By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Siegel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Philadelphia: Lippincott-Raven; 1999.

Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition.
Show detailsThe indolealkylamine 5-hydroxytryptamine, serotonin, was identified initially because of interest in its cardiovascular effects
It has been known since the mid-nineteenth century that after blood clots the resulting serum possesses a substance that constricts vascular smooth muscle so as to increase vascular tone. Around the turn of this century, platelets were identified as the source of this substance. In the late 1940s, Rapport and collaborators [1] isolated, purified and identified this “tonic” substance in “serum” (hence, serotonin) as the substituted indole 5-hydroxytryptamine (5-HT).
The structures of serotonin and related compounds are shown in Figure 13-1. The combination of the hydroxyl group in the 5 position of the indole nucleus and a primary amine nitrogen serving as a proton acceptor at physiological pH makes 5-HT a hydrophilic substance. As such, it does not pass the lipophilic blood—brain barrier readily. Thus, its discovery in brain indicated that 5-HT is synthesized in brain, where it might play an important role in brain function. The observation, at about the same time, that the psychedelic drug (+)lysergic acid diethylamide (LSD) antagonized a response produced by 5-HT further substantiated the idea that 5-HT might have important behavioral effects, even though the response was contraction of gastrointestinal smooth muscle. Subsequently, various theories arose linking abnormalities of 5-HT function to the development of a number of psychiatric disorders, particularly schizophrenia and depression. Psychotherapeutic drugs are now available that are effective in depression (see Chap. 52), anxiety disorders and schizophrenia; some of these drugs have potent, and in some cases selective, effects on serotonin neurons in brain.
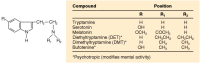
Figure 13-1
Chemical structures of 5-hydroxytryptamine and related indolealkylamines. The indole ring structure consists of the benzene ring and the attached five-member ring structure containing nitrogen.
Understanding the neuroanatomical organization of serotonergic cells in brain provides insight into the functions of this neurotransmitter
Serotonin-containing neuronal cell bodies are restricted to discrete clusters or groups of cells located along the midline of the brainstem. Their axons, however, innervate nearly every area of the CNS (Fig. 13-2). In 1964, Dahlstrom and Fuxe (discussed in [2]), using the Falck-Hillarp technique of histofluorescence, observed that the majority of serotonergic soma are found in cell body groups, which previously had been designated as the raphe nuclei. This earlier description of the raphe nuclei was based on cell body structural characteristics and organization. Dahlstrom and Fuxe described nine groups of serotonin-containing cell bodies, which they designated B1 through B9, and which correspond for the most part with the raphe nuclei (discussed in [2]) (Table 13-1). Some serotonergic neuronal cell bodies, however, are found outside the raphe nuclei, and not all of the cell bodies in the raphe nuclei are serotonergic. In most of the raphe nuclei, the majority of neurons are nonserotonergic. For example, the dorsal raphe contains the largest number of serotonergic neurons; however, only 40 to 50% of the cell bodies in the dorsal raphe are serotonergic (Fig. 13-3).
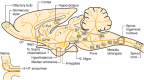
Figure 13-2
Schematic drawing depicting the location of the serotonergic cell body groups in a sagittal section of the rat central nervous system and their major projections. OT, olfactory tuberculum; Sept, septum; C. Put, nucleus caudate-putamen; G. Pal, globus (more...)
Table 13-1
Classification of Serotonergic Cell Body Groups According to Dahlstrom and Fuxe and Corresponding Anatomical Structure.
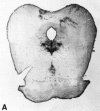
Figure 13-3
Serotonergic cell bodies in the midbrain raphe nuclei demonstrated by 5-hydroxytryptamine immunocytochemistry. A: Low magnification of transverse section through rat midbrain. The serotonergic cell body groups shown give rise to widespread serotonergic (more...)
Since the late 1960s, a variety of techniques have been used to characterize the neuronal circuitry of serotonin cells in the CNS. The density of serotonergic innervation in the forebrain was underestimated initially because the original histofluorescence method was limited in sensitivity and did not permit the detection of many fine axons and terminals. Subsequent anatomical techniques, such as immunohistochemical localization of either 5-HT or tryptophan hydroxylase, an enzyme unique to the synthesis of 5-HT, in addition to retrograde and anterograde axonal transport studies, have allowed a more complete and accurate characterization of the serotonergic innervation of forebrain areas.
The largest group of serotonergic cells is B7 (Fig. 13-2), which is continuous with a smaller group of serotonergic cells, B6. Groups B6 and B7 often are considered together as the dorsal raphe nucleus, with B6 being its caudal extension. Another prominent serotonergic cell body group is B8, which corresponds to the median raphe nucleus, also termed the nucleus central superior. Group B9, part of the ventrolateral tegmentum of the pons and midbrain, forms a lateral extension of the median raphe (Fig. 13-2) and, therefore, is not considered one of the midline raphe nuclei. Ascending serotonergic projections innervating the cerebral cortex and other regions of the forebrain arise primarily from the dorsal raphe, median raphe and B9 cell group.
Two distinct ascending projections arise from the rostral serotonergic system. The two main ascending serotonergic pathways emerging from the midbrain raphe nuclei to the forebrain are the dorsal periventricular path and the ventral tegmental radiations. Both pathways converge in the caudal hypothalamus, where they join the medial forebrain bundle. Axons of both dopaminergic and noradrenergic neurons course anteriorly through the medial forebrain bundle as well (see Chap. 12).
Ascending projections from the raphe nuclei to forebrain structures are organized in a topographical manner. The dorsal and median raphe nuclei give rise to distinct projections to forebrain regions (Fig. 13-4). The median raphe projects heavily to hippocampus, septum and hypothalamus, whereas the striatum is innervated predominantly by the dorsal raphe. The dorsal and median raphe nuclei send overlapping neuronal projections to the neocortex. Within the dorsal and median raphe, cells are organized in particular zones or groups that send axons to specific areas of brain. For example, the frontal cortex receives heavy innervation from the rostral and lateral subregions of the dorsal raphe nucleus. Raphe neurons send collateral axons to areas of brain that are related in function, such as the amygdala and hippocampus or the substantia nigra and caudate putamen. The specific and highly organized innervation of forebrain structures by raphe neurons implies independent functions of sets of serotonergic neurons dependent on their origin and terminal projections, as opposed to a nonselective or general role for serotonin in the CNS.
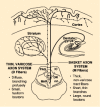
Figure 13-4
Simplified diagram of the main features of the dual serotonergic system innervating the forebrain. The thin varicose axon system (D fibers) arises from the dorsal raphe (DR) nucleus with fibers that branch profusely in their target areas. It is difficult (more...)
Serotonergic axon terminals, labeled by uptake of [3H]5-HT or studied with immunohistochemical techniques, appear to exhibit morphological differences related to the raphe nucleus of origin (Fig. 13-4). Serotonergic axons from the median raphe nucleus, type M, look relatively coarse with large spherical varicosities. By contrast, axons from the dorsal raphe, type D, are very fine and typically have small, pleomorphic varicosities. Dorsal raphe axons appear to be more vulnerable to certain neurotoxic amphetamine derivatives, such as d-fenfluramine, 3,4-methylenedioxymethamphetamine (MDMA, commonly termed Ecstasy) or parachloroamphetamine (PCA). Median raphe axons appear to be more resistant to the neurotoxic effects of these drugs. Blockade of the serotonin transporter prevents the neurotoxic effects of these amphetamine derivatives, indicating that activity of this transporter is critical for the neurotoxic effects of these drugs.
As expected, serotonergic terminals make the usual specialized synaptic contacts with target neurons and release serotonin following nerve stimulation. In some areas of the mammalian CNS, there are sites where 5-HT is released and no evidence for synaptic specialization has been found. For example, it is difficult to demonstrate the synaptic contacts of the fine varicose fibers, whereas large terminals make well defined synapses with target cells. The percentage of 5-HT terminals associated with synaptic specializations appears to vary in particular brain regions. This may have important implications for the type of information processing in which 5-HT is involved in these brain areas. The appearance of specialized synaptic contacts suggests relatively stable and strong associations between a presynaptic neuron and its target. Conversely, the lack of synaptic specialization implies a dynamic, and perhaps less specific, interaction with target neurons. For example, neurotransmitter is released and then diffuses over distances as great as several hundred microns. In this case, 5-HT may act as a neuromodulator to adjust or tune ongoing synaptic activity.
The other raphe nuclei, B1 to B4, are situated more caudally in the midpons to caudal medulla and contain a smaller number of serotonergic cells. These cell body groups give rise to serotonergic axons that project within the brainstem and to the spinal cord (Fig. 13-2). The spinal cord receives a strong serotonergic innervation. Three principal descending pathways have been described: (i) from the raphe magnus nucleus (B3) to laminae I and II of the dorsal horn, (ii) from the raphe obscurus nucleus (B2, B) to lamina IX of the ventral horn and (iii) from the rostral ventrolateral medulla and the lateral paragigantocellular reticular nucleus (B3) to the interomediolateral cell column. Projections from the caudal ventrolateral medulla (B1) are not known at present [3].
Afferent connections to the raphe nuclei include those between the dorsal and median raphe nuclei, B9, B1 and B3. Connections between the raphe nuclei have been described by retrograde tracing techniques using horseradish peroxidase and wheat germ agglutinin. Such innervation may have considerable physiological and/or pharmacological importance as serotonin released in the vicinity of serotonergic cell bodies regulates the firing of serotonergic neurons through the activation of somatodendritic autoreceptors. The raphe nuclei also receive input from other cell body groups in the brainstem, such as the substantia nigra and ventral tegmental area (dopamine), superior vestibular nucleus (acetylcholine), locus ceruleus (norepinephrine) and nucleus prepositus hypoglossi and nucleus of the solitary tract (epinephrine). Other afferents include neurons from the hypothalamus, thalamus and limbic forebrain structures (see [2–4] for a comprehensive review of serotonergic neuroanatomy).
The amino acid l-tryptophan serves as the precursor for the synthesis of 5-hydroxytryptamine
Not all cells that contain 5-HT synthesize it. For example, platelets do not synthesize 5-HT; rather, they accumulate 5-HT from plasma by an active-transport mechanism found on the platelet membrane. Certain brain cells do synthesize 5-HT. The synthesis and primary metabolic pathways of 5-HT are shown in Figure 13-5. The initial step in the synthesis of serotonin is the facilitated transport of the amino acid l-tryptophan from blood into brain. The primary source of tryptophan is dietary protein. Certain other neutral amino acids, such as phenylalanine, leucine and methionine, are transported into brain by the same carrier. The entry of tryptophan into brain is related not only to its concentration in blood but is also a function of its concentration in relation to the concentrations of other neutral amino acids. Consequently, lowering the dietary intake of tryptophan while raising intake of the amino acids that tryptophan competes with for transport into brain lowers the content of 5-HT in brain and changes certain behaviors associated with 5-HT function. This strategy for lowering the brain content of 5-HT has been used clinically to evaluate the importance of brain 5-HT in the mechanism of action of psychotherapeutic drugs [5].
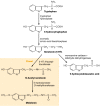
Figure 13-5
The biosynthesis and catabolism of serotonin. Note that in the pineal gland, serotonin is converted enzymatically to melatonin.
Serotonergic neurons contain the enzyme l-tryptophan-5-monooxygenase (EC 1.14.16.4), more commonly termed tryptophan hydroxylase, which converts tryptophan to 5-hydroxytryptophan (5-HTP) (Fig. 13-5). This enzyme is synthesized in serotonergic cell bodies of the raphe nuclei and is found only in cells that synthesize 5-HT; its distribution in brain is similar to that of 5-HT itself. The enzyme requires both molecular oxygen and a reduced pteridine cofactor, such as l-erythro-tetrahydrobiopterin (BH4), for activity. In the enzymatic reaction, one atom of oxygen is used to form 5-HTP and the other is reduced to water. The pteridine cofactor donates electrons, and the unstable quinonoid dihydrobiopterin that results is regenerated immediately to the tetrahydrobiopterin form by a NADPH-linked enzymatic reaction:
The Km of partially purified tryptophan hydroxylase for tryptophan is approximately 30 to 60 μM, a concentration comparable to that of tryptophan in brain. If the concentration of tryptophan in serotonergic neurons is assumed to be comparable to that in whole brain, the enzyme would not be saturated with substrate and the formation of 5-HT in brain would be expected to rise as the brain concentration of tryptophan increases. This occurs specifically in response to raising the dietary intake of tryptophan. However, the relationship among tryptophan availability, total tissue 5-HT concentration and 5-HT release is not fully understood.
cDNAs encoding tryptophan hydroxylase from both brain and pineal gland have been cloned and sequenced. Some biochemical differences between the enzyme(s) obtained from brain and from pineal gland, such as molecular weight, substrate specificity and isoelectric point, have been reported previously. However, cDNAs isolated from both tissue sources appear to have identical nucleotide sequences, making it likely that tissue-specific differences in the properties of tryptophan hydroxylase result from differential post-translational processing. Tryptophan hydroxylase contains 444 amino acids, corresponding to a molecular weight of about 51,000, and is 50% homologous with tyrosine hydroxylase, the rate-limiting enzyme in catecholamine (CA) biosynthesis (see Chap. 12). The greatest homology resides in the central and C-terminus regions of these enzymes, making it likely that these areas contain the catalytic site. Substrate specificity may reside in those amino acids nearer the N-terminus.
The other enzyme involved in the synthesis of serotonin, aromatic l-amino acid decarboxylase (AADC) (EC 4.1.1.28), is a soluble pyridoxal-5′-phosphate-dependent enzyme which converts 5-HTP to 5-HT (Fig. 13-5). It has been demonstrated that administration of pyridoxine increases the rate of synthesis of 5-HT in monkey brain, as revealed using position emission tomography. This presumably reflects a regulatory effect of pyridoxine on AADC activity and raises the interesting issue of the use of pyridoxine supplementation in situations associated with 5-HT deficiency.
AADC is present not only in serotonergic neurons but also in catecholaminergic neurons, where it converts 3,4-dihydroxyphenylalanine (DOPA) to dopamine (see Chap. 12). However, different pH optima or concentrations of substrate or cofactor are required for optimal activity of the enzyme in brain homogenates when using either 5-HTP or DOPA as the substrate. cDNAs encoding AADC have been cloned from various species. The encoded protein contains 480 amino acids and has a molecular weight of 54,000 but it appears to exist as a dimer. Characterization of the protein expressed in cells transfected with the cDNA shows that it decarboxylates either DOPA or 5-HTP. Also, in situ hybridization of the mRNA for the enzyme revealed its presence both in serotonergic cells in the dorsal raphe nucleus and in catecholaminergic cells in brain regions containing catecholaminergic soma [6]. Taken together, these results support the idea that the enzymatic decarboxylation of both DOPA and 5-HTP is catalyzed by the same enzyme.
Because the decarboxylase enzyme is not saturated with 5-HTP under physiological conditions, that is, the concentration of 5-HTP is much less than the Km of 10 μM, it is possible to raise the content of 5-HT in brain not only by increasing the dietary intake of tryptophan but also by raising the intake of 5-HTP. This procedure, though, results in the formation of 5-HT in cells that would not normally contain it, such as catecholaminergic neurons, because of the nonselective nature of AADC.
The initial hydroxylation of tryptophan, rather than the decarboxylation of 5-HTP, appears to be the rate-limiting step in serotonin synthesis. Evidence in support of this view includes the fact that 5-HTP is found only in trace amounts in brain, presumably because it is decarboxylated about as rapidly as it is formed. As might be expected if the hydroxylation reaction is rate-limiting, inhibition of this reaction results in a marked depletion of the content of 5-HT in brain. The enzyme inhibitor most widely used in experiments is parachlorophenylalanine (PCPA). In vivo, PCPA irreversibly inhibits tryptophan hydroxylase, presumably by incorporating itself into the enzyme to produce an inactive protein. This results in a long-lasting reduction of 5-HT levels. Recovery of enzyme activity and 5-HT biosynthesis requires the synthesis of new enzyme. Marked increases in levels of mRNA for tryptophan hydroxylase are found in the raphe nuclei 1 to 3 days after administration of PCPA [7].
The synthesis of 5-hydroxytryptamine can increase markedly under conditions requiring a continuous supply of the neurotransmitter
Plasticity is an important concept in neurobiology. In general, this refers to the ability of neuronal systems to conform to either short- or long-term demands placed upon their activity or function. One of the processes contributing to neuronal plasticity is the ability to increase the rate of neurotransmitter synthesis and release in response to increased neuronal activity. Serotonergic neurons have this capability; the synthesis of 5-HT from tryptophan is increased in a frequency-dependent manner in response to electrical stimulation of serotonergic soma [8]. The increase in synthesis results from the enhanced conversion of tryptophan to 5-HTP and has an absolute dependence on extracellular Ca2+. It is likely that the increased synthesis results in part from alterations in the kinetic properties of tryptophan hydroxylase, perhaps due to calcium-dependent phosphorylation of the enzyme. The enzyme can be phosphorylated directly by the action of calmodulin-dependent protein kinase II; an activator protein appears to be required for this interaction. In the presence of the activator, tryptophan hydroxylase also may be a substrate for cAMP-dependent protein kinase (PKA). The increased activity of tryptophan hydroxylase does not result from the removal of enzyme inhibition caused by either 5-HT or 5-HTP.
Short-term requirements for increases in the synthesis of 5-HT can be met by processes that change the kinetic properties of tryptophan hydroxylase, such as phosphorylation, without necessitating the synthesis of more molecules of tryptophan hydroxylase. By contrast, situations requiring long-term increases in the synthesis and release of 5-HT result in the synthesis of tryptophan hydroxylase protein. For example, partial but substantial destruction of >60% of central serotonergic neurons results in an increase in the synthesis of 5-HT in residual terminals. The increase in synthesis initially results from activation of existing tryptophan hydroxylase molecules, but the increased synthesis of 5-HT seen weeks after the lesion results from more tryptophan hydroxylase being present in the residual terminals. An increase in tryptophan hydroxylase mRNA has been reported in residual raphe serotonergic neurons after partial lesioning, consistent with the idea of an increase in the synthesis of tryptophan hydroxylase molecules in residual neurons.
As with other biogenic amine transmitters, 5-hydroxytryptamine is stored primarily in vesicles and released by an exocytotic mechanism
Peripheral sources of monoamine-containing cells have been utilized to study the properties of storage vesicles, such as chromaffin cells of the adrenal medulla for CAs and parafollicular cells of the thyroid gland for 5-HT. In some respects, the vesicles that store 5-HT resemble those that store CAs. For example, drugs such as reserpine and tetrabenazine, which inhibit the activity of the transporter localized to the vesicular membrane, deplete the brain content of 5-HT as well as of CAs. Storage of 5-HT in vesicles requires its active transport from the cytoplasm. The vesicular transporter uses the electrochemical gradient generated by a vesicular H+-ATPase to drive transport, such that a cytoplasmic amine is exchanged for a luminal proton; that is, uptake of 5-HT is coupled to efflux of H+ (Fig. 13-6).
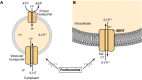
Figure 13-6
The substituted amphetamine fenfluramine inhibits the transport of 5-hydroxytryptamine (5-HT) by both (A) the vesicular transporter and (B) the serotonin transporter (SERT). Substituted amphetamines, such as fenfluramine and 3,4-methylenedioxymethamphetamine (more...)
Two synaptic vesicle transporters have been cloned, with the predicted amino acid sequence containing 12 transmembrane domains. The first transporter, cloned from chromaffin granules, has been termed vesicular membrane transporter1 (VMAT1). It contains 521 amino acids and a large loop between transmembrane domains 1 and 2, which faces the lumen of the vesicle, contains sites for glycosylation. Both the NH2 and COOH termini face the cytoplasm. A second vesicular transporter (VMAT2), cloned from rat brain, is 62% identical to VMAT1. Human chromosome 8 contains the gene for VMAT1 and chromosome 10 contains the gene for VMAT2. These transporters show homology to a family of drug-resistant transporters in bacteria and yeast. All members of this family function as antiporters to remove toxic compounds from the cytoplasm. It has been suggested that this family, including VMAT1 and VMAT2, be termed T oxin-Extruding Antiporters (TEXANs) [9].
Although there are clear structural differences between the vesicular transporter and the Na+-dependent plasma membrane transporter for 5-HT (described below), there are also striking structural similarities. Nevertheless, drugs that inhibit the vesicular transporter generally do not block the plasma membrane transporter and vice versa. However, two drugs that have effects at both the vesicular transporter and the plasma membrane transporter are the anorectic agent fenfluramine and MDMA. Fenfluramine inhibits the vesicular transporter directly by competing for its substrate-binding site. It also dissipates the transmembrane pH gradient to inhibit further 5-HT uptake into the vesicle (Fig. 13-6). MDMA has these pharmacological effects as well. The effects of these drugs on the plasma membrane transporter are described below. The consequence of such pharmacological actions is stimulation of 5-HT release by a nonexocytotic mechanism.
Vesicles storing 5-HT exhibit some differences from those storing CAs. In contrast to CA-containing vesicles, there is virtually no ATP in serotonin vesicles. Also, serotonergic synaptic vesicles, but not chromaffin granules, contain a specific protein that binds 5-HT with high affinity. This serotonin-binding protein is present in serotonergic cells derived ontogenetically from the neuroectoderm [10]. It binds 5-HT with high affinity in the presence of Fe2+. There are three isoforms of serotonin-binding protein. The 45-kDa isoform appears to be packaged in secretory vesicles along with 5-HT, which probably accounts for the observation that newly taken up [3H]5-HT is rapidly complexed with this isoform in brain in situ. This isoform is secreted along with 5-HT by a calcium-dependent process.
There is considerable evidence that the release of 5-HT occurs by exocytosis, that is, by the discharge from the cell of the entire contents of individual storage vesicles. First, 5-HT is ionized sufficiently at physiological pH so that it does not cross plasma membranes by simple diffusion. Second, most intraneuronal 5-HT is contained in storage vesicles and other contents of the vesicle, including serotonin-binding protein (SPB), are released together with serotonin. By contrast, cytosolic proteins do not accompany electrical stimulation-elicited release of 5-HT. Third, the depolarization-induced release of 5-HT occurs by a calcium-dependent process; indeed, it appears that the influx of Ca2+ with or without membrane depolarization can increase the release of 5-HT. Ca2+ has been reported to stimulate the fusion of vesicular membranes with the plasma membrane.
The rate of serotonin release is dependent on the firing rate of serotonergic soma in the raphe nuclei. Numerous studies have revealed that an increase in raphe cell firing enhances the release of 5-HT in terminal fields. The opposite effect is observed when raphe cell firing decreases. This means that drugs that change the firing rate of serotonergic soma modify the release of serotonin as well. An important target for such drugs is the somatodendritic autoreceptor, which, as discussed later, is the 5-HT1A receptor subtype (see Fig. 13-8). Administration of 5-HT1A agonists, such as 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT), into the dorsal raphe nucleus slows the rate of firing of serotonergic soma. Using the technique of in vivo microdialysis, application of 8-OH-DPAT in the dorsal raphe nucleus decreases the release of 5-HT in the striatum. Depending on the species, serotonergic autoreceptors in terminal fields appear to be either the 5-HT1B or the 5-HT1D subtype. Administration of agonists of these receptors into areas receiving serotonergic innervation decreases the synthesis and release of 5-HT measured in vitro or in situ, using the technique of microdialysis. However, in contrast to the activation of somatodendritic autoreceptors, such effects are not due to decreases in the firing rate of serotonergic soma.
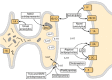
Figure 13-8
Effects of psychoactive drugs on serotonergic neurotransmission. Drugs that act as agonists are indicated by solid-line arrows, whereas antagonists or inhibitors are shown with broken-line arrows. The 5-hydroxytryptamine (5-HT)1A receptor acts as both (more...)
The activity of 5-hydroxytryptamine in the synapse is terminated primarily by its re-uptake into serotonergic terminals
Synaptic effects of many amino acid and monoaminergic neurotransmitters, including 5-HT, are terminated by binding of these molecules to specific transporter proteins. The serotonin transporter (SERT) is located on serotonergic neurons. Evidence for this comes from studies showing that the selective lesioning of serotonergic neurons in brain markedly reduces both the high-affinity uptake of [3H]5-HT in areas of brain receiving serotonergic innervation and the specific binding of radioligands to the serotonin transporter. Activity of the SERT regulates the concentration of 5-HT in the synapse, thereby influencing synaptic transmission.
The uptake system for 5-HT is saturable and of high affinity, with a Km value for 5-HT of approximately 0.2 to 0.5 μM. Uptake of 5-HT is an active process that is temperature-dependent and has an absolute requirement for external Na+ and C1−; it is inhibited by metabolic inhibitors as well as by inhibitors of Na/K ATPase activity. From these and other data, it has been inferred that the energy requirement for 5-HT uptake is not used directly to transport 5-HT but rather is necessary to maintain the gradient of Na+ across the plasma membrane, upon which 5-HT uptake is dependent. The current model of transport has one Na+, one C1− and one protonated 5-HT binding to the transporter extracellularly prior to translocation to form a quaternary complex that subsequently undergoes a conformational change to release the neurotransmitter and the ions into the cytoplasm. The conformational change may involve the “opening” of a pore formed by some portion of the transmembrane domains of the SERT (see below). In the cytoplasm, K+ associates with the SERT to promote reorientation of the unloaded carrier for another transport cycle (Fig. 13-6).
The cloning, sequencing and expression of several transporter proteins, including that for 5-HT, has aided considerably in understanding structure/function relationships of transporter proteins [11,12]. The cDNA for the SERT isolated from rat brain predicts a protein containing 630 amino acids with a molecular weight of about 68,000. The SERT is encoded by a single gene on the long arm of chromosome 17. The mRNA for the SERT has been localized in brain exclusively to the serotonergic cells in the raphe nuclei. Of interest is the fact that mRNA for the SERT has not yet been detected in glia, even though primary cultures of astrocytes in vitro can take up 5-HT. The inability to detect message for the SERT in glia raises questions about the physiological relevance of the uptake of 5-HT by glia in vitro.
The putative structure of the SERT has 12 transmembrane domains (TMDs) with both the amino- and carboxy-termini being intracellular, and a large extracellular loop containing canonical glycosylation sites connecting TMDs 3 and 4 (Fig. 13-7). Glycosylation seems necessary for optimal stability of the transporter in the membrane but not for 5-HT transport or ligand binding. There are also six potential sites of phosphorylation by PKA and protein kinase C (PKC) on the human SERT. The predicted structure of the serotonin transporter is similar to that of other cloned neurotransmitter transporters and quite distinct, for example, from the seven-transmembrane domain structure of G protein-linked receptors. These transporters are considered members of the Na+ and C1−-dependent neurotransporter family, distinct from the vesicular transporter family described earlier. All members of this family are fragmented by multiple introns, raising the possibility of multiple transcripts by alternative RNA processing.
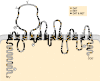
Figure 13-7
Putative structure of the rat serotonin transporter showing homologous amino acids with the rat dopamine transporter (DAT), human norepinephrine transporter (NET) or both. Possible phosphorylation  sites are shown, as are possible glycosylation sites (more...)
sites are shown, as are possible glycosylation sites (more...)
SERT exhibits about 50% absolute homology with the transporters for norepinephrine (NET) and dopamine (DAT), with the greatest homology being found in TMDs 1 and 2 and TMDs 4–8. The least conserved regions are the intracellular amino- and carboxy-terminal tails (Fig. 13-7). It has been proposed that the conserved regions may be involved in general transport functions and the least conserved regions in the unique attributes of each carrier, such as pharmacological specificity. However, studies in which the terminal regions were exchanged between the SERT and the NET revealed no alternation in substrate or antagonist selectivity. Thus, the NH2 and COOH termini may not be important for ligand recognition.
Of considerable interest is whether the SERT is subject to physiological regulation. Such regulation could occur at the level of transcription or translation or by post-translational modifications, such as glycosylation or phosphorylation. The focus of much of this research, carried out in vitro using cells that naturally express the SERT or cells stably transfected with the SERT, has been on the role of second-messenger systems, particularly those activating protein kinases. It has been established that SERT gene expression is (i) influenced by cAMP-dependent pathways and (ii) rapidly regulated by changes in intracellular Ca2+, treatment with calmodulin inhibitors or activation of PKC as well as nitric oxide synthase (NOS)/cGMP pathways. Most of the kinetic alterations reflect changes in maximal transport capacity (Vmax) rather than in apparent affinity (Km). Activation of PKC causes a loss of SERT protein on the cell surface. It remains to be established, however, whether such processes contribute to modulation of SERT activity in vivo.
Although there is structural homology among the transporters for 5-HT, NE and DA, some drugs exhibit great selectivity at inhibiting the activity of just one of these proteins. For example, secondary amine tricyclic antidepressants, such as desipramine, are 25- to 150-fold more potent at inhibiting transport of NE than 5-HT. By contrast, some of the newer antidepressants, appropriately termed selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, sertraline and paroxetine, are 15 to 75 times more potent at inhibiting the uptake of 5-HT than the uptake of NE. However, there are also drugs, such as cocaine, that are nonselective inhibitors of all three transporters.
In addition to facilitating the removal of transmitters from the synapse, plasma membrane transporters function under certain circumstances to release transmitter by a non-Ca2+-dependent, that is, nonexocytotic, process. In contrast to exocytotic release, this release process is not modulated by presynaptic receptors. However, such transporter-mediated release is Na+-dependent and sensitive to transport inhibitors. The role of such transporter-mediated release in physiological circumstances is speculative. However, certain drugs elicit the release of 5-HT, at least in part, through such a mechanism. For example, both fenfluramine and MDMA act as substrates for the SERT and not only inhibit the transport of 5-HT into the cell but also facilitate its outward transport. The action of these drugs on 5-HT-storing vesicles raises cytoplasmic 5-HT and facilitates SERT-mediated efflux of 5-HT (Fig. 13-6). The release of 5-HT caused by drugs such as fenfluramine is prevented by inhibitors of the SERT.
The primary catabolic pathway for 5-hydroxytryptamine is oxidative deamination by the enzyme monoamine oxidase
Monoamine oxidase (MAO) (E.C.1.4.3.4.) converts serotonin to 5-hydroxyindoleacetaldehyde, and this product is oxidized by an NAD+-dependent aldehyde dehydrogenase to form 5-hydroxyindoleacetic acid (5-HIAA) (Fig. 13-5). The intermediate acetaldehyde also can be reduced by an NADH-dependent aldehyde reductase to form the alcohol 5-hydroxytryptophol. Whether oxidation or reduction takes place depends on the ratio of NAD+ to NADH in the tissue. In brain, 5-HIAA is the primary metabolite of serotonin.
There are at least two isoenzymes of MAO, referred to as MAO-A and MAO-B. These isoenzymes are integral flavoproteins of outer mitochondrial membranes in neurons, glia and other cells. Evidence for the existence of isoenzymes was based initially on differing substrate specificities and sensitivities to inhibitors of MAO. For example, both 5-HT and NE are metabolized preferentially by MAO-A. Selective inhibitors of each form of MAO exist: clorgyline or moclobemide for type A and deprenyl for type B. Definitive proof of the existence of these two forms of MAO comes from the cloning of cDNAs encoding subunits of MAO-A and MAO-B from human liver [13]. The deduced amino acid sequences of MAO-A and MAO-B show about 70% homology and have masses of 59.7 and 58.8 kDa, respectively. When each cDNA was cloned into an expression vector and transfected independently into a cell line, the activity of the proteins expressed resembled that of the endogenous enzymes from human brain, such that the expressed MAO-A preferred 5-HT as a substrate and was inhibited preferentially by clorgyline. From such data it was inferred that the functional differences between these two enzymes exist in their primary structures.
Several different techniques have been used to study the neuroanatomical localization of the two forms of MAO in brain. Originally, both histochemical and immunohistochemical techniques were used. More recently, in situ hybridization histochemistry has been used to demonstrate the location of the mRNAs for the two isoenzymes. The development of radioligands selective for each form has enabled their distribution to be revealed by quantitative autoradiography. In general, the results from the use of these different techniques are similar. It is of interest that there is more MAO-A than MAO-B throughout rat brain, whereas human brain contains more MAO-B than MAO-A. Serotonergic cell bodies contain predominantly MAO-B, for which 5-HT is not a preferred substrate. This has led to the hypothesis that MAO-B in serotonergic neurons prevents the cell from accumulating various other natural substrates, such as DA, that could interfere with the storage, release and uptake of 5-HT. Furthermore, treatment of rats with clorgyline, a selective inhibitor of MAO-A, raises the brain content of 5-HT and reduces the conversion of 5-HT to 5-HIAA in brain. Thus, 5-HT may well be oxidized preferentially by MAO-A in vivo, as it is in vitro, even though serotonergic neurons do not contain much of this form of the enzyme.
The use of transgenic mice permits the selective elimination, or “knockout,” of either MAO type [14]. In the brains of mice deficient in MAO-A, the content of 5-HT is elevated markedly for about 12 days after birth and then slowly declines, reaching values comparable to those in normal mice after about 7 months. In MAO-A-deficient mice, the selective inhibitor of MAO-B, deprenyl, had a greater effect on serotonin metabolism than it did in normal mice. Such observations indicate that, in the absence of MAO-A, MAO-B can metabolize 5-HT in vivo. However, mice lacking the MAO-B isoenzyme do not have elevated levels of 5-HT in brain. Of interest are the aggressive behaviors exhibited by mice deficient in MAO-A, consistent with a postulated role of serotonergic neurons in human aggressive behaviors.
- The indolealkylamine 5-hydroxytryptamine, serotonin, was identified initially because of interest in its cardiovascular effects
- Understanding the neuroanatomical organization of serotonergic cells in brain provides insight into the functions of this neurotransmitter
- The amino acid l-tryptophan serves as the precursor for the synthesis of 5-hydroxytryptamine
- The synthesis of 5-hydroxytryptamine can increase markedly under conditions requiring a continuous supply of the neurotransmitter
- As with other biogenic amine transmitters, 5-hydroxytryptamine is stored primarily in vesicles and released by an exocytotic mechanism
- The activity of 5-hydroxytryptamine in the synapse is terminated primarily by its re-uptake into serotonergic terminals
- The primary catabolic pathway for 5-hydroxytryptamine is oxidative deamination by the enzyme monoamine oxidase
- Serotonin - Basic NeurochemistrySerotonin - Basic Neurochemistry
Your browsing activity is empty.
Activity recording is turned off.
See more...