fies (Stocker 1994; Stocker et al. 1990). In fies, neurons differ in their morphology and the innervated glomeruli, with several genetic differences that allow for the generation of specific markers of LN groups (Das et al. 2008). Both GABAergic (inhibitory) and cholinergic (exhitatory) neurons have been characterized (Shang et al. 2007; Silbering et al. 2008).
GABA-like immunoreactivity in LNs has been shown in bees (Schäfer and Bicker 1986), moths (Hoskins et al. 1986), Drosophila (Buchner 1991; Jackson et al. 1990), cockroaches (Distler et al. 1998b; Malun 1991b), and locusts (Ignell et al. 2001; Leitch and Laurent 1996). In bees, there is a small population of histaminergic LNs (Bornhauser and Meyer 1997), which likely acts as an inhibitory transmitter (Sachse and Galizia 2002). Since OSNs in flies express G-protein-coupled glutamate receptors, there probably is a population of glutamatergic LNs that synapse onto (at least some) OSN terminals (Ramaekers et al. 2001). In addition, there are cholinergic LNs (Shang et al. 2007).
LNs often express or coexpress peptides. Among these, allatotropin, allatostatins, tachykinins, FMRF-amide, and other RF-amide peptides have been found. The patterns of peptide antiserum staining differ widely among the species (Davis et al. 1996; Homberg 2002; Homberg et al. 1990; Homberg and Müller 1999; Iwano and Kanzaki 2005; Nässel 1993, 2000; Schachtner et al. 2004). Some neuropeptides are coexpressed with GABA, while others are not.
LNs have sodium action potential in honeybees (Galizia and Kimmerle 2004; Sun et al. 1993), moths (Christensen et al. 1993), and flies (Wilson et al. 2004). In locusts, only nonspiking LNs have been reported so far (Laurent and Davidowitz 1994). In cockroaches, in addition to spiking LNs (Ernst and Boeckh 1983), there is a population of LNs without voltage-dependent sodium channels, and strong intracellular calcium activity (Husch et al. 2009). In bees and moths, intracellular recordings often show multiple spike amplitudes, suggesting either the presence of multiple spike initiation zones, or that LNs are electrically coupled. In some cases, artifcial electrical connections created by the penetrating sharp electrode may create multiple spike heights (Galizia and Kimmerle 2004). Asymmetric LNs in honeybees have distinct odor-response profiles and respond to the odors associated with their main glomerulus (Galizia and Kimmerle 2004). In contrast, homoLNs studied in Drosophila have very broad response profiles, responding to most odors, with activity spread across large parts of arborization (Ng et al. 2002; Silbering et al. 2008; Wilson et al. 2004).
2.2.8. Projection Neurons (PNs)
In honeybees, the lACT and mACT count about 400 fibers each, the mlACT is smaller, giving a total of less than 1000 PNs altogether (Rybak 1994). PNs in bees have also been estimated at 500 (Bicker et al. 1993) or 800 (Hammer 1997). The total number of PNs in Drosophila is estimated to be 150–200 (Stocker et al. 1997). The proportion of multiglomerular PNs with respect to uniglomerular PNs is below 10% in Drosophila.
Uniglomerular PNs branch in a single glomerulus within the AL and innervate both the mushroom bodies (MBs) and the lateral protocerebrum (LP). In most species, uniglomerular PNs form two distinct tracts, one located close to the brain midline (mACT in bees, iACT in other species), and the other traveling laterally (lACT). Multiglomerular neurons branch in several, if not all glomeruli, and generally do not innervate the MBs, but a variety of other areas in the protocerebrum. Generally, multiglomerular PNs use a group of smaller, intermediate tracts. PNs have both input and output synapses within olfactory glomeruli.
In bees, the two uniglomerular PN tracts, lACT and mACT (medial ACT) (Mobbs 1982), innervate a distinct AL hemilobe each. Interestingly, each hemilobe is also innervated by different tracts of the antennal (sensory) nerve: lACT glomeruli are innervated by T1, mACT glomeruli are innervated by T2–T4 (Abel et al. 2001; Bicker et al. 1993; Kirschner et al. 2006). In Manduca, tracts are iACT (inner, with uniglomerular PNs), mACT (medial, multiglomerular PNs, do not innervate the MBs), and oACT (outer, multiglomerular PNs, innervate the MB calyx) (Homberg et al. 1988). Fly tracts are named inner tract (iACT, traveling along the brain midline), which forms the strongest input to the MB calyces, mACT and inner-medial tract (imACT), which have some axons that innervate the MB calyces, while most axons target only the LP, and outer tract (oACT), which does not innervate the MB calyces (Figure 2.1C). All tracts innervate the LP (Stocker 1994; Stocker et al. 1990; Strausfeld et al. 2003). The GH146 line labels PNs with somata in the anterodorsal cluster (adPN) and lateral cluster (lPN), which have axons that use the iACT and send their dendrites to about 30–35 stereotypical glomeruli (Jefferis et al. 2001; Marin et al. 2002; Wong et al. 2002). GH146 also labels at least four PNs that use the mACT and at least one PN that uses the oACT. PNs from a given glomerulus always branch in the same MB target area (Jefferis et al. 2007). The POU domain transcription factors, Acj6 and Drifter, are expressed in adPNs and lPNs, respectively, and are required for their dendritic targeting (Komiyama et al. 2003). Interestingly, these genes are also necessary for OSN targeting (see above).
The situation in locusts is clearly different from that in other insects. In locusts, only a single ACT close to the brain midline connects the ALs to the MB (Leitch and Laurent 1996), and a few minor tracts connect the AL to other areas (Ignell et al. 2001). PNs are not uniglomerular, but branch in a limited number of glomeruli within the AL (Anton et al. 2002; Laurent et al. 1996). This is reminiscent of the multiple glomeruli innervated by individual OSNs in this species, but the two do not form corresponding groups, i.e., PNs innervate groups of glomeruli that do not correspond to groups innervated by an OSN (Anton et al. 2002). Therefore, glomerular groups in locusts cannot be functionally analogous to single glomeruli in other insects.
Many, but possibly not all, uniglomerular PNs have ACh as their transmitter. In bees, mACT PNs are AChE positive, showing that they use ACh (Kreissl and Bicker 1989). Furthermore, Kenyon cells (KCs) express nicotinic ACh receptors, and are activated by ACh (Bicker and Kreissl 1994). In moths, ~67 somata associated with the AL stain for AChE, and the outer ACT leading to the LP and the MB is stained (Homberg et al. 1995). The situation in Drosophila is similar, with at least one tract strongly stained for AChE (Yasuyama and Salvaterra 1999). In locusts, the ACT tract stains for AChE (Homberg 2002). However, whether the remaining PNs are also cholinergic and do not stain for some biochemical reason, or whether they use another—as yet unknown—transmitter remains to be elucidated.
GABAergic multiglomerular PNs have been shown in bees (Schäfer and Bicker 1986), moths (Hoskins et al. 1986), and flies (K. Ito pers. comm.).
Responses in insect PNs have been measured at the single cell level with electrophysiological techniques in a variety of species (Abel et al. 2001; Christensen et al. 1998a, 1998b; Galizia and Kimmerle 2004; Hansson et al. 1991; Müller et al. 2002; Wilson and Laurent 2005), including fruit flies (Bhandawat et al. 2007; Olsen and Wilson 2008; Schlief and Wilson 2007). Optical methods were successful in bees after loading PNs with calcium-sensitive dyes (Sachse and Galizia 2002, 2003), and in flies after genetically expressing activity-sensitive proteins (Fiala et al. 2002; Ng et al. 2002; Silbering and Galizia 2007; Silbering et al. 2008; Wang et al. 2003; Yu et al. 2004) (Figure 2.2). PNs are spontaneously active in insects, including honeybees (Abel et al. 2001; Galán et al. 2006), moths (Christensen et al. 1998b), Drosophila (Wilson et al. 2004), cockroaches (Boeckh et al. 1987), and locusts (Perez-Orive et al. 2002). Against this activity background, responses to odors can be both excitatory and inhibitory. PN responses are shaped by inhibitory networks within the AL (Bhandawat et al. 2007; Christensen et al. 1998a; Olsen and Wilson 2008; Sachse and Galizia 2002; Schlief and Wilson 2007; Silbering and Galizia 2007; Silbering et al. 2008; Wilson and Laurent 2005).
Instantaneous response frequencies of excitatory responses can be several hundreds of Hertz, and persist over the duration of a 2-s stimulus.
2.2.9. Feedback Neurons and Biogenic Amines
Insect brains have characteristic, very large neurons that stain with antibodies against biogenic amines (dopamine, serotonin, octopamine, histamine) and innervate large areas of the brain (Bicker 1999a; Homberg 2002; Monastirioti 1999; Nässel 1999; Pfüger and Stevenson 2005; Roeder 1999; Stevenson and Sporhase-Eichmann 1995). These neurons are believed to have a modulatory function, including up and down regulation, thresholding, motivational states, attention, and learning (Bicker 1999a; Hammer and Menzel 1998; Homberg and Müller 1999). Thus, they also form part of a feedback channel from higher brain areas to the ALs. Other known feedback neurons include the honeybee AL-1 neurons, which originate in the MB α lobe and project widely through the ALs (Rybak and Menzel 1993).
2.2.10. Glial Cells
Glial cells have been intensively studied in moths (Kretzschmar and Pfugfelder 2002; Oland and Tolbert 2003; Tolbert et al. 2004). Other species with information about glial cells are bees (Hähnlein and Bicker 1996), Drosophila (Awasaki et al. 2008; Jhaveri and Rodrigues 2002; Jhaveri et al. 2000, 2004), cockroaches (Prillinger 1981), and locust ALs (Hähnlein et al. 1996). The cells are prominent on the outside of the AL, and form thin processes that digitate between glomeruli, creating a boundary between them. In species where glomeruli are arranged around a central, non–glomerular neuropil (coarse neuropil, e.g., honeybees, moths), glial cells do not form a border between glomeruli and the central neuropil.
There are several different types of glial cells. Along the antennal nerve, they enwrap axon fascicles with long processes and multiple expansions. OSN axons travel as parallel bundles within the antennal nerve, but as they approach the AL, they reach a so-called “sorting zone,” where they form a dense and complex network and rearrange, in order to target the correct glomeruli, a process that necessitates functional glial cells (Oland et al. 1988; Rössler et al. 1999). The distinct nerves that innervate the AL (e.g., T1–T4 in bees) do not correspond to the distinct branches of the antennal nerve within the antenna (dorsal and ventral nerve). Within the AL, there are at least two glial cell types that form borders around glomeruli: one has large cell bodies and branching, vellate arbors. The other has multiple, mostly unbranched processes with many lamellate expansions along their length, which surround glomeruli as part of a multilamellar envelope (Oland et al. 1999). Glial cells are needed to form protoglomeruli and for correct AL development (Baumann et al. 1996).
Functions that have been proposed for glial cells in the adult system include the control of ion diffusion across glomeruli, or the formation of NO sinks (Gibson et al. 2001; Goriely et al. 2002).
2.2.11. Odor-Evoked Activity
Given the circuitry of AL neurons known so far, it is possible to attribute putative functions to different cell types (Sachse and Galizia 2006). Thus, homogeneous LNs might serve as a gain control mechanism (either as inhibitory neurons or as excitatory neurons or both), asymmetric LNs would compute glomerulus-specific information, multiglomerular PNs respond to global activity, and thus give information about stimulus timing (onset/offset) and odor concentration, while uniglomerular PNs encode odors in their combinatorial activity pattern across their axons’ identities.
As a result of different odor-response profiles across receptor cells, an odor stimulus leads to a characteristic activity pattern across individual glomeruli (Figure 2.2). Importantly, these activity patterns are not binary (on/off), but continuous (each glomerulus can be activated to varying degrees). From these patterns, it is possible to identify the stimulating odor, and therefore it would be sufficient as an olfactory code. However, it is not known what information is really used by the brain, and therefore the biological olfactory code remains to be elucidated.
Odor-evoked combinatorial patterns can be measured with imaging techniques, which afford measuring many glomeruli at the same time. Such responses have been measured in D. melanogaster by using genetically encoded reporter proteins. synaptopHluorin was used to measure synaptic vesicle release (Ng et al. 2002; Yu et al. 2004) and cameleon or G-CaMP for intracellular calcium (Fiala et al. 2002; Suh et al. 2004; Wang et al. 2003; Silbering and Galizia 2007; Silbering et al. 2008). These proteins were expressed under the control of specific promoters, which allow for a good reproducibility of the measured cells. For example, using the GAL4-line, GH146, a reporter protein can be expressed in a population of brain cells that within the AL only consists of PNs. Similarly, expressing the reporter in subpopulations of LNs allows the dissection of their relative contribution to odor-evoked activity.
Apart from genetically encoded reporter proteins, a large number of synthetic reporters are available, and have been used, in particular, in species other than Drosophila. Such dyes can be washed into the brain, as done for calcium-sensitive dyes in bees (Galizia and Menzel 2001; Joerges et al. 1997), ants (Galizia et al. 1999b), and moths (Carlsson et al. 2002; Galizia et al. 2000b; Hansson et al. 2003), and for NO release in moths (Collmann et al. 2004). Here, NO activity patterns and calcium activity patterns were similar, as expected, given that in moths, NO is produced by OSNs (Gibson and Nighorn 2000). Odor responses can also be measured with voltage-sensitive dyes (Galizia et al. 1997, 2000a). Cell-specific measurements can be obtained by staining specific cell populations, as done for PNs (Sachse and Galizia 2002, 2003). These selective stainings lead to calcium responses that are fast, with a steep rise at stimulus onset and a steep decay at stimulus offset. As in mammals (Leon and Johnson 2003; Xu et al. 2000), the spatial odor-response patterns have also been recorded using 2-deoxyglucose labeling in fruit flies (Buchner and Rodrigues 1983; Rodrigues and Buchner 1984), and in Calliphora (Distler et al. 1998a). In insects, because olfactory glomeruli are few and often easily recognizable by their relative position, shape, and size, functional atlases have been created of glomerular responses (e.g., http://neuro.unikonstanz.de/honeybeeALatlas). Using multielectrode arrays affords the simultaneous extracellular recording of many neurons, but the neurons involved cannot be identified (Christensen et al. 2000).
2.2.12. Contrast Enhancement
Information from input neurons (OSN) is transformed by internal connections (LNs) into the activity of output neurons (PNs). Consequently, the AL has often been modeled as a prototypical neural network in computational neuroscience (Bazhenov et al. 2001; Getz 1991; Getz and Lutz 1999; Linster et al. 1994; Rabinovich et al. 2000). The question of what role the hidden layers play in such a network can easily be addressed by comparing the glomerular activity of PNs with the glomerular activity in the OSNs, which in this system is particularly elegant given that both have the same number of dimensions (i.e., the same number of glomeruli). This approach has been taken in several studies, with surprisingly disparate results. In some optical imaging studies in Drosophila, the difference between PNs and OSNs was so small that no apparent processing was deducible (Ng et al. 2002; Wang et al. 2003). In an electrophysiological study comparing input and output of glomerulus DM2, also in Drosophila, the response spectrum was apparently broader in PNs than in OSNs (Wilson et al. 2004). In another study in Drosophila, presynaptic inhibition onto receptor cell terminals led to an inhibitory network activity (Olsen and Wilson 2008). Comparing input and output for odor concentration dose-response curves in honeybees showed that glomeruli with a low threshold for an odor have almost identical dose-response curves in PNs and in OSNs, while less sensitive glomeruli have a shifted dose-response curve in PNs, showing that at higher concentrations, LNs suppress these PN responses (Sachse and Galizia 2003). Linster and colleagues created a computer model of the AL and ran experimental data under different assumptions about LN network connectivity, comparing three network architectures: one where LNs interconnect neighboring glomeruli, one with stochastic connections, and one based on the odor-response properties of glomeruli. The results show that inhibitory connections are strongest among glomeruli that have similar odor-response profiles, and weakest among glomeruli that do not overlap in their odor-response profiles, irrespective of their spatial position (Linster et al. 2005). This leads to an amelioration of odor responses in PNs across odors, by reducing response overlap to similar odors. Interestingly, the system need not be symmetrical: an inhibitory connection from glomerulus A to a glomerulus B does not imply a similar connection from B to A. Physiological evidence for such a nonsymmetrical connectivity has been found (Sachse and Galizia 2002), and behavioral experiments confirm such an asymmetry: similarity from an odor X to Y can be different from the similarity of Y to X (Guerrieri et al. 2005).
2.2.13. Sensitivity Optimization in the Antennal Lobe (AL)
PNs are spontaneously active with a pronounced temporal complexity, resulting in continuously changing, low-level glomerular activity patterns, even in the absence of sensory stimulation (Galán et al. 2006; Sachse and Galizia 2002). If the response magnitude were normalized, it would be difficult or even impossible to distinguish individual events of spontaneous activity from odor-evoked responses. The driving force for this spontaneous activity might come from background activity in OSNs. Within the AL network, background activity can be tightly controlled to be kept just at threshold, a scenario that would increase the sensitivity to weak odors (Sachse and Galizia 2006; Shang et al. 2007). Conceptionally, PNs can be compared to a loaded spring. A tight regulation of inhibitory and excitatory LNs keeps PNs at firing threshold, so that a minimal olfactory stimulus would already elicit an odor-evoked pattern. PNs are maintained close to threshold by constantly probing their depolarization, which results in a level of spontaneous activity. Even though this system leads to a continuous shift of the baseline, odor–concentration coding is not affected, because the odor–concentration magnitude of a stimulus remains available in the first derivative of the odor response (i.e., in the steepness of the response). Odor concentration information is much more affected by receptor adaptation at the periphery, a phenomenon known from all sensory systems. As with most sensory systems, the olfactory system is better at measuring concentration changes rather than absolute concentrations. The “loaded spring model” of the AL ensures that small increases in odor concentration will lead to a strong response, even when background odors are present.
2.2.14. Temporal Activity Structures
In the natural environment, odors are temporally complex due to air turbulence (Justus et al. 2005; Murlis et al. 1992), and temporal odor-response patterns predominantly reflect stimulus variation (Vickers et al. 2001). In addition, OSNs already have temporally complex response properties: some have phasic, some tonic responses, some have activity that outlasts the odor stimulus, and some reduce activity upon olfactory stimulation (de Bruyne et al. 2001). As a consequence, even an olfactory stimulus that is temporally uniform leads to a temporally complex pattern of activity (see also Chapters 12 and 13).
Slow temporal structures consisting of sequences of bursts and inhibitory events have been observed in PNs of all insects studied so far, including moths (Christensen et al. 1998b; see also Chapter 3), locusts (Laurent 1996), and honeybees (Abel et al. 2001; Müller and Hildebrandt 2002).
Behavioral studies have shown that olfactory discrimination is in the range of ~200 ms in rats (Abraham et al. 2004; Uchida and Mainen 2003) and ~690 ms in bees (Ditzen et al. 2003), a time that includes the time needed for the motor responses and physical displacement of the animal in that particular task. Odor similarity or odor concentration have no or only a small effect on the time needed for olfactory discrimination (Abraham et al. 2004; Ditzen et al. 2003; Uchida and Mainen 2003). Physiological studies of PN responses show that 200–300 ms in locusts (Stopfer et al. 2003) and maximally 400 ms in bees (Galán et al. 2004) are needed to reach the most distinct odor classification in the AL. Therefore, all phases of slow activity components after this time are irrelevant for odor discrimination. However, late activities might be important for olfactory learning or other aspects of olfactory processing; in fact, odor representation is ameliorated during the first 2 s after stimulus onset, leading to a clearer distinction of odors (Friedrich and Laurent 2001; Galizia et al. 2000a).
Fast temporal structures are evident in odor-evoked oscillations, which are found almost ubiquitously in olfactory systems. Within insects, oscillations have been shown in cockroaches, locusts, bees, wasps, flies, and moths (Heinbockel et al. 1998; Stopfer et al. 1999). The chloride channel blocker, picrotoxin, abolishes these oscillations (MacLeod and Laurent 1996; Stopfer et al. 1997), but also modifies combinatorial spatial activity patterns (Sachse and Galizia 2002). Individual PNs do not fire in every oscillation cycle, and action potentials also occur out of the synchrony pattern. Therefore, odor identity could be encoded in sequences of changing PN ensembles (Laurent 1999). Alternatively, or in addition, synchrony could be related to odor concentration or intermittency rather than odor quality (Christensen et al. 2000).
2.2.15. Combinatorial Odor Codes
Odors evoke combinatorial patterns of activated glomeruli, with each glomerulus participating in the activity patterns of many odors. These patterns are conserved among individuals (Galizia et al. 1999c; Wang et al. 2003), which is a consequence of the innate mapping of OSNs that express a given OR to individual glomeruli (Couto et al. 2005; Fishilevich and Vosshall 2005; Vosshall et al. 2000). Molecular receptive ranges (MRR) are best described by the response range to many odors (Sachse et al. 1999), as is the case for the receptors themselves (Hallem and Carlson 2006; Pelz et al. 2006). There are no glomeruli for functional groups or other chemical parameters (e.g., “aldehyde” or “C6-carbon-chain”). Therefore, the response profile of individual glomeruli is not determined by particular “features” of the odorant (sometimes referred to as odotopes), such as “ketone group” or “aldehyde,” and the olfactory code is not a building set, where 1-heptanol would be coded in an “alcohol glomerulus” plus a “C7 aliphatic chain” glomerulus.
2.2.16. Odor Concentration and Mixtures
With increasing odor concentration, responses increase both in magnitude and in number of active glomeruli. Similarly, with decreasing concentration, activity decreases, and may consist of a single glomerulus being active at very low concentration. This is a direct consequence of the receptors’ response properties: each receptor has a few substances to which it responds with higher affinity than any other receptor, and at its lowest effective concentration, that substance will elicit a combinatorial pattern of activity in the AL, which consists of that single glomerulus being active, and all other glomeruli being silent. PN responses in honeybees are qualitatively stable over a concentration range of up to 4 log units, because higher odor concentrations increase total response intensity without changing the relative intensity across glomeruli; at the input level, however, the activity patterns are more affected by concentration differences (Sachse and Galizia 2003). Thus, the neural network within the AL contributes to concentration invariance. Comparable results were found in Drosophila (Silbering et al. 2008).
When an odor consists of many substances, the task for the organism might be either to recognize that particular mixture (e.g., the characteristic bouquet of coffee), or to extract a component (e.g., the presence of garlic in the food). Physiologically, the presence of an odor Y can interfere with the normally strong response to an odor X, which is termed mixture suppression. Conversely, synergism indicates when a neuron or glomerulus responds to a binary mixture with a response that exceeds the summed responses to the single components. An inhibitory network within the AL, optimized for sharpening odor-response patterns, should create stronger mixture interactions when similar odors are mixed than when dissimilar odors are mixed. Psychophysically, similar odors in a mixture “compose” a new odor, which makes it difficult for the olfactory system to extract the identity of the odor components (synthetic representation), while mixtures of dissimilar substances are represented as the sum of the optimized representation of each component (analytical representation) (Wilson and Stevenson 2003). Behavioral data in rats support this idea (Wiltrout et al. 2003). By increasing the number of components in an odor mixture, mixture interactions increase and further reduce the similarity to the single component patterns (Deisig et al. 2003; Silbering and Galizia 2007). With this coding strategy of odor mixtures, the olfactory system implements a logic that allows a unique representation of odor mixtures without saturating the olfactory code, at the expense of losing analytical capacity.
2.2.17. Special Cases: Sexual Pheromones
Generally, OSNs responding to sexual pheromones are highly specific, and form labeled lines for each pheromone component. The labeled line property is dependent on the environment: if, in the natural environment of the animal, there are no other, alternative ligands, the receptor functions as a labeled line, even though a chemist’s collection might find other effective substances. For example, in M. sexta, the sexual pheromone is a blend of two chemicals, the main component (E,Z)-10,12-hexadecadienal, and the secondary component (E,E,Z)-10,12,14-hexadecatrienal. A different and more stable molecule, (E,Z)-11,13-pentadecadienal, is a good mimic and is routinely used instead in physiological experiments (Christensen and Hildebrand 1997). Coding of sexual pheromones is combinatorial, because each component is necessary for identifying that the pheromone is the species-specific blend.
Sexual pheromones in Drosophila are not involved in long-distance navigation, but rather are part of a “close-encounter” olfactory display, which is detected by contact chemoreceptors. The only candidate for a volatile pheromone is cis-vaccenyl acetate, though its precise behavioral significance remains to be elucidated (Amrein 2004; Amrein 2004). OSNs sensitive to cis-vaccenyl acetate reside in T1 sensilla on the antenna (Xu et al. 2005).
2.2.18. Special Cases: Carbon Dioxide
Most arthropods have CO2 sensitive systems with a wide variability in structure and function (Bogner et al. 1986; Kleineidam and Tautz 1996; Stange and Stowe 1999). The sensilla can be hairs, pegs, plugged or open grooves, they can be on the surface, or they can be located within a depression or a pit with a restricted opening (Keil 1996; Stange and Stowe 1999). The relevance of CO2 also differs across species. In blood-sucking insects such as mosquitoes, CO2 sensitivity is often relevant for finding the host (Dekker et al. 2002; Grant et al. 1995). For nocturnal moths feeding on nectar, CO2 might be a component of the attractive flower odor, since fowers release considerable amounts of metabolic CO2 (Guerenstein et al. 2004; Raguso 2004; Thom et al. 2004). Insects that live in confned spaces, such as centipedes or beetle larvae, sense CO2 to ensure sufficient respiration. Similarly, social insects (ants, bees, and termites) monitor CO2 in their hives and control its concentration (Lacher 1964; Stange and Stowe 1999; Weidenmüller et al. 2002).
Drosophila is repelled by CO2, and a role of this gas as a component of a stress signal has been suggested (Suh et al. 2004). However, CO2 is also produced by rotting fruit, and by fly aggregations on such fruit that might indicate good ovipositioning sites. In the Drosophila, AL CO2 activates the V glomerulus, suggesting a labeled-line-like system for this substance (Suh et al. 2004).
2.3. THE MUSHROOM BODIES (MBs)
MBs are multimodal structures in the insect protocerebrum. They are involved in learning (Davis 2004; Heisenberg 2003; Strausfeld and Gilbert 1992), and receive both olfactory and visual input in most insect species (Farris 2005; Strausfeld et al. 1998). Their names derive from the massive peduncles with large, cup-shaped protuberances, which are called the calyces. In hymenoptera, the calyces are subdivided into lip, collar, and basal ring, which correspond to three separate bands in the α lobe (or vertical lobe). In Drosophila, subdivisions from the calyces can be traced to a concentrically circular arrangement in the α/β lobe and to a layered structure in the α’/β’ lobes (Tanaka et al. 2004). The peduncles generally branch into two lobes, the vertical (α) lobe and the horizontal (β) lobe. In addition, in Drosophila, they form the α’ and the β’ lobes (Strausfeld et al. 2003). A third lobe, the γ lobe, is physically attached to the vertical lobe (the α lobe) in honeybees, but is morphologically distinct in Drosophila (Farris et al. 2004; Strausfeld 2002). The intrinsic neurons in the MBs are called KCs.
In the olfactory pathway, uniglomerular PNs form the input to MB calyces. In hymenoptera, this input is targeted at the lip and the basal ring of the calyces, while the intermediate area, the collar, receives input from the optic lobes (Gronenberg 1999, 2001). In Drosophila, each PN axon travels over large areas of the calyx, forming synapses with many intrinsic KCs (Marin et al. 2002; Wong et al. 2002). This is reminiscent of the situation in the mammalian olfactory cortex (Zou et al. 2005), and allows for a combinatorial readout of PN response patterns. PNs within the MB calyx region occupy concentric layers (Tanaka et al. 2004). PNs from identified glomeruli branch in a stereotypical manner within the MB calyces, and segregate in a functionally dictated way. For example, fruit odors and pheromone odors target other areas (Jefferis et al. 2007). On average, three uniglomerular PNs innervate each glomerulus, and these have the same projection pattern in MB and LP, suggesting that they are not functionally distinct (Wong et al. 2002).
Schematically, MB input can be described as a scaffold, with arrays of PN axons crossing arrays of KCs, and forming synapses with some, but not all KCs (Heisenberg 2003). In neural network language, this is ideal for combinatorial readout across PNs.
KC numbers differs widely among species: in honeybees, there are ~170,000 KCs in each hemisphere (Mobbs 1982; Witthöft 1967), Drosophila counts give ~2500 KCs (Stocker 1994). In adult cockroaches, the number is ~175,000 (Neder 1959), but juveniles have much smaller numbers (Farris and Strausfeld 2001), adult locusts have ~50,000 KCs (Farivar 2005). KC somata lie close to the MB calyces, in a densely packed manner. Their axons are long and thin and form the peduncles. The γ lobe is formed by the axons of the clawed KCs, the first subpopulation of KCs to occur in development (Farris et al. 2004; Mobbs 1982; Rybak and Menzel 1993; Strausfeld 2002). The name “clawed” derives from their claw-like dendritic shapes within the calyces.
KC morphology, pharmacology, and peptide expression shows a considerable variability across all species studied so far (Iwasaki et al. 1999; Sinakevitch et al. 2001; Strausfeld 2002; Strausfeld et al. 2000, 2003; Strausfeld and Li 1999a, 1999b). Glutamate labels a KC subpopulation of bees (Bicker et al. 1988). Aspartate, glutamate, and taurine immunocytochemistry label different KC populations in Drosophila (Strausfeld et al. 2003) and in cockroaches (Sinakevitch et al. 2001). In addition, in Drosophila, KCs produce NO (Schürmann 2000), but ACh and GABA are excluded as KC transmitters (Yusuyama et al. 2002).
2.3.1. Local Inhibitory and Modulatory Neurons
Synaptic arrangements in the MBs form microglomeruli with very local computational capabilities. In Drosophila, each MB microglomerulus comprises a large cholinergic bouton formed by a PN axon from the AL, which is surrounded by tiny vesicle-free KC dendrites and several GABAergic terminals (Yusuyama et al. 2002). GABAergic terminals contact both KC dendrites and PN axon terminals, suggesting that PN input is modulated both pre and postsynaptically (Ganeshina and Menzel 2001; Leitch and Laurent 1996; Yusuyama et al. 2002). MB microglomeruli have no glial sheath (Ganeshina and Menzel 2001; Yusuyama et al. 2002).
In addition to microglomerular circuits, there are GABAergic feedback neurons from the MB lobes back onto their calyces. These neurons are few in number, in honeybees ~55. Each feedback neuron innervates a subcompartment in the calyx, with each subcompartment in the calyx being connected to its specific, corresponding layer in the α lobe (Grünewald 1999). These neurons have been found in bees (Bicker et al. 1985; Schäfer and Bicker 1986), moths (Homberg and Hildebrand 1994), Drosophila (Yusuyama et al. 2002), cockroaches (Farris and Strausfeld 2001), and locusts (Leitch and Laurent 1996).
Octopaminergic cellular processes sparsely but uniformly innervate MB calyces (Strausfeld et al. 2003). In honeybees, most of these processes are formed by the VUMmx1 neuron that represents the conditioned stimulus (CS) during olfactory learning (Hammer 1997). VUMmx1 also branches in the AL, the LP, and the subesophageal ganglion (SEG).
2.3.2. Output Lobe Circuitry
The pathways from AL to MB, and the internal circuitry of the MB, have been studied in more detail than the MB output to other brain areas, notably the LP. Generally, the output is believed to target premotor areas, and the pathway OSN-AL-uniglomerularPN-MB-LP-premotor areas-motor neuron would run in parallel to the pathway OSN-AL-multiglomerularPNs-LP-premotor areas-motor neuron loop, which bypasses the MBs. Several subregions within the protocerebrum have been identified, including the superior medial protocerebrum, the inferior medial protocerebrum, and the superior LP (Ito et al. 1998; Tanaka et al. 2004). MB output neurons in honeybees branch unilaterally or bilaterally (Rybak and Menzel 1993). A prominent large neuron in honeybee brains is PE1, which is a single neuron in each brain hemisphere, and connects the α lobe to the LP and the ring neuropil around the α lobe (Brandt et al. 2005; Rybak and Menzel 1998). Olfactory learning modifies the response properties of PE1 neurons (Mauelshagen 1993) (Okada et al. 2007).
2.3.3. Odor-Evoked Activity in the Mushroom Bodies (MBs)
As compared to PNs, KCs respond to odors with few spikes, if any (Stopfer et al. 2003): while PNs had a response probability of p=.64, KCs responded with p=.11 to a given odor set (Perez-Orive et al. 2002). Also, PNs respond with trains of spikes, but KCs respond with single or only very few spikes (Figure 2.2B). Similar results were found in flies and honeybees (Szyszka et al. 2005; Wang et al. 2004). Thus, odor representation in KCs is sparse in the sense of population sparseness (a low proportion of units active at any time) and in the sense of lifetime sparseness (few spikes in each neuron with narrow tuning) (Laurent 2002; Olshausen and Field 2004). This sparsening is a progressive feature of MB circuitry: activity trains arriving at the MB terminals are inhibited pre-synaptically by GABAergic glomerular microcircuits, so that only the first APs are likely to drive activity in KCs (Assisi et al. 2007; Szyszka et al. 2005). In addition, the inhibitory feedback loop from the MB output lobes onto the calyces further sharpens that response (Szyszka et al. 2005). This feedback also generates a global oscillatory rhythm (Perez-Orive et al. 2002), which, in turn, favors the extraction of synchronized APs from PNs (Perez-Orive et al. 2004).
2.3.4. Olfactory Coding
If only the initial firing pattern leads to KC activity in each odor puff (Szyszka et al. 2005), then most APs that PNs generate in an odor response cannot contribute to odor-information decoding in the MBs. Are the remaining APs wasted? Such a waste would be quite inefficient, considering that producing APs is among the most energy-costly activities of the brain (Attwell and Laughlin 2001). However, the “surplus” spikes may be relevant outside the MB, e.g., within the AL and in the LP, where PNs have other output synapses.
The massive expansion from relatively few PNs to many KCs in insect olfactory systems has been likened to a support vector machine (Galán et al. 2004). With an integrating neuron at the PN output level that reads across PN activities, it is only possible to perform a limited classification, which statistically corresponds to a linear classification in a multidimensional space. However, when the same number of PNs are first combinatorially mapped onto a very large number of KCs, an integrating neuron that would read across these activities could extract much more complex pattern topologies. Thus, the large number of KCs allows for the computation of highly nonlinear classification schemes across PNs (Huerta et al. 2004). If temporal complexity is added to the code, the theoretical capacity of the system increases even further (Laurent et al. 2001).
2.4. OLFACTORY MEMORY AND PLASTICITY
2.4.1. The Antennal Lobe (AL)
Brains respond to experience with changed behavior, a process generally called learning. Here, associative and nonassociative forms of plasticity are differentiated, because they differ both conceptionally, behaviorally, and in the cellular processes involved. Associative paradigms include classical conditioning and operand conditioning. Among the nonassociative processes, habituation, and sensitization are the most important ones. There are several sites in the brain where learning induces cellular changes. In the olfactory system, the AL as the first olfactory neuropil is already involved in substantial experience-induced changes. Some of these changes in odor responses occur without being directly attributable to a particular form of behavioral change (so far). For example, repeated exposure to an odor leads to changes in odor responses in PNs: the number of APs is reduced, but their temporal precision is increased, which may mean that their coding is more efficient for later puffs (Stopfer and Laurent 1999). This sensory memory trace decays within 10–15 min after the last puff (Stopfer and Laurent 1999). In honeybees, a single odor exposure leads to a change in the ongoing spontaneous activity across PNs: a pattern corresponding to the experienced odor reoccurs repeatedly during the next 1–2 min, showing that the AL network creates an ephimeral neural attractor for this pattern (Galán et al. 2006).
Application of sucrose (or water) to the honeybee antenna leads to olfactory sensitization. It elicits a transient increase of PKA activity in the AL, but odor stimulation alone does not (Hildebrandt and Müller 1995a, 1995b). This effect is mediated by octopamine, and reverts to baseline within 3 s (Hildebrandt and Müller 1995a).
In classical conditioning, an odor is associated with a punishment (e.g., electroshock, aversive, and learning) or a reward (e.g., sugar water, and appetitive learning). Aversive conditioning leads to changes in the fly AL, where PNs in some glomeruli change their odor responses for about 3 min after conditioning (Yu et al. 2004). Appetitive associative learning is also mediated by octopamine, which represents the unconditioned stimulus (US). In bees, the US can be replaced by octopamine injections into the AL (Hammer and Menzel 1998). Blocking octopaminergic transmission either by injecting an octopamine receptor antagonist (mianserin) or by injecting double-stranded octopamine receptor RNA into the AL, interferes both with odor memory acquisition and with odor memory recall. Consequently, we must assume that octopamine is important for memory consolidation but not only, and that octopaminergic neurons become reactivated during memory recall (Farooqui et al. 2003). In honeybees, short-term memory (STM) can be generated by single-trial conditioning, and long-term memory (LTM) by multiple conditioning trials during training. Multiple conditioning trials lead to an elevated PKA response in the AL, which is mediated by the NO/cGMP system. STM can be converted into LTM when PKA activity is artifcially increased after single-trial learning, suggesting that it is not the presence of PKA, but its concentration that is important for generating LTM (Müller 2000). Differential conditioning (one odor was rewarded, the other not) leads to a modification of odor-response patterns in the AL in the time window of 5–15 min after conditioning, as shown with calcium imaging in bees (Faber et al. 1999), and with extracellular recordings in moths (Daly et al. 2004).
Other studies have not found changes in the AL that are attributable to olfactory associative memory. In Drosophila, the MBs have been shown not only to be necessary, but also to be sufficient for short-term learning of odors, leaving no space for a memory trace in the AL (Gerber et al. 2004b). In honeybees, the uniglomerular PNs from the lACT tract have very stable odor responses that are not affected by single-odor training or differential appetitive training (Peele 2005). These neurons might represent a processing channel that ensures reliable transfer of odor-related information to higher order brain centers, a hypothesis that remains to be investigated. Together, these currently available data show that odor learning occurs in the AL and affects spike timing and/or relative activities to different odors. These effects, however, only occur in very limited time windows, are limited to specific cell populations, and only represent a part of the memory trace.
2.4.2. The Mushroom Bodies (MBs)
MBs play an important role in olfactory memory, as already shown by experiments in which memory retrieval was impaired when the MBs were cooled in honeybees (Erber et al. 1980). Similarly, learning deficits are observed in Drosophila mutants where MB structure is altered (MB deranged, mbd, and MB miniature, mbm) (Heisenberg et al. 1985). Blocking synaptic activity or disrupting MB physiology also leads to memory defcits (Connolly et al. 1996; Dubnau et al. 2001; McGuire et al. 2001). MBs can be chemically ablated by applying hydroxyurea, a DNA-synthesis inhibitor, during the early proliferation phase of KCs. This leads to olfactory memory impairment in Drosophila (de Belle and Heisenberg 1994). In bees, partial ablation of MBs only impairs complex tasks with several odors, but not easy learning tasks (Komischke et al. 2005; Malun et al. 2002).
It should be noted, though, that MBs are not indispensable. There are many olfactory tasks that can be solved without MBs, and indeed, fly mutants that lack MBs are remarkably normal: they feed, lay eggs, are alert, court and copulate, are well oriented in space, and respond to odors (Heisenberg 2003). Furthermore, MBs have many tasks beyond olfaction: they are also used for spatial memory and navigation without olfactory cues (Kwon et al. 2004; Mizunami et al. 1998; Strausfeld et al. 1998).
In honeybees, the VUMmx1 neuron that represents the appetitive reinforcer also innervates the MB calyces. Because the VUMmx1 neuron represents the US, it is probably involved in the necessary mechanism of coincidence detection, which means that for appetitive olfactory learning in honeybees, coincidence detection occurs in the MB input region (Menzel and Giurfa 2001). Optical imaging experiments show that a rewarded odor leads to increased calcium responses in the MB calyces as compared to before learning (Faber and Menzel 2001; Szyszka et al. 2008). Morphological changes are also observed in honeybee MBs after learning: worker bees that forage have KC dendrites with more branches than age-matched bees that do not forage, while the density of dendritic spines remained constant (Farris et al. 2001).
The best cellular analysis of olfactory memory traces in MBs comes from Drosophila (Davis 2004; Dubnau et al. 2003; Heisenberg 2003; Waddell and Quinn 2001). Memories can be categorized based on how long they last: STM decays within 1 h, middle-term memory (MTM) within 3 h. Anesthesia-resistant memory (ARM) and LTM are two forms of LTM that differ in their training procedures: ARM occurs after massed training, and the protein-synthesis-dependent LTM occurs after spaced training, i.e., a training protocol where individual learning events occur with longer intervals in between (Tully et al. 1994). However, even though the nomenclature of this classifcation is based on time, the real classification should be based on the biochemical pathways associated with STM, MTM, ARM, and LTM.
KCs are the cells where associative olfactory memory is located in flies (Gerber et al. 2004b). Mutants for the genes dunce (dnc, which is a cAMP phosphodiesterase), DC0 (which is a PKA catalytic subunit), or CREB (cAMP response element binding protein) are impaired in STM tasks, showing that a necessary second messenger in STM is cAMP. The Drosophila gene rutabaga (rut) codes for a Ca/CaM-dependent adenylyl cyclase (Levin et al. 1992). rut mutants are olfactory learning defective, and expressing rut in MB cells restores learning, showing that MB cells are sufficient for learning (Zars et al. 2000). Several different Drosophila lines were used in these experiments that all differed in exactly which cells were restored. The cell population common to all successful rescue groups were the clawed KCs, suggesting that these are suffcient for short-term olfactory learning. A loss-of-function study showed that clawed KCs are also necessary for olfactory learning (Connolly et al. 1996). rut is only necessary in adult animals, but not during development (Mao et al. 2004; McGuire et al. 2003). rut acts presynaptically at the output synapses of KCs, and blocking synaptic release of these cells impairs retrieval, but not acquisition (Dubnau et al. 2001; Schwaerzel et al. 2002).
Thus, at first sight, this appears to be a difference between flies and bees (see above): in fies, the learning site is at the output synapse of KCs, while in bees it is at the input site. Possibly, this finding is not a species-related difference, but rather a task-related difference: most experiments in flies are made using aversive conditioning (with electric shock as US), most experiments in bees are made using appetitive conditioning (with food reward as US). Indeed, when appetitive conditioning is performed in flies, both PNs and KCs are independently sufficient for successful memory performance (Thum et al. 2007). The neuronal pathways for appetitive and aversive conditioning are clearly different, in particular for US representation. Therefore, it is not surprising that coincidence detection may occur in different places. In honeybees, appetitive learning is mediated by octopamine (Hammer and Menzel 1998), consistent with findings in Drosophila, while the aversive US is mediated by dopaminergic neurons (Schwaerzel et al. 2003).
The gene amnesiac (amn) is strongly expressed in the dorsal paired medial (DPM) neurons, of which there are two in each fly. amn codes for a neuropeptide (PACAP) that modulates rut activity in KCs. Disruption of amn or silencing of DPM neurons leads to loss of MTM. The activity of DPM is necessary at different times for some odors, but not for others. For the odors octanol and methyl-cyclohexanol, DPM neuron activity is necessary during storage and possibly consolidation, but not during acquisition and recall, while memories for benzaldehyde need DPM neuron activity during acquisition (Keene et al. 2004). These data show that not only the cells and the neural networks have a high diversity in memory research, but even the odors used affect which cell, memory phases, genetic, and neural networks are used and relevant. Furthermore, the entire network needs to be functioning. Even if the engram was at a single synapse, without the network, memory would not be formed. For example, inhibitory MB output/feedback neurons are necessary for memory formation, and when they are silenced, no memory is formed (Liu et al. 2009).
LTM includes ARM and LTM. These are not sequential: ARM is formed even in amn mutants that show no MTM, and at least partially also in rut mutants that show no STM (Isabel et al. 2004). Different KC populations are needed for STM/MTM (γ lobe) and ARM/LTM (α/β lobe) (Isabel et al. 2004; Pascual and Preat 2001; Zars et al. 2000). The ubiquitin ligase, Neuralized, is only used in α/β lobe and is necessary for LTM, but not for ARM (Pavlopoulos et al. 2008). Nevertheless, LTM and ARM are not entirely independent pathways: rather, LTM induces an active erasure of ARM memory (Isabel et al. 2004). The cyclic AMP response element CREB appears to be related to LTM (Yin and Tully 1996), though the scientific evidence has recently been questioned (Perazzona et al. 2004).
2.4.3. Activity-Dependent Plasticity
The ability of the brain to adapt structurally and functionally in response to sensory experience is a striking property across animal phyla. However, several studies have shown that the olfactory system of Drosophila possesses a remarkable wiring stability. Ablation of the olfactory input to the AL by cutting the antennae in the adult fly has no effect on dendritic or axonal arborization of the ascending PNs (Berdnik et al. 2006; Tanaka et al. 2004; Wong et al. 2002). The adult olfactory circuit seems to respect the glomerular boundaries imposed by the rules of development.
Despite this apparent wiring rigidity, the olfactory circuit appears to have the capacity for experience-dependent plasticity within the confines of a glomerulus. In honeybee workers, the volume of identified olfactory glomeruli is modified during adult life dependent on the behavioral task (Winnington et al. 1996). Studies from Drosophila show that deprivation of the input from one antenna reveals the existence of activity-dependent competition in the extent of axonal arborization (Berdnik et al. 2006). Moreover, continuous exposure of adult flies to single odors for several days causes either a stimulus-dependent decrease of the nonactivated, possibly inhibited glomeruli (Devaud et al. 2001, 2003), or results in a drastic volume increase of glomeruli activated by the stimulus used for long-term exposure (Sachse et al. 2007). Interestingly, chronic olfactory exposure does not affect the morphology or function of the OSNs, while one class of inhibitory LNs and the output of the PNs are functionally modulated in response to the exposed odor (Sachse et al. 2007). Long-term odor exposure has also been shown to affect the odor-guided behavior of different insects. The spontaneous aversive tendency of Drosophila to some specific odors can be reduced by exposing adult flies to these odors beforehand (Hershberger and Smith 1967). Studies in honeybees have shown that bees prefer an odor when they have previously associated that odor to a sugar reward, but not if they have just been exposed to that odor without reinforcement (Sandoz et al. 2000).
2.5. THE LARVAL SYSTEM
In this chapter, we have only looked at the adult olfactory system in insects, and not at the maggot, for which there are several recent reviews that can be accessed (Cobb 1999; Heimbeck et al. 1999; Kreher et al. 2005; Marin et al. 2005; Scherer et al. 2003; Stocker 2001; Gerber and Stocker 2007). The main difference between the two systems is the greater simplicity in larvae, both in terms of cell numbers and organization, even though important principles are shared (Python and Stocker 2002a, 2002b). Odor perception is good in larvae, and odor learning is robust (Gerber et al. 2004a; Hendel et al. 2005; Scherer et al. 2003). The main pathway, OSN-PN-KC, is also realized in larvae, but the main difference is that—at least in Drosophila—each glomerulus only receives input from a single OSN axon, and is innervated only by a single PN (Kreher et al. 2005; Ramaekers et al. 2005). The larval MBs also share several features of their adult counterparts (Marin et al. 2005). In Drosophila larvae, there are 21 OSNs that express ~25 OR genes, of which only 13 are also expressed in adults (Kreher et al. 2005; Ramaekers et al. 2005), suggesting that the olfactory space of the larva is substantially different from the adult one.
ABBREVIATIONS
- ACh:
acetylcholine
- AL:
antennal lobe
- ARM:
anesthesia-resistant memory
- CS:
conditioned stimulus
- GPCR:
G-protein-coupled receptor
- KC:
Kenyon cell
- LN:
local neurons
- LP:
lateral protocerebrum
- LTM:
long-term memory
- MB:
mushroom bodies
- MTM:
middle-term memory
- NO:
nitric oxide
- OBP:
odorant–binding protein
- OR:
odorant receptor
- OSN:
olfactory sensory neuron
- PBP:
pheromone binding protein
- SEG:
subesophageal ganglion
- STM:
short-term memory
- US:
unconditioned stimulus
REFERENCES
- Abel R., Rybak J., Menzel R. Structure and response patterns of olfactory interneurons in the honeybee, Apis mellifera. J Comp Neurol. 2001;437:363–83. [PubMed: 11494262]
- Abraham N.M., Spors H., Carleton A., Margrie T.W., Kuner T., Schaefer A.T. Maintaining accuracy at the expense of speed; stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–76. [PubMed: 15572116]
- Amrein H. Pheromone perception and behavior in Drosophila. Curr Opin Neurobiol. 2004;14:435–42. [PubMed: 15321064]
- Ang L.H., Kim J., Stepensky V., Hing H. Dock and Pak regulate olfactory axon pathfinding in Drosophila. Development. 2003;130:1307–16. [PubMed: 12588847]
- Anton S., Hansson B.S. Antennal lobe interneurons in the desert locust Schistocerca gregaria (Forskal): Processing of aggregation pheromones in adult males and females. J Comp Neurol. 1996;370:85–96. [PubMed: 8797159]
- Anton S., Homberg U. Antennal lobe structure. In: Hansson B.S., editor. Insect Olfaction. Berlin: Springer; 1999. pp. 97–124.
- Anton S., Ignell R., Hansson B.S. Developmental changes in the structure and function of the central olfactory system in gregarious and solitary desert locusts. Microsc Res Tech. 2002;56:281–91. [PubMed: 11877803]
- Arnold G., Masson C., Budharugsa S. Spatial organization of the sensory antennal system in the honeybee by using a cobalt ions marketing method. Apidologie. 1983;14:127–35.
- Arnold G., Masson C., Budharugsa S. Comparative study of the antennal lobes and their afferent pathway in the worker bee and the drone (Apis mellifera). Cell Tissue Res. 1985;242:593–605.
- Assisi C., Stopfer M., Laurent G., Bazhenov M. Adaptive regulation of sparseness by feedforward inhibition. Nat Neurosci. 2007;10:1176–84. [PMC free article: PMC4061731] [PubMed: 17660812]
- Attwell D., Laughlin S.B. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. [PubMed: 11598490]
- Awasaki T., Lai S.L., Ito K., Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci. 2008;28:13742–53. [PMC free article: PMC6671902] [PubMed: 19091965]
- Baumann P.M., Oland L.A., Tolbert L.P. Glial cells stabilize axonal protoglomeruli in the developing olfactory lobe of the moth Manduca sexta. J Comp Neurol. 1996;373:118–28. [PubMed: 8876467]
- Bazhenov M., Stopfer M., Rabinovich M., Abarbanel H.D., Sejnowski T.J., Laurent G. Model of cellular and network mechanisms for odor-evoked temporal patterning in the locust antennal lobe. Neuron. 2001;30:569–81. [PMC free article: PMC2907737] [PubMed: 11395015]
- Benton R., Sachse S., Michnick S.W., Vosshall L.B. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. [PMC free article: PMC1334387] [PubMed: 16402857]
- Berdnik D., Chihara T., Couto A., Luo L. Wiring stability of the adult Drosophila olfactory circuit after lesion. J Neurosci. 2006;26:3367–76. [PMC free article: PMC6673868] [PubMed: 16571743]
- Berg B.G., Galizia C.G., Brandt R., Mustaparta H. Digital atlases of the antennal lobe in two species of tobacco budworm moths, the Oriental Helicoverpa assulta (male) and the American Heliothis virescens (male and female). J Comp Neurol. 2002;446:123–34. [PubMed: 11932931]
- Bhandawat V., Olsen S.R., Gouwens N.W., Schlief M.L., Wilson R.I. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–82. [PMC free article: PMC2838615] [PubMed: 17922008]
- Bicker G. Biogenic amines in the brain of the honeybee: Cellular distribution, development, and behavioral functions. Microsc Res Tech. 1999a;44:166–78. [PubMed: 10084823]
- Bicker G. Histochemistry of classical neurotransmitters in antennal lobes and mushroom bodies of the honeybee. Microsc Res Tech. 1999b;45:174–83. [PubMed: 10344769]
- Bicker G. Sources and targets of nitric oxide signalling in insect nervous systems. Cell Tissue Res. 2001;303:137–46. [PubMed: 11291761]
- Bicker G., Kreissl S. Calcium imaging reveals nicotinic acetylcholine receptors on cultured mushroom body neurons. J Neurophysiol. 1994;71:808–10. [PubMed: 8176442]
- Bicker G., Kreissl S., Hofbauer A. Monoclonal antibody labels olfactory and visual pathways in Drosophila and Apis brains. J Comp Neurol. 1993;335:413–24. [PubMed: 8227528]
- Bicker G., Schäfer S., Kingan T.G. Mushroom body feedback interneurones in the honeybee show GABA-like immunoreactivity. Brain Res. 1985;360:394–97. [PubMed: 3907757]
- Bicker G., Schäfer S., Ottersen O.P., StormMathisen J. Glutamate-like immunoreactivity in identified neuronal populations of insect nervous systems. J Neurosci. 1988;8:2108–22. [PMC free article: PMC6569325] [PubMed: 2898517]
- Boeckh J., Ernst K.D., Selsam P. Neurophysiology and neuroanatomy of the olfactory pathway in the cockroach. Ann N Y Acad Sci. 1987;510:39–43. [PubMed: 3481237]
- Boeckh J., Tolbert L.P. Synaptic organization and development of the antennal lobe in insects. Microsc Res Tech. 1993;24:260–80. [PubMed: 8431606]
- Bogner F., Boppre M., Ernst K.D., Boeckh J. CO2 sensitive receptors on labial palps of rhodogastria moths (Lepidoptera, Arctiidae) – Physiology, fine-structure and central projection. J Comp Physiol A. 1986;158:741–49. [PubMed: 3090241]
- Bornhauser B.C., Meyer E.P. Histamine-like immunoreactivity in the visual system and brain of an orthopteran and a hymenopteran insect. Cell Tissue Res. 1997;287:211–21. [PubMed: 9011397]
- Brandt R., Rohlfing T., Rybak J., Krofczik S., Maye A., et al. Three-dimensional average-shape atlas of the honeybee brain and its applications. J Comp Neurol. 2005;492:1–19. [PubMed: 16175557]
- Buchner E. Genes expressed in the adult brain of Drosophila and effects of their mutations on behavior: A survey of transmitter and second messenger-related genes. J Neurogenet. 1991;7:153–92. [PubMed: 1679453]
- Buchner E., Rodrigues V. Autoradiographic localization of [3H]choline uptake in the brain of Drosophila melanogaster. Neurosci Lett. 1983;42:25–31. [PubMed: 6318163]
- Buck L., Axel R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell. 1991;65:175–87. [PubMed: 1840504]
- Carlson J.R. Functional expression of a Drosophila odor receptor. Proc Natl Acad Sci USA. 2001;98:8936–37. [PMC free article: PMC55351] [PubMed: 11481464]
- Carlsson M.A., Galizia C.G., Hansson B.S. Spatial representation of odours in the antennal lobe of the moth Spodoptera littoralis (Lepidoptera: Noctuidae). Chem Senses. 2002;27:231–44. [PubMed: 11923186]
- Chapman R.F. Development of phenotypic differences in sensillum populations on the antennae of a grasshopper, Schistocerca americana. J Morphol. 2002;254:186–94. [PubMed: 12353300]
- Chess A., Buck L., Dowling M.M., Axel R., Ngai J. Molecular biology of smell: Expression of the multigene family encoding putative odorant receptors. Cold Spring Harb Symp Quant Biol. 1992;57:505–16. [PubMed: 1339687]
- Christensen T.A., Harrow I.D., Cuzzocrea C., Randolph P.W., Hildebrand J.G. Distinct projections of two populations of olfactory receptor axons in the antennal lobe of the sphinx moth Manduca sexta. Chem Senses. 1995;20:313–23. [PubMed: 7552040]
- Christensen T.A., Hildebrand J.G. Coincident stimulation with pheromone components improves temporal pattern resolution in central olfactory neurons. J Neurophysiol. 1997;77:775–81. [PubMed: 9065849]
- Christensen T.A., Hildebrand J.G. Pheromonal and host-odor processing in the insect antennal lobe: How different? Curr Opin Neurobiol. 2002;12:393–99. [PubMed: 12139986]
- Christensen T.A., Pawlowski V.M., Lei H., Hildebrand J.G. Multi-unit recordings reveal context-dependent modulation of synchrony in odor-specific neural ensembles. Nat Neurosci. 2000;3:927–31. [PubMed: 10966624]
- Christensen T.A., Waldrop B.R., Harrow I.D., Hildebrand J.G. Local interneurons and information processing in the olfactory glomeruli of the moth Manduca sexta. J Comp Physiol A. 1993;173:385–99. [PubMed: 8254565]
- Christensen T.A., Waldrop B.R., Hildebrand J.G. GABAergic mechanisms that shape the temporal response to odors in moth olfactory projection neurons. Ann N Y Acad Sci. 1998a;855:475–81. [PubMed: 9929641]
- Christensen T.A., Waldrop B.R., Hildebrand J.G. Multitasking in the olfactory system: Context-dependent responses to odors reveal dual GABAregulated coding mechanisms in single olfactory projection neurons. J Neurosci. 1998b;18:5999–6008. [PMC free article: PMC6793051] [PubMed: 9671685]
- Clyne P.J., Warr C.G., Freeman M.R., Lessing D., Kim J., Carlson J.R. A novel family of divergent seven-transmembrane proteins: Candidate odorant receptors in Drosophila. Neuron. 1999;22:327–38. [PubMed: 10069338]
- Cobb M. What and how do maggots smell? Biol Rev. 1999;74:425–59.
- Collmann C., Carlsson M.A., Hansson B.S., Nighorn A. Odorant-evoked nitric oxide signals in the antennal lobe of Manduca sexta. J Neurosci. 2004;24:6070–77. [PMC free article: PMC1794326] [PubMed: 15240798]
- Connolly J.B., Roberts I.J., Armstrong J.D., Kaiser K., Forte M., et al. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–7. [PubMed: 8953046]
- Costa R. Esterase-6 and the pheromonal effects of cis-vaccenyl acetate in Drosophila melanogaster. J Evol Biol. 1989;2:395–407.
- Couto A., Alenius M., Dickson B.J. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–47. [PubMed: 16139208]
- Dahanukar A., Hallem E.A., Carlson J.R. Insect chemoreception. Curr Opin Neurobiol. 2005;15:423–30. [PubMed: 16006118]
- Daly K.C., Christensen T.A., Lei H., Smith B.H., Hildebrand J.G. Learning modulates the ensemble representations for odors in primary olfactory networks. Proc Natl Acad Sci USA. 2004;101:10476–81. [PMC free article: PMC478594] [PubMed: 15232007]
- Das A., Sen S., Lichtneckert R., Okada R., Ito K., et al. Drosophila olfactory local interneurons and projection neurons derive from a common neuroblast lineage specified by the empty spiracles gene. Neural Dev. 2008;3(33) [PMC free article: PMC2647541] [PubMed: 19055770]
- Davis N.T., Homberg U., Teal P.E.A., Altstein M., Agricola H.J., Hildebrand J.G. Neuroanatomy and immunocytochemistry of the median neuroendocrine cells of the subesophageal ganglion of the tobacco hawkmoth, Manduca sexta: Immunoreactivities to PBAN and other neuropeptides. Microsc Res Tech. 1996;35:201–29. [PubMed: 8956271]
- Davis R.L. Olfactory learning. Neuron. 2004;44:31–48. [PubMed: 15450158]
- de Belle J.S., Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–95. [PubMed: 8303280]
- de Bruyne M., Foster K., Carlson J.R. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–52. [PubMed: 11395013]
- Deisig N., Lachnit H., Sandoz J.C., Lober K., Giurfa M. A modified version of the unique cue theory accounts for olfactory compound processing in honeybees. Learn Mem. 2003;10:199–208. [PMC free article: PMC202310] [PubMed: 12773584]
- Dekker T., Steib B., Cardé R.T., Geier M. L-lactic acid: A human-signifying host cue for the anthropophilic mosquito Anopheles gambiae. Med Vet Entomol. 2002;16:91–98. [PubMed: 11963986]
- Devaud J.M., Acebes A., Ferrus A. Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J Neurosci. 2001;21:6274–82. [PMC free article: PMC6763130] [PubMed: 11487650]
- Devaud J.M., Acebes A., Ramaswami M., Ferrus A. Structural and functional changes in the olfactory pathway of adult Drosophila take place at a critical age. J Neurobiol. 2003;56:13–23. [PubMed: 12767029]
- Distler P.G., Bausenwein B., Boeckh J. Localization of odor-induced neuronal activity in the antennal lobes of the blowfly Calliphora vicina: A [3H] 2-deoxyglucose labeling study. Brain Res. 1998a;805:263–66. [PubMed: 9733981]
- Distler P.G., Boeckh J. Synaptic connection between olfactory receptor cells and uniglomerular projection neurons in the antennal lobe of the American cockroach, Periplaneta americana. J Comp Neurol. 1996;370:35–46. [PubMed: 8797155]
- Distler P.G., Boeckh J. Synaptic connections between identified neuron types in the antennal lobe glomeruli of the cockroach, Periplaneta americana: II. Local multiglomerular interneurons. J Comp Neurol. 1997;383:529–40. [PubMed: 9208997]
- Distler P.G., Gruber C., Boeckh J. Synaptic connections between GABA-immunoreactive neurons and uniglomerular projection neurons within the antennal lobe of the cockroach, Periplaneta americana. Synapse. 1998b;29:1–13. [PubMed: 9552171]
- Ditzen M., Evers J.F., Galizia C.G. Odor similarity does not influence the time needed for odor processing. Chem Senses. 2003;28:781–89. [PubMed: 14654446]
- Dobritsa A.A., van der Goes, van Naters W., Warr C.G., Steinbrecht R.A., Carlson J.R. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–41. [PubMed: 12628173]
- Dubnau J., Chiang A.S., Tully T. Neural substrates of memory: From synapse to system. J Neurobiol. 2003;54:238–53. [PubMed: 12486707]
- Dubnau J., Grady L., Kitamoto T., Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–80. [PubMed: 11373680]
- Elphick M., Rayne R., Riveros-Moreno V.V., Moncada S., Shea M. Nitric oxide synthesis in locust olfactory interneurones. J Exp Biol. 1995;198:821–29. [PubMed: 9318598]
- Engsontia P., Sanderson A.P., Cobb M., Walden K.K., Robertson H.M., Brown S. The red flour beetle’s large nose: An expanded odorant receptor gene family in Tribolium castaneum. Insect Biochem Mol Biol. 2008;38:387–97. [PubMed: 18342245]
- Erber J., Masuhr T., Menzel R. Localization of short-term-memory in the brain of the bee, Apis mellifera. Physiol Entomol. 1980;5:343–58.
- Ernst K.D., Boeckh J. A neuroanatomical study on the organization of the central antennal pathways in insects. 3. Neuroanatomical characterization of physiologically defined response types of deutocerebral neurons in Periplaneta americana. Cell Tissue Res. 1983;229:1–22. [PubMed: 6831538]
- Ernst K.D., Boeckh J., Boeckh V. A neuroanatomical study on the organization of the central antennal pathways in insects. Cell Tissue Res. 1977;176:285–306. [PubMed: 832298]
- Esslen J., Kaissling K.E. Number and distribution of sensilla on antennal flagellum of honeybee (Apis mellifera L). Zoomorphologie. 1976;83:227–51.
- Faber T., Joerges J., Menzel R. Associative learning modifies neural representations of odors in the insect brain. Nat Neurosci. 1999;2:74–78. [PubMed: 10195183]
- Faber T., Menzel R. Visualizing mushroom body response to a conditioned odor in honeybees. Naturwissenschaften. 2001;88:472–76. [PubMed: 11771476]
- Farivar S.S. Cytoarchitecture of the locust olfactory system. 2005 Dissertation, http://resolver
.caltech.edu/ - Farooqui T., Robinson K., Vaessin H., Smith B.H. Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. J Neurosci. 2003;23:5370–80. [PMC free article: PMC6741157] [PubMed: 12832563]
- Farris S.M. Evolution of insect mushroom bodies: Old clues, new insights. Arthropod Struct Dev. 2005;34:211–34.
- Farris S.M., Abrams A.I., Strausfeld N.J. Development and morphology of class II Kenyon cells in the mushroom bodies of the honey bee, Apis mellifera. J Comp Neurol. 2004;474:325–39. [PubMed: 15174077]
- Farris S.M., Robinson G.E., Fahrbach S.E. Experience and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J Neurosci. 2001;21:6395–6404. [PMC free article: PMC6763189] [PubMed: 11487663]
- Farris S.M., Strausfeld N.J. Development of laminar organization in the mushroom bodies of the cockroach: Kenyon cell proliferation, outgrowth, and maturation. J Comp Neurol. 2001;439:331–51. [PubMed: 11596058]
- Feinstein P., Bozza T., Rodriguez I., Vassalli A., Mombaerts P. Axon guidance of mouse olfactory sensory neurons by odorant receptors and the beta2 adrenergic receptor. Cell. 2004;117:833–46. [PubMed: 15186782]
- Feinstein P., Mombaerts P. A contextual model for axonal sorting into glomeruli in the mouse olfactory system. Cell. 2004;117:817–31. [PubMed: 15186781]
- Fiala A., Spall T., Diegelmann S., Eisermann B., Sachse S., et al. Genetically expressed cameleon in Drosophila melanogaster is used to visualize olfactory information in projection neurons. Curr Biol. 2002;12:1877–84. [PubMed: 12419190]
- Fishilevich E., Vosshall L.B. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–53. [PubMed: 16139209]
- Flanagan D., Mercer A.R. An atlas and 3-D reconstruction of the antennal lobes in the worker honey bee, Apis mellifera L. (Hymenoptera: Apidae). Int J Insect Morphol Embryol. 1989a;18:145–59.
- Flanagan D., Mercer A.R. Morphology and response characteristics of neurones in the deutocerebrum of the brain in the honeybee Apis mellifera. J Comp Physiol A. 1989b;164:483–94.
- Friedrich R.W., Laurent G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science. 2001;291:889–94. [PubMed: 11157170]
- Galán R.F., Sachse S., Galizia C.G., Herz A.V. Odor-driven attractor dynamics in the antennal lobe allow for simple and rapid olfactory pattern classification. Neural Comput. 2004;16:999–1012. [PubMed: 15070507]
- Galán R.F., Weidert M., Menzel R., Herz A.V.M., Galizia C.G. Sensory memory for odors is encoded in spontaneous correlated activity between olfactory glomeruli. Neural Comput. 2006;18:10–25. [PubMed: 16354378]
- Galizia C.G., Joerges J., Küttner A., Faber T., Menzel R. A semi in-vivo preparation for optical recording of the insect brain. J Neurosci Methods. 1997;76:61–69. [PubMed: 9334940]
- Galizia C.G., Kimmerle B. Physiological and morphological characterization of honeybee olfactory neurons combining electrophysiology, calcium imaging and confocal microscopy. J Comp Physiol A. 2004;190:21–38. [PubMed: 14639486]
- Galizia C.G., Küttner A., Joerges J., Menzel R. Odour representation in honeybee olfactory glomeruli shows slow temporal dynamics: An optical recording study using a voltage-sensitive dye. J Insect Physiol. 2000a;46:877–86. [PubMed: 10802099]
- Galizia C.G., McIlwrath S.L., Menzel R. A digital three-dimensional atlas of the honeybee antennal lobe based on optical sections acquired by confocal microscopy. Cell Tissue Res. 1999a;295:383–94. [PubMed: 10022959]
- Galizia C., Menzel R. The role of glomeruli in the neural representation of odours: Results from optical recording studies. J Insect Physiol. 2001;47:115–30. [PubMed: 11064019]
- Galizia C.G., Menzel R., Hölldobler B. Optical imaging of odor-evoked glomerular activity patterns in the antennal lobes of the ant Camponotus rufipes. Naturwissenschaften. 1999b;86:533–37. [PubMed: 10551948]
- Galizia C., Sachse S., Mustaparta H. Calcium responses to pheromones and plant odours in the antennal lobe of the male and female moth Heliothis virescens. J Comp Physiol A. 2000b;186:1049–63. [PubMed: 11195281]
- Galizia C.G., Sachse S., Rappert A., Menzel R. The glomerular code for odor representation is species specific in the honeybee Apis mellifera. Nat Neurosci. 1999c;2:473–78. [PubMed: 10321253]
- Ganeshina O., Menzel R. GABA-immunoreactive neurons in the mushroom bodies of the honeybee: An electron microscopic study. J Comp Neurol. 2001;437:335–49. [PubMed: 11494260]
- Gao Q., Chess A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics. 1999;60:31–39. [PubMed: 10458908]
- Gascuel J., Masson C. A quantitative ultrastructural study of the honeybee antennal lobe. Tissue Cell. 1991;23:341–55. [PubMed: 18621165]
- Gerber B., Scherer S., Neuser K., Michels B., Hendel T., et al. Visual learning in individually assayed Drosophila larvae. J Exp Biol. 2004a;207:179–88. [PubMed: 14638844]
- Gerber B., Stocker R.F. The Drosophila larva as a model for studying chemosensation and chemosensory learning: A review. Chem Senses. 2007;32:65–89. [PubMed: 17071942]
- Gerber B., Tanimoto H., Heisenberg M. An engram found? Evaluating the evidence from fruit flies. Curr Opin Neurobiol. 2004b;14:737–44. [PubMed: 15582377]
- Getz W.M. A neural network for processing olfactory-like stimuli. Bull Math Biol. 1991;53:805–23. [PubMed: 1958892]
- Getz W.M., Lutz A. A neural network model of general olfactory coding in the insect antennal lobe. Chem Senses. 1999;24:351–72. [PubMed: 10480672]
- Gewecke M. Central projection of antennal afferents for the flight motor in Locusta migratoria (Orthoptera, Acrididae). Entomol Gen. 1979;5:317–20.
- Gibson N.J., Nighorn A. Expression of nitric oxide synthase and soluble guanylyl cyclase in the developing olfactory system of Manduca sexta. J Comp Neurol. 2000;422:191–205. [PubMed: 10842227]
- Gibson N.J., Rossler W., Nighorn A.J., Oland L.A., Hildebrand J.G., Tolbert L.P. Neuron-glia communication via nitric oxide is essential in establishing antennal-lobe structure in Manduca sexta. Dev Biol. 2001;240:326–39. [PubMed: 11784067]
- Goldman A.L., Van der Goes van Naters W., Lessing D., Warr C.G., Carlson J.R. Coexpression of two functional odor receptors in one neuron. Neuron. 2005;45:661–66. [PubMed: 15748842]
- Goriely A.R., Secomb T.W., Tolbert L.P. Effect of the glial envelope on extracellular K+ diffusion in olfactory glomeruli. J Neurophysiol. 2002;87:1712–22. [PubMed: 11929893]
- Grant A.J., Wigton B.E., Aghajanian J.G., O’Connell R.J. Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J Comp Physiol A. 1995;177:389–96. [PubMed: 7674195]
- Grimaldi D.A., Engel M.S. Evolution of the Insects. Cambridge: Cambridge University Press; 2004.
- Gronenberg W. Modality-specific segregation of input to ant mushroom bodies. Brain Behav Evol. 1999;54:85–95. [PubMed: 10529521]
- Gronenberg W. Subdivisions of hymenopteran mushroom body calyces by their afferent supply. J Comp Neurol. 2001;435:474–89. [PubMed: 11406827]
- Grünbaum L., Müller U. Induction of a specific olfactory memory leads to a long-lasting activation of protein kinase C in the antennal lobe of the honeybee. J Neurosci. 1998;18:4384–92. [PMC free article: PMC6792810] [PubMed: 9592115]
- Grünewald B. Morphology of feedback neurons in the mushroom body of the honeybee, Apis mellifera. J Comp Neurol. 1999;404:114–26. [PubMed: 9886029]
- Guerenstein P.G., Yepez E.A., van Haren J., Williams D.G., Hildebrand J.G. Floral CO2 emission may indicate food abundance to nectar-feeding moths. Naturwissenschaften. 2004;91:329–33. [PubMed: 15257387]
- Guerrieri F., Schubert M., Sandoz J.C., Giurfa M. Perceptual and neural olfactory similarity in honeybees. PLoS Biol. 2005;3:e60. [PMC free article: PMC1043859] [PubMed: 15736975]
- Hähnlein I., Bicker G. Morphology of neuroglia in the antennal lobes and mushroom bodies of the brain of the honeybee. J Comp Neurol. 1996;367:235–45. [PubMed: 8708007]
- Hähnlein I., Hartig W., Bicker G. Datura stramonium lectin staining of glial associated extracellular material in insect brains. J Comp Neurol. 1996;376:175–87. [PubMed: 8951635]
- Hallem E.A., Carlson J.R. Coding of odors by a receptor repertoire. Cell. 2006;125:143–60. [PubMed: 16615896]
- Hammer M. The neural basis of associative reward learning in honeybees. Trends Neurosci. 1997;20:245–52. [PubMed: 9185305]
- Hammer M., Menzel R. Multiple sites of associative odor learning as revealed by local brain micro-injections of octopamine in honeybees. Learn Mem. 1998;5:146–56. [PMC free article: PMC311245] [PubMed: 10454379]
- Hansson B.S., Carlsson M.A., Kalinova B. Olfactory activation patterns in the antennal lobe of the sphinx moth, Manduca sexta. J Comp Physiol A. 2003;189:301–8. [PubMed: 12743734]
- Hansson B.S., Christensen T.A., Hildebrand J.G. Functionally distinct subdivisions of the macroglomerular complex in the antennal lobe of the male sphinx moth Manduca sexta. J Comp Neurol. 1991;312:264–78. [PubMed: 1748732]
- Heimbeck G., Bugnon V., Gendre N., Haberlin C., Stocker R.F. Smell and taste perception in Drosophila melanogaster larva: Toxin expression studies in chemosensory neurons. J Neurosci. 1999;19:6599–6609. [PMC free article: PMC6782832] [PubMed: 10414987]
- Heinbockel T., Kloppenburg P., Hildebrand J.G. Pheromone-evoked potentials and oscillations in the antennal lobes of the sphinx moth Manduca sexta. J Comp Physiol A. 1998;182:703–14. [PubMed: 9631552]
- Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4:266–75. [PubMed: 12671643]
- Heisenberg M., Borst A., Wagner S., Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet. 1985;2:1–30. [PubMed: 4020527]
- Hendel T., Michels B., Neuser K., Schipanski A., Kaun K., et al. The carrot, not the stick: Appetitive rather than aversive gustatory stimuli support associative olfactory learning in individually assayed Drosophila larvae. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:265–79. [PubMed: 15657743]
- Hershberger W.A., Smith M.P. Conditioning in Drosophila melanogaster. Anim Behav. 1967;15:259–62. [PubMed: 6030950]
- Hildebrand J.G., Shepherd G.M. Mechanisms of olfactory discrimination: Converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. [PubMed: 9056726]
- Hildebrandt H., Müller U. Octopamine mediates rapid stimulation of protein kinase A in the antennal lobe of honeybees. J Neurobiol. 1995a;27:44–50. [PubMed: 7643074]
- Hildebrandt H., Müller U. PKA activity in the antennal lobe of honeybees is regulated by chemosensory stimulation in vivo. Brain Res. 1995b;679:281–88. [PubMed: 7633889]
- Hill E.S., Iwano M., Gatellier L., Kanzaki R. Morphology and physiology of the serotonin-immunoreactive putative antennal lobe feedback neuron in the male silkmoth Bombyx mori. Chem Senses. 2002;27:475–83. [PubMed: 12052784]
- Homberg U. Neurotransmitters and neuropeptides in the brain of the locust. Microsc Res Tech. 2002;56:189–209. [PubMed: 11810722]
- Homberg U., Hildebrand J.G. Postembryonic development of gamma-aminobutyric acid-like immunoreactivity in the brain of the sphinx moth Manduca-sexta. J Comp Neurol. 1994;339:132–49. [PubMed: 8106658]
- Homberg U., Hoskins S.G., Hildebrand J.G. Distribution of acetylcholinesterase activity in the deutocerebrum of the sphinx moth Manduca sexta. Cell Tissue Res. 1995;279:249–59. [PubMed: 7895267]
- Homberg U., Kingan T.G., Hildebrand J.G. Distribution of FMRFamide-like immunoreactivity in the brain and suboesophageal ganglion of the sphinx moth Manduca sexta and colocalization with SCPB-, B.P.P-, and GABA-like immunoreactivity. Cell Tissue Res. 1990;259:401–19. [PubMed: 2180574]
- Homberg U., Montague R.A., Hildebrand J.G. Anatomy of antenno-cerebral pathways in the brain of the sphinx moth Manduca sexta. Cell Tissue Res. 1988;254:255–81. [PubMed: 3197087]
- Homberg U., Müller U. Neuroactive substances in the antennal lobe. In: Hansson B.S., editor. Insect Olfaction. Berlin: Springer; 1999. pp. 181–206.
- Hoskins S.G., Homberg U., Kingan T.G., Christensen T.A., Hildebrand J.G. Immunocytochemistry of GABA in the antennal lobes of the sphinx moth Manduca sexta. Cell Tissue Res. 1986;244:243–52. [PubMed: 3521878]
- Hösl M. Pheromone-sensitive neurons in the deutocerebrum of Periplaneta americana – Receptive fields on the antenna. J Comp Physiol A. 1990;167:321–27.
- Hosler J.S., Buxton K.L., Smith B.H. Impairment of olfactory discrimination by blockade of GABA and nitric oxide activity in the honey bee antennal lobes. Behav Neurosci. 2000;114:514–25. [PubMed: 10883802]
- Huerta R., Nowotny T., GarciaSanchez M., Abarbanel H.D., Rabinovich M.I. Learning classification in the olfactory system of insects. Neural Comput. 2004;16:1601–40. [PubMed: 15228747]
- Huetteroth W., Schachtner J. Standard three-dimensional glomeruli of the Manduca sexta antennal lobe: A tool to study both developmental and adult neuronal plasticity. Cell Tissue Res. 2005;319:513–24. [PubMed: 15672266]
- Hummel T., Vasconcelos M.L., Clemens J.C., Fishilevich Y., Vosshall L.B., Zipursky S.L. Axonal targeting of olfactory receptor neurons in Drosophila is controlled by Dscam. Neuron. 2003;37:221–31. [PubMed: 12546818]
- Hummel T., Zipursky S.L. Afferent induction of olfactory glomeruli requires N-cadherin. Neuron. 2004;42:77–88. [PubMed: 15066266]
- Husch A., Paehler M., Fusca D., Paeger L., Kloppenburg P. Calcium current diversity in physiologically different local interneuron types of the antennal lobe. J Neurosci. 2009;29:716–26. [PMC free article: PMC6665175] [PubMed: 19158298]
- Ignell R., Anton S., Hansson B.S. The antennal lobe of orthoptera – anatomy and evolution. Brain Behav Evol. 2001;57:1–17. [PubMed: 11359044]
- Ignell R., Dekker T., Ghaninia M., Hansson B.S. Neuronal architecture of the mosquito deutocerebrum. J Comp Neurol. 2005;493:207–40. [PubMed: 16255032]
- Isabel G., Pascual A., Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–27. [PubMed: 15143285]
- Ito K., Suzuki K., Estes P., Ramaswami M., Yamamoto D., Strausfeld N.J. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster Meigen. Learn Mem. 1998;5:52–77. [PMC free article: PMC311240] [PubMed: 10454372]
- Iwano M., Kanzaki R. Immunocytochemical identification of neuroactive substances in the antennal lobe of the male silkworm moth Bombyx mori. Zoolog Sci. 2005;22:199–211. [PubMed: 15738640]
- Iwasaki M., Mizunami M., Nishikawa M., Itoh T., Tominaga Y. Ultrastructural analyses of modular subunits in the mushroom bodies of the cockroach. J Electron Microsc. 1999;48:55–62.
- Jackson F.R., Newby L.M., Kulkarni S.J. Drosophila GABAergic systems: Sequence and expression of glutamic acid decarboxylase. J Neurochem. 1990;54:1068–78. [PubMed: 1689376]
- Jefferis G., Marin E.C., Stocker R.F., Luo L.Q. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414:204–8. [PubMed: 11719930]
- Jefferis G., Vyas R.M., Berdnik D., Ramaekers A., Stocker R.F., et al. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development. 2004;131:117–30. [PubMed: 14645123]
- Jefferis G.S., Potter C.J., Chan A.M., Marin E.C., Rohlfing T., et al. Comprehensive maps of Drosophila higher olfactory centers: Spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. [PMC free article: PMC1885945] [PubMed: 17382886]
- Jhaveri D., Rodrigues V. Sensory neurons of the Atonal lineage pioneer the formation of glomeruli within the adult Drosophila olfactory lobe. Development. 2002;129:1251–60. [PubMed: 11874920]
- Jhaveri D., Saharan S., Sen A., Rodrigues V. Positioning sensory terminals in the olfactory lobe of Drosophila by Robo signaling. Development. 2004;131:1903–12. [PubMed: 15056612]
- Jhaveri D., Sen A., Rodrigues V. Mechanisms underlying olfactory neuronal connectivity in Drosophila – The atonal lineage organizes the periphery while sensory neurons and glia pattern the olfactory lobe. Dev Biol. 2000;226:73–87. [PubMed: 10993675]
- Joerges J., Küttner A., Galizia C.G., Menzel R. Representations of odours and odour mixtures visualized in the honeybee brain. Nature. 1997;387:285–88.
- Jones W.D., Nguyen T.A., Kloss B., Lee K.J., Vosshall L.B. Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr Biol. 2005;15:R119–21. [PubMed: 15723778]
- Justus K.A., Cardé R.T., French A.S. Dynamic properties of antennal responses to pheromone in two moth species. J Neurophysiol. 2005;93:2233–39. [PubMed: 15537812]
- Kaissling K.E. Olfactory perireceptor and receptor events in moths: A kinetic model. Chem Senses. 2001;26:125–50. [PubMed: 11238244]
- Kasowski H.J., Kim H., Greer C.A. Compartmental organization of the olfactory bulb glomerulus. J Comp Neurol. 1999;407:261–74. [PubMed: 10213094]
- Keene A.C., Stratmann M., Keller A., Perrat P.N., Vosshall L.B., Waddell S. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron. 2004;44:521–33. [PubMed: 15504331]
- Keil T.A. Sensilla on the maxillary palps of Helicoverpa armigera caterpillars: In search of the CO2receptor. Tissue Cell. 1996;28:703–17. [PubMed: 18621342]
- Kirschner S., Kleineidam C.J., Zube C., Rybak J., Grunewald B., Rössler W. Dual olfactory pathway in the honeybee, Apis mellifera. J Comp Neurol. 2006;499:933–52. [PubMed: 17072827]
- Kleineidam C., Tautz J. Perception of carbon dioxide and other “air-condition” parameters in the leaf cutting ant Atta cephalotes. Naturwissenschaften. 1996;83:566–68.
- Kloppenburg P., Camazine S.M., Sun X.J., Randolph P., Hildebrand J.G. Organization of the antennal motor system in the sphinx moth Manduca sexta. Cell Tissue Res. 1997;287:425–33. [PubMed: 8995213]
- Komischke B., Sandoz J.C., Malun D., Giurfa M. Partial unilateral lesions of the mushroom bodies affect olfactory learning in honeybees Apis mellifera L. Eur J Neurosci. 2005;21:477–85. [PubMed: 15673446]
- Komiyama T., Carlson J.R., Luo L. Olfactory receptor neuron axon targeting: Intrinsic transcriptional control and hierarchical interactions. Nat Neurosci. 2004;7:819–25. [PubMed: 15247920]
- Komiyama T., Johnson W.A., Luo L., Jefferis G.S. From lineage to wiring specificity. POU domain transcription factors control precise connections of Drosophila olfactory projection neurons. Cell. 2003;112:157–67. [PubMed: 12553905]
- Kreher S., Kwon J.Y., Carlson J.R. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46:445–56. [PubMed: 15882644]
- Kreissl S., Bicker G. Histochemistry of acetylcholinesterase and immunocytochemistry of an acetylcholine receptor-like antigen in the brain of the honeybee. J Comp Neurol. 1989;286:71–84. [PubMed: 2768558]
- Kretzschmar D., Pflugfelder G.O. Glia in development, function, and neurodegeneration of the adult insect brain. Brain Res Bull. 2002;57:121–31. [PubMed: 11827744]
- Krieger J., Breer H. Olfactory reception in invertebrates. Science. 1999;286:720–23. [PubMed: 10531050]
- Krieger J., Klink O., Mohl C., Raming K., Breer H. A candidate olfactory receptor subtype highly conserved across different insect orders. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2003;189:519–26. [PubMed: 12827420]
- Kwon H.W., Lent D.D., Strausfeld N.J. Spatial learning in the restrained American cockroach Periplaneta americana. J Exp Biol. 2004;207:377–83. [PubMed: 14668321]
- Lacher V. Elektrophysiologische Untersuchungen an einzelnen Rezeptoren für Geruch, Kohlendioxyd, Luftfeuchtigkeit und Temperatur auf den Antennen der Arbeitsbiene und der Drohne (Apis mellifica L.) [Electrophysiological single-receptor analysis for odor, CO2, humidity and temperature on the antennae of worker and drone honeybees] Z Vergl Physiol. 1964;48:587–623.
- Laissue P.P., Reiter C., Hiesinger P.R., Halter S., Fischbach K.F., Stocker R.F. Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J Comp Neurol. 1999;405:543–52. [PubMed: 10098944]
- Larsson M.C., Domingos A.I., Jones W.D., Chiappe M.E., Amrein H., Vosshall L.B. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–14. [PubMed: 15339651]
- Lattemann M., Zierau A., Schulte C., Seidl S., Kuhlmann B., Hummel T. Semaphorin-1a controls receptor neuron-specific axonal convergence in the primary olfactory center of Drosophila. Neuron. 2007;53:169–84. [PubMed: 17224401]
- Laurent G. Dynamical representation of odors by oscillating and evolving neural assemblies. Trends Neurosci. 1996;19:489–96. [PubMed: 8931275]
- Laurent G. A systems perspective on early olfactory coding. Science. 1999;286:723–28. [PubMed: 10531051]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3:884–95. [PubMed: 12415296]
- Laurent G., Davidowitz H. Encoding of olfactory information with oscillating neural assemblies. Science. 1994;265:1872–75. [PubMed: 17797226]
- Laurent G., Stopfer M., Friedrich R.W., Rabinovich M.I., Volkovskii A., Abarbanel H.D. Odor encoding as an active, dynamical process: Experiments, computation, and theory. Annu Rev Neurosci. 2001;24:263–97. [PubMed: 11283312]
- Laurent G., Wehr M., Davidowitz H. Temporal representations of odors in an olfactory network. J Neurosci. 1996;16:3837–47. [PMC free article: PMC6578619] [PubMed: 8656278]
- Leitch B., Laurent G. GABAergic synapses in the antennal lobe and mushroom body of the locust olfactory system. J Comp Neurol. 1996;372:487–514. [PubMed: 8876449]
- Leon M., Johnson B.A. Olfactory coding in the mammalian olfactory bulb. Brain Res Brain Res Rev. 2003;42:23–32. [PubMed: 12668289]
- Levin L.R., Han P.L., Hwang P.M., Feinstein P.G., Davis R.L., Reed R.R. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–89. [PubMed: 1739965]
- Linster C., Kerszberg M., Masson C. How neurons may compute: The case of insect sexual pheromone discrimination. J Comput Neurosci. 1994;1:231–38. [PubMed: 8792232]
- Linster C., Sachse S., Galizia C.G. Computational modeling suggests that response properties rather than spatial position determine connectivity between olfactory glomeruli. J Neurophysiol. 2005;93:3410–17. [PubMed: 15673548]
- Liu X., Buchanan M.E., Han K.A., Davis R.L. The GABAA receptor RDL suppresses the conditioned stimulus pathway for olfactory learning. J Neurosci. 2009;29:1573–79. [PMC free article: PMC2665291] [PubMed: 19193904]
- MacLeod K., Laurent G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science. 1996;274:976–79. [PubMed: 8875938]
- Malun D. Inventory and distribution of synapses of identified uniglomerular projection neurons in the antennal lobe of Periplaneta americana. J Comp Neurol. 1991a;305:348–60. [PubMed: 1709183]
- Malun D. Synaptic relationships between GABA-immunoreactive neurons and an identified uniglomerular projection neuron in the antennal lobe of Periplaneta americana: A double-labeling electron microscopic study. Histochemistry. 1991b;96:197–207. [PubMed: 1917576]
- Malun D., Giurfa M., Galizia C.G., Plath N., Brandt R. et al. Hydroxyurea-induced partial mushroom body ablation does not affect acquisition and retention of olfactory differential conditioning in honeybees. J Neurobiol. 2002;53:343–60. [PubMed: 12382262]
- Mao Z., Roman G., Zong L., Davis R.L. Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Switch. Proc Natl Acad Sci USA. 2004;101:198–203. [PMC free article: PMC314162] [PubMed: 14684832]
- Marin E.C., Jefferis G.S., Komiyama T., Zhu H., Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–55. [PubMed: 12007410]
- Marin E.C., Watts R.J., Tanaka N.K., Ito K., Luo L.Q. Developmentally programmed remodeling of the Drosophila olfactory circuit. Development. 2005;132:725–37. [PubMed: 15659487]
- Masante-Roca I., Gadenne C., Anton S. Three-dimensional antennal lobe atlas of male and female moths, Lobesia botrana (Lepidoptera: Tortricidae) and glomerular representation of plant volatiles in females. J Exp Biol. 2005;208:1147–59. [PubMed: 15767314]
- Matsumoto S.G., Hildebrand J.G. Olfactory mechanisms in the moth Manduca sexta – response characteristics and morphology of central neurons in the antennal lobes. Proc R Soc Lond B Biol Sci. 1981;213:249–77.
- Mauelshagen J. Neural correlates of olfactory learning paradigms in an identified neuron in the honeybee brain. J Neurophysiol. 1993;69:609–25. [PubMed: 8459289]
- McGuire S.E., Le P.T., Davis R.L. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–33. [PubMed: 11397912]
- McGuire S., Le P., Osborn A., Matsumoto K., Davis R.L. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–68. [PubMed: 14657498]
- Menzel R., Giurfa M. Cognitive architecture of a mini-brain: The honeybee. Trends Cogn Sci. 2001;5:62–71. [PubMed: 11166636]
- Mizunami M., Weibrecht J.M., Strausfeld N.J. Mushroom bodies of the cockroach: Their participation in place memory. J Comp Neurol. 1998;402:520–37. [PubMed: 9862324]
- Mobbs P.G. The brain of the honeybee Apis mellifera 1. The connections and spatial organization of the mushroom bodies. Philos T Roy Soc B. 1982;298:309–54.
- Mobbs P.G. Brain structure. In: Kerkut G.A., Gilbert L.I., editors. Comprehensive Insect Physiology Biochemistry and Pharmacology. Nervous System: Structure and Motor Function. Vol. 5. Oxford: Pergamon Press; 1985. pp. 299–370.
- Monastirioti M. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc Res Tech. 1999;45:106–21. [PubMed: 10332728]
- Müller D., Abel R., Brandt R., Zockler M., Menzel R. Differential parallel processing of olfactory information in the honeybee, Apis mellifera L. J Comp Physiol A. 2002;188:359–70. [PubMed: 12073081]
- Müller U. Prolonged activation of cAMP-dependent protein kinase during conditioning induces longterm memory in honeybees. Neuron. 2000;27:159–68. [PubMed: 10939339]
- Müller U., Hildebrandt H. Nitric oxide/cGMP-mediated protein kinase A activation in the antennal lobes plays an important role in appetitive reflex habituation in the honeybee. J Neurosci. 2002;22:8739–47. [PMC free article: PMC6757786] [PubMed: 12351749]
- Murlis J., Elkinton J.S., Cardé R.T. Odor plumes and how insects use them. Annu Rev Entomol. 1992;37:505–32.
- Nässel D.R. Neuropeptides in the insect brain: A review. Cell Tissue Res. 1993;273:1–29. [PubMed: 8364953]
- Nässel D.R. Histamine in the brain of insects: A review. Microsc Res Tech. 1999;44:121–36. [PubMed: 10084821]
- Nässel D.R. Functional roles of neuropeptides in the insect central nervous system. Naturwissenschaften. 2000;87:439–49. [PubMed: 11129943]
- Neder R. Allometrisches Wachstum von Hirnteilen bei drei verschieden grossen Schabenarten. [Allometric growth of brain structures in three cockroaches of different size.] Zool Jahrb Anat. 1959;77:411–64.
- Neuhaus E.M., Gisselmann G., Zhang W., Dooley R., Stortkuhl K., Hatt H. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat Neurosci. 2005;8:15–17. [PubMed: 15592462]
- Ng M., Roorda R.D., Lima S.Q., Zemelman B.V., Morcillo P., Miesenbock G. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–74. [PubMed: 12408848]
- Ochieng S.A., Hallberg E., Hansson B.S. Fine structure and distribution of antennal sensilla of the desert locust, Schistocerca gregaria (Orthoptera: Acrididae). Cell Tissue Res. 1998;291:525–36. [PubMed: 9477309]
- Okada R., Rybak J., Manz G., Menzel R. Learning-related plasticity in PE1 and other mushroom body-extrinsic neurons in the honeybee brain. J Neurosci. 2007;27:11736–47. [PMC free article: PMC6673233] [PubMed: 17959815]
- Oland L.A., Marrero H.G., Burger I. Glial cells in the developing and adult olfactory lobe of the moth Manduca sexta. Cell Tissue Res. 1999;297:527–45. [PubMed: 10460499]
- Oland L.A., Tolbert L.P. Effects of hydroxyurea parallel the effects of radiation in developing olfactory glomeruli in insects. J Comp Neurol. 1988;278:377–87. [PubMed: 3216049]
- Oland L.A., Tolbert L.P. Key interactions between neurons and glial cells during neural development in insects. Annu Rev Entomol. 2003;48:89–110. [PubMed: 12194908]
- Oland L.A., Tolbert L.P., Mossman K.L. Radiation-induced reduction of the glial population during development disrupts the formation of olfactory glomeruli in an insect. J Neurosci. 1988;8:353–67. [PMC free article: PMC6569375] [PubMed: 3339417]
- Olsen S.R., Wilson R.I. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–60. [PMC free article: PMC2824883] [PubMed: 18344978]
- Olshausen B.A., Field D.J. Sparse coding of sensory inputs. Curr Opin Neurobiol. 2004;14:481–87. [PubMed: 15321069]
- Ott S.R., Elphick M.R. Nitric oxide synthase histochemistry in insect nervous systems: Methanol/formalin fixation reveals the neuroarchitecture of formaldehyde-sensitive NADPH diaphorase in the cockroach Periplaneta americana. J Comp Neurol. 2002;448:165–85. [PubMed: 12012428]
- Pareto A. Die zentrale Verteilung der Fühlerrafferenz bei Arbeiterinnen der Honigbiene, Apis mellifera L. [Spatial distribution of sensory antennal fibres in the central nervous system of worker bees.] Z Zellforsch Mikrosk Anat. 1972;131:109–40. [PubMed: 5073638]
- Pascual A., Preat T. Localization of long-term memory within the Drosophila mushroom body. Science. 2001;294:1115–17. [PubMed: 11691997]
- Pavlopoulos E., Anezaki M., Skoulakis E.M. Neuralized is expressed in the alpha/beta lobes of adult Drosophila mushroom bodies and facilitates olfactory long-term memory formation. Proc Natl Acad Sci USA. 2008;105:14674–79. [PMC free article: PMC2567224] [PubMed: 18794519]
- Peele P. Freie Universität Berlin; Berlin: 2005. Stable odor coding is ensured by uniglomerular output neurons throughout olfactory learning in the honeybee Apis mellifera. PhD Thesis.
- Pelz D., Roeske C., Galizia C.G. Dijon; France: Sep, 2004. Odour response spectrum of a population of genetically identified olfactory sensory neurons: Characterizing the input to a single glomerulus in Drosophila melanogaster; pp. 12–15. ECRO.
- Pelz D., Roeske T., Syed Z., de Bruyne M., Galizia C.G. The molecular receptive range of an olfactory receptor in vivo (Drosophila melanogaster Or22a). J Neurobiol. 2006;66:1544–63. [PubMed: 17103386]
- Perazzona B., Isabel G., Preat T., Davis R.L. The role of cAMP response element-binding protein in Drosophila long-term memory. J Neurosci. 2004;24:8823–28. [PMC free article: PMC6729945] [PubMed: 15470148]
- PerezOrive J., Bazhenov M., Laurent G. Intrinsic and circuit properties favor coincidence detection for decoding oscillatory input. J Neurosci. 2004;24:6037–47. [PMC free article: PMC6729236] [PubMed: 15229251]
- PerezOrive J., Mazor O., Turner G.C., Cassenaer S., Wilson R.I., Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–65. [PubMed: 12130775]
- Pflüger H.J., Stevenson P.A. Evolutionary aspects of octopaminergic systems with emphasis on arthropods. Arthropod Struct Dev. 2005;34:379–96.
- Pitts R.J., Fox A.N., Zwiebel L.J. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 2004;101:5058–63. [PMC free article: PMC387373] [PubMed: 15037749]
- Pophof B. Octopamine enhances moth olfactory responses to pheromones, but not those to general odorants. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:659–62. [PubMed: 12355242]
- Prillinger L. Postembryonic development of the antennal lobes in Periplaneta americana L. Cell Tissue Res. 1981;215:563–75. [PubMed: 7214496]
- Python F., Stocker R.F. Adult-like complexity of the larval antennal lobe of D. melanogaster despite markedly low numbers of odorant receptor neurons. J Comp Neurol. 2002a;445:374–87. [PubMed: 11920714]
- Python F., Stocker R.F. Immunoreactivity against choline acetyltransferase, gamma-aminobutyric acid, histamine, octopamine, and serotonin in the larval chemosensory system of Dosophila melanogaster. J Comp Neurol. 2002b;453:157–67. [PubMed: 12373781]
- Rabinovich M.I., Huerta R., Volkovskii A., Abarbanel H.D., Stopfer M., Laurent G. Dynamical coding of sensory information with competitive networks. J Physiol Paris. 2000;94:465–71. [PubMed: 11165913]
- Raguso R.A. Flowers as sensory billboards: Progress toward an integrated understanding of floral advertisement. Curr Opin Plant Biol. 2004;7:434–40. [PubMed: 15231267]
- Ramaekers A., Magnenat E., Marin E.C., Gendre N., Jefferis G.S., et al. Glomerular maps without cellular redundancy at successive levels of the Drosophila larval olfactory circuit. Curr Biol. 2005;15:982–92. [PubMed: 15936268]
- Ramaekers A., Parmentier M.L., Lasnier C., Bockaert J., Grau Y. Distribution of metabotropic glutamate receptor DmGlu-A in Drosophila melanogaster central nervous system. J Comp Neurol. 2001;438:213–25. [PubMed: 11536189]
- RiesgoEscovar J., Raha D., Carlson J.R. Requirement for a phospholipase C in odor response: Overlap between olfaction and vision in Drosophila. Proc Natl Acad Sci USA. 1995;92:2864–68. [PMC free article: PMC42319] [PubMed: 7708738]
- Robertson H.M., Wanner K.W. The chemoreceptor superfamily in the honey bee, Apis mellifera: Expansion of the odorant, but not gustatory, receptor family. Genome Res. 2006;16:1395–1403. [PMC free article: PMC1626641] [PubMed: 17065611]
- Robertson H.M., Warr C.G., Carlson J.R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;2 100 Suppl:14537–42. [PMC free article: PMC304115] [PubMed: 14608037]
- Rodrigues V., Buchner E. [3H]2-deoxyglucose mapping of odor-induced neuronal activity in the antennal lobes of Drosophila melanogaster. Brain Res. 1984;324:374–78. [PubMed: 6442179]
- Rodrigues V., Hummel T. Development of the Drosophila olfactory system. Adv Exp Med Biol. 2008;628:82–101. [PubMed: 18683640]
- Roeder T. Octopamine in invertebrates. Prog Neurobiol. 1999;59:533–61. [PubMed: 10515667]
- Rospars J.P. Structure and development of the insect antennodeutocerebral system. Int J Insect Morphol Embryol. 1988;17:243–94.
- Rospars J.P., Hildebrand J.G. Anatomical identification of glomeruli in the antennal lobes of the male sphinx moth Manduca sexta. Cell Tissue Res. 1992;270:205–27. [PubMed: 1451169]
- Rössler W., Oland L.A., Higgins M.R., Hildebrand J.G., Tolbert L.P. Development of a glia-rich axon-sorting zone in the olfactory pathway of the moth Manduca sexta. J Neurosci. 1999;19:9865–77. [PMC free article: PMC6782967] [PubMed: 10559396]
- Røstelien T., Borg-Karlson A.K., Fäldt J., Jacobsen U., Mustaparta H. The plant sesquiterpene germacrene D specifically activates a major type of antennal receptor neruons of the tobacco budworm moth Heliothis virescens. Chem Senses. 2000;25:141–48. [PubMed: 10781020]
- Rybak J. Freie Universität; Berlin: 1994. Die strukturelle Organisation der Pilzkîrper und synaptische Konnektivität protocerebraler Interneurone im Gehirn der Honigbiene, Apis mellifera. Eine lichtund elektronenmikroskopische Studie. PhD Thesis.
- Rybak J., Menzel R. Anatomy of the mushroom bodies in the honey bee brain: The neuronal connections of the alpha-lobe. J Comp Neurol. 1993;334:444–65. [PubMed: 8376627]
- Rybak J., Menzel R. Integrative properties of the Pe1 neuron, a unique mushroom body output neuron. Learn Mem. 1998;5:133–45. [PMC free article: PMC311236] [PubMed: 10454378]
- Sachse S., Galizia C.G. Role of inhibition for temporal and spatial odor representation in olfactory output neurons: A calcium imaging study. J Neurophysiol. 2002;87:1106–17. [PubMed: 11826074]
- Sachse S., Galizia C.G. The coding of odour-intensity in the honeybee antennal lobe: Local computation optimizes odour representation. Eur J Neurosci. 2003;18:2119–32. [PubMed: 14622173]
- Sachse S., Galizia C.G. Topography and Dynamics of the Olfactory System. In: Grillner S., editor. Microcircuits: The Interface between Neurons and Global Brain Function, Dahlem Workshop Report 93. Cambridge: MIT Press; 2006. pp. 251–73.
- Sachse S., Rappert A., Galizia C.G. The spatial representation of chemical structures in the antennal lobe of honeybees: Steps toward the olfactory code. Eur J Neurosci. 1999;11:3970–82. [PubMed: 10583486]
- Sandoz J.C., Laloi D., Odoux J.F., Pham-Delegue M.H. Olfactory information transfer in the honeybee: Compared efficiency of classical conditioning and early exposure. Anim Behav. 2000;59:1025–34. [PubMed: 10860530]
- Sanes J.R., Hildebrand J.G. Acetylcholine and its metabolic enzymes in developing antennae of moth, Manduca sexta. Dev Biol. 1976a;52:105–20. [PubMed: 183992]
- Sanes J.R., Hildebrand J.G. Origin and morphogenesis of sensory neurons in an insect antenna. Dev Biol. 1976b;51:300–19. [PubMed: 955261]
- Sanes J.R., Hildebrand J.G. Structure and development of antennae in a moth, Manduca sexta. Dev Biol. 1976c;51:282–99. [PubMed: 955260]
- Sato K., Pellegrino M., Nakagawa T., Vosshall L.B., Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–6. [PubMed: 18408712]
- Schachtner J., Schmidt M., Homberg U. Organization and evolutionary trends of primary olfactory brain centers in Tetraconata (Crustacea+Hexapoda). Arthropod Struct Dev. 2005;34:257–99.
- Schachtner J., Trosowski B., D’Hanis W., Stubner S., Homberg U. Development and steroid regulation of RFamide inummoreactivity in antennal lobe neurons of the sphinx moth Manduca sexta. J Exp Biol. 2004;207:2389–2400. [PubMed: 15184511]
- Schafer R., Sanchez T.V. Antennal sensory system of the cockroach, Periplaneta americana: Postembryonic development and morphology of the sense organs. J Comp Neurol. 1973;149:335–54. [PubMed: 4714572]
- Schäfer S., Bicker G. Distribution of GABA-like immunoreactivity in the brain of the honeybee. J Comp Neurol. 1986;246:287–300. [PubMed: 3700720]
- Scherer S., Stocker R.F., Gerber B. Olfactory learning in individually assayed Drosophila larvae. Learn Mem. 2003;10:217–25. [PMC free article: PMC202312] [PubMed: 12773586]
- Schlief M.L., Wilson R.I. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat Neurosci. 2007;10:623–30. [PMC free article: PMC2838507] [PubMed: 17417635]
- Schürmann F.W. Acetylcholine, G.ABA, glutamate and NO as putative transmitters indicated by immunocytochemistry in the olfactory mushroom body system of the insect brain. Acta Biol Hung. 2000;51:355–62. [PubMed: 11034160]
- Schwaerzel M., Heisenberg M., Zars T. Extinction antagonizes olfactory memory at the subcellular level. Neuron. 2002;35:951–60. [PubMed: 12372288]
- Schwaerzel M., Monastirioti M., Scholz H., FriggiGrelin F., Birman S., Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–502. [PMC free article: PMC6740930] [PubMed: 14627633]
- Seidel C., Bicker G. Colocalization of NADPH-diaphorase and GABA-immunoreactivity in the olfactory and visual system of the locust. Brain Res. 1997;769:273–80. [PubMed: 9374195]
- Shang Y., ClaridgeChang A., Sjulson L., Pypaert M., Miesenbock G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–12. [PMC free article: PMC2866183] [PubMed: 17289577]
- Shirokova E., Schmiedeberg K., Bedner P., Niessen H., Willecke K., et al. Identification of specific ligands for orphan olfactory receptors. G protein-dependent agonism and antagonism of odorants. J Biol Chem. 2005;280:11807–15. [PubMed: 15598656]
- Silbering A.F., Galizia C.G. Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J Neurosci. 2007;27:11966–77. [PMC free article: PMC6673347] [PubMed: 17978037]
- Silbering A.F., Okada R., Ito K., Galizia C.G. Olfactory information processing in the Drosophila antennal lobe: Anything goes? J Neurosci. 2008;28:13075–87. [PMC free article: PMC6671615] [PubMed: 19052198]
- Sinakevitch I., Farris S.M., Strausfeld N.J. Taurine-,aspartateand glutamate-like immunoreactivity identifies chemically distinct subdivisions of Kenyon cells in the cockroach mushroom body. J Comp Neurol. 2001;439:352–67. [PubMed: 11596059]
- Skiri H.T., Galizia C.G., Mustaparta H. Representation of primary plant odorants in the antennal lobe of the moth Heliothis virescens using calcium imaging. Chem Senses. 2004;29:253–67. [PubMed: 15047600]
- Skiri H.T., Ro H., Berg B.G., Mustaparta H. Consistent organization of glomeruli in the antennal lobes of related species of heliothine moths. J Comp Neurol. 2005;491:367–80. [PubMed: 16175552]
- Stange G., Stowe S. Carbon-dioxide sensing structures in terrestrial arthropods. Microsc Res Tech. 1999;47:416–27. [PubMed: 10607381]
- Steinbrecht R.A. Structure and function of insect olfactory sensilla. Ciba Found Symp. 1996;200:158–74. discussion 74–77. [PubMed: 8894297]
- Stengl M., Homberg U., Hildebrand J.G. Acetylcholinesterase activity in antennal receptor neurons of the sphinx moth Manduca sexta. Cell Tissue Res. 1990;262:245–52. [PubMed: 2076532]
- Stensmyr M.C., Giordano E., Balloi A., Angioy A.M., Hansson B.S. Novel natural ligands for Drosophila olfactory receptor neurones. J Exp Biol. 2003;206:715–24. [PubMed: 12517989]
- Stevenson P.A., SporhaseEichmann U. Localization of octopaminergic neurones in insects. Comp Biochem Physiol A Physiol. 1995;110:203–15. [PubMed: 7712064]
- Stocker R.F. The organization of the chemosensory system in Drosophila melanogaster: A review. Cell Tissue Res. 1994;275:3–26. [PubMed: 8118845]
- Stocker R.F. Drosophila as a focus in olfactory research: Mapping of olfactory sensilla by fine structure, odor specificity, odorant receptor expression, and central connectivity. Microsc Res Tech. 2001;55:284–96. [PubMed: 11754508]
- Stocker R.F., Heimbeck G., Gendre N., de Belle J.S. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–56. [PubMed: 9110257]
- Stocker R.F., Lienhard M.C., Borst A., Fischbach K.F. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. [PubMed: 2124174]
- Stocker R.F., Singh R.N., Schorderet M., Siddiqi O. Projection patterns of different types of antennal sensilla in the antennal glomeruli of Drosophila melanogaster. Cell Tissue Res. 1983;232:237–48. [PubMed: 6411344]
- Stopfer M., Bhagavan S., Smith B.H., Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. [PubMed: 9363891]
- Stopfer M., Jayaraman V., Laurent G. Intensity versus identity coding in an olfactory system. Neuron. 2003;39:991–1004. [PubMed: 12971898]
- Stopfer M., Laurent G. Short-term memory in olfactory network dynamics. Nature. 1999;402:664–68. [PubMed: 10604472]
- Stopfer M., Wehr M., MacLeod K., Laurent G. Neural dynamics, oscillatory synchronisation, and odour codes. In: Hansson B.S., editor. Insect Olfaction. Berlin: Springer; 1999. pp. 163–80.
- Strausfeld N.J. Atlas of an Insect Brain. Berlin: Springer-Verlag; 1976.
- Strausfeld N.J. Organization of the honey bee mushroom body: Representation of the calyx within the vertical and gamma lobes. J Comp Neurol. 2002;450:4–33. [PubMed: 12124764]
- Strausfeld N.J., Gilbert C. Small-field neurons associated with oculomotor control in muscoid flies: Cellular organization in the lobula plate. J Comp Neurol. 1992;316:56–71. [PubMed: 1573051]
- Strausfeld N.J., Hansen L., Li Y., Gomez R.S., Ito K. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn Mem. 1998;5:11–37. [PMC free article: PMC311242] [PubMed: 10454370]
- Strausfeld N.J., Hildebrand J.G. Olfactory systems: Common design, uncommon origins? Curr Opin Neurobiol. 1999;9:634–39. [PubMed: 10508748]
- Strausfeld N.J., Homberg U., Kloppenburg P. Parallel organization in honey bee mushroom bodies by peptidergic Kenyon cells. J Comp Neurol. 2000;424:179–95. [PubMed: 10888747]
- Strausfeld N.J., Li Y. Organization of olfactory and multimodal afferent neurons supplying the calyx and pedunculus of the cockroach mushroom bodies. J Comp Neurol. 1999a;409:603–25. [PubMed: 10376743]
- Strausfeld N.J., Li Y. Representation of the calyces in the medial and vertical lobes of cockroach mushroom bodies. J Comp Neurol. 1999b;409:626–46. [PubMed: 10376744]
- Strausfeld N.J., Sinakevitch I., Vilinsky I. The mushroom bodies of Drosophila melanogaster: An immunocytological and golgi study of Kenyon cell organization in the calyces and lobes. Microsc Res Tech. 2003;62:151–69. [PubMed: 12966500]
- Suh G.S., Wong A.M., Hergarden A.C., Wang J.W., Simon A.F., et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–59. [PubMed: 15372051]
- Sun X.J., Fonta C., Masson C. Odour quality processing by bee antennal lobe interneurones. Chem Senses. 1993;18:355–77.
- Suzuki H. Convergence of olfactory inputs from both antennae in the brain of the honeybee. J Exp Biol. 1975;62:11–26. [PubMed: 1151274]
- Szyszka P., Ditzen M., Galkin A., Galizia C.G., Menzel R. Sparsening and temporal sharpening of olfactory representations in the honeybee mushroom bodies. J Neurophysiol. 2005;94:3303–13. [PubMed: 16014792]
- Szyszka P., Galkin A., Menzel R. Associative and non-associative plasticity in Kenyon cells of the honeybee mushroom body. Front Syst Neurosci. 2008;2:3. [PMC free article: PMC2526274] [PubMed: 18958247]
- Tanaka N.K., Awasaki T., Shimada T., Ito K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr Biol. 2004;14:449–57. [PubMed: 15043809]
- Thom C., Guerenstein P.G., Mechaber W.L., Hildebrand J.G. Floral CO2 reveals flower profitability to moths. J Chem Ecol. 2004;30:1285–88. [PubMed: 15303329]
- Thum A.S., Jenett A., Ito K., Heisenberg M., Tanimoto H. Multiple memory traces for olfactory reward learning in Drosophila. J Neurosci. 2007;27:11132–38. [PMC free article: PMC6672858] [PubMed: 17928455]
- Tolbert L.P., Oland L.A., Tucker E.S., Gibson N.J., Higgins M.R., Lipscomb B.W. Bidirectional influences between neurons and glial cells in the developing olfactory system. Prog Neurobiol. 2004;73:73–105. [PubMed: 15201035]
- Tully T., Preat T., Boynton S.C., Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. [PubMed: 7923375]
- Uchida N., Mainen Z.F. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6:1224–29. [PubMed: 14566341]
- Venkatesh S., Singh R. Sensilla on the third antennal segment of Drosophila melanogaster meigen. Int J Insect Morphol Embryol. 1984;13:51–63.
- Vetter R.S., Sage A.E., Justus K.A., Cardé K.A., Galizia C.G. Temporal integrity of an airborne odor stimulus is greatly affected by physical aspects of the odor delivery system. Chem Senses. 2006;31:359–69. [PubMed: 16510844]
- Vickers N.J., Christensen T.A., Baker T.C., Hildebrand J.G. Odour-plume dynamics influence the brain’s olfactory code. Nature. 2001;410:466–70. [PubMed: 11260713]
- Vosshall L.B., Amrein H., Morozov P.S., Rzhetsky A., Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–36. [PubMed: 10089887]
- Vosshall L.B., Wong A.M., Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–59. [PubMed: 10943836]
- Waddell S., Quinn W.G. Flies, genes, and learning. Annu Rev Neurosci. 2001;24:1283–1309. [PubMed: 11520934]
- Waldrop B., Hildebrand J.G. Physiology and pharmacology of acetylcholinergic responses of interneurons in the antennal lobes of the moth Manduca sexta. J Comp Physiol A. 1989;164:433–41. [PubMed: 2926690]
- Wang F., Nemes A., Mendelsohn M., Axel R. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93:47–60. [PubMed: 9546391]
- Wang J.W., Wong A.M., Flores J., Vosshall L.B., Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–82. [PubMed: 12553914]
- Wang Y.L., Guo H.F., Pologruto T.A., Hannan F., Hakker I., et al. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J Neurosci. 2004;24:6507–14. [PMC free article: PMC6729867] [PubMed: 15269261]
- Weidenmüller A., Kleineidam C., Tautz J. Collective control of nest climate parameters in bumblebee colonies. Anim Behav. 2002;63:1065–71.
- Wicher D., Schafer R., Bauernfeind R., Stensmyr M.C., Heller R., et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–11. [PubMed: 18408711]
- Wilson D.A., Stevenson R.J. The fundamental role of memory in olfactory perception. Trends Neurosci. 2003;26:243–47. [PubMed: 12744840]
- Wilson R.I., Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–79. [PMC free article: PMC6725763] [PubMed: 16207866]
- Wilson R.I., Turner G.C., Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–70. [PubMed: 14684826]
- Wiltrout C., Dogra S., Linster C. Configurational and nonconfigurational interactions between odorants in binary mixtures. Behav Neurosci. 2003;117:236–45. [PubMed: 12708520]
- Winnington A.P., Napper R.M., Mercer A.R. Structural plasticity of identified glomeruli in the antennal lobes of the adult worker honey bee. J Comp Neurol. 1996;365:479–90. [PubMed: 8822183]
- Witthöft W. Absolute Anzahl und Verteilung der Zellen im Hirn der Honigbiene [Absolute number and distribution of cells in the brain of honeybees.] Z Morphol Tiere. 1967;61:160–84.
- Wong A.M., Wang J.W., Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–41. [PubMed: 12007409]
- Xu F., Greer C.A., Shepherd G.M. Odor maps in the olfactory bulb. J Comp Neurol. 2000;422:489–95. [PubMed: 10861521]
- Xu P., Atkinson R., Jones D.N., Smith D.P. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron. 2005;45:193–200. [PubMed: 15664171]
- Yasuyama K., Kitamoto T., Salvaterra P.M. Localization of choline acetyltransferase-expressing neurons in the larval visual system of Drosophila melanogaster. Cell Tissue Res. 1995;282:193–202. [PubMed: 8565051]
- Yasuyama K., Meinertzhagen I.A., Schurmann F.W. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J Comp Neurol. 2002;445:211–26. [PubMed: 11920702]
- Yasuyama K., Salvaterra P.M. Localization of choline acetyltransferase-expressing neurons in Drosophila nervous system. Microsc Res Tech. 1999;45:65–79. [PubMed: 10332725]
- Yin J.C., Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6:264–68. [PubMed: 8725970]
- Yu D., Ponomarev A., Davis R.L. Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron. 2004;42:437–49. [PubMed: 15134640]
- Zars T., Fischer M., Schulz R., Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–75. [PubMed: 10784450]
- Zhu H., Hummel T., Clemens J.C., Berdnik D., Zipursky S.L., Luo L. Dendritic patterning by Dscam and synaptic partner matching in the Drosophila antennal lobe. Nat Neurosci. 2006;9:349–55. [PubMed: 16474389]
- Zou Z., Li F., Buck L.B. Odor maps in the olfactory cortex. Proc Natl Acad Sci USA. 2005;102:7724–29. [PMC free article: PMC1138263] [PubMed: 15911779]
- Zube C., Kleineidam C.J., Kirschner S., Neef J., Rossler W. Organization of the olfactory pathway and odor processing in the antennal lobe of the ant Camponotus floridanus. J Comp Neurol. 2008;506:425–41. [PubMed: 18041786]
- Review Engineering Aspects of Olfaction.[Neuromorphic Olfaction. 2013]Review Engineering Aspects of Olfaction.Persaud KC. Neuromorphic Olfaction. 2013
- Morphological and functional heterogeneity in olfactory perception between antennae and maxillary palps in the pumpkin fruit fly, Bactrocera depressa.[Arch Insect Biochem Physiol. 2...]Morphological and functional heterogeneity in olfactory perception between antennae and maxillary palps in the pumpkin fruit fly, Bactrocera depressa.Oh HW, Jeong SA, Kim J, Park KC. Arch Insect Biochem Physiol. 2019 Aug; 101(4):e21560. Epub 2019 May 31.
- Spatial organization of antennal olfactory sensory neurons in the female Spodoptera littoralis moth: differences in sensitivity and temporal characteristics.[Chem Senses. 2012]Spatial organization of antennal olfactory sensory neurons in the female Spodoptera littoralis moth: differences in sensitivity and temporal characteristics.Binyameen M, Anderson P, Ignell R, Seada MA, Hansson BS, Schlyter F. Chem Senses. 2012 Sep; 37(7):613-29. Epub 2012 Mar 29.
- Review Elements of olfactory reception in adult Drosophila melanogaster.[Anat Rec (Hoboken). 2013]Review Elements of olfactory reception in adult Drosophila melanogaster.Martin F, Boto T, Gomez-Diaz C, Alcorta E. Anat Rec (Hoboken). 2013 Sep; 296(9):1477-88. Epub 2013 Jul 31.
- Inactivation of olfactory sensilla of a single morphological type differentially affects the response of Drosophila to odors.[J Neurobiol. 2002]Inactivation of olfactory sensilla of a single morphological type differentially affects the response of Drosophila to odors.Park SK, Shanbhag SR, Dubin AE, de Bruyne M, Wang Q, Yu P, Shimoni N, D'Mello S, Carlson JR, Harris GL, et al. J Neurobiol. 2002 Jun 5; 51(3):248-60.
- Odor Coding in Insects - The Neurobiology of OlfactionOdor Coding in Insects - The Neurobiology of Olfaction
Your browsing activity is empty.
Activity recording is turned off.
See more...