By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Siegel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Philadelphia: Lippincott-Raven; 1999.

Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition.
Show detailsThe cytoskeleton is one of several biological elements that define eukaryotic cells
Other defining elements include the nucleus and mitochondria. The term “cytoskeleton” is often used as if it described a single, unified structure, but the cytoskeleton of neurons and other eukaryotic cells comprises three distinct, interacting structural complexes that have very different properties: microtubules (MTs), neurofilaments (NFs) and microfilaments (MFs). Each has a characteristic composition, structure and organization that may be further specialized in a particular cell type or subcellular domain. The defining structural elements have long been identifiable in electron micrographs (Fig. 8-1), and a considerable amount is known about the detailed organization of these components in neurons and glia. Each set of cytoskeletal structures is considered in turn.

Figure 8-1
The cytoskeleton and organization of the axon in cross-section. Left: Electron micrograph of a myelinated toad axon in cross-section taken near a Schmidt-Lanterman cleft; axon diameter is slightly reduced and the different domains within the axoplasm (more...)
Microtubules act as both dynamic structural elements and tracks for organelle traffic
Neuronal MTs are structurally similar to those found in other eukaryotic cells [1]. The core structure is a polymer of 50-kDa tubulin subunits. Heterodimers of α- and β-tubulin align end to end to form protofilaments, 13 of which join laterally to form a hollow tube with an outer diameter of 25 nm (Fig. 8-2). Examples also exist of MTs with 12 and 14 protofilaments. The α- and β-tubulins are the best known members of a unique protein family, the members of which have significant sequence similarity [2]. There is approximately 40% sequence identity between α- and β-tubulins and even greater identity within the α and β gene subfamilies. Conservation of the primary sequence for tubulins is also high across species so that tubulins from yeast can readily co-assemble with tubulins from human brain. Tubulin dimers bind two molecules of GTP and exhibit GTPase activity that is closely linked to assembly and disassembly of MTs [1,3]. While many questions remain about tubulin and its interactions, the structure of the αβ-tubulin dimer has recently been derived from electron diffraction studies [3], providing a basis for dissection of the functional architecture of MTs.
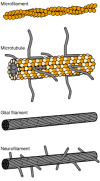
Figure 8-2
Microfilaments, microtubules and intermediate filaments in the nervous system. Each cytoskeletal structure has a distinctive ultrastructure. This schematic illustrates the major features of the core fibrils. The microfilament consists of two strands of (more...)
Heterodimers in a MT are oriented in the same direction, so the resulting MT has asymmetrical ends that differ in assembly properties [4]. The β-tubulin subunit is exposed at the “plus” end, which is the preferred end for addition of tubulin dimers. The opposite, “minus,” end grows more slowly at physiological concentrations of tubulin. In the case of free MTs, the balance between assembly and disassembly at each end defines a critical concentration for net growth. MT assembly under in vitro conditions involves a slow nucleation step followed by a more rapid, net growth phase interspersed with occasional, rapid shrinkage, a kinetic pattern described as dynamic instability. In glia and most other non-neuronal cells, however, the minus ends of MTs are usually bound at the site of nucleation, which is associated with the pericentriolar complex of the cell, a site often called the microtubule-organizing center (MTOC) [5]. Anchoring of MT minus ends helps to establish and maintain the polarity of cellular MTs. Anchoring and nucleation of MTs appear to require a third class of tubulin, γ-tubulin, which is detectable only as part of the pericentriolar complex [5].
The organization of MTs in neurons differs in several ways from that seen in non-neuronal cells (Fig. 8-3) [6]. Axonal and dendritic MTs are not continuous back to the cell body nor are they associated with any visible MTOC. Axonal MTs can be more than 100 μm long, but they have uniform polarity, with all plus ends distal to the cell body. Dendritic MTs are typically shorter and often exhibit mixed polarity, with only about 50% of the MTs oriented with the plus end distal. Recent work suggests that MTs in both axons and dendrites are nucleated normally at the MTOC but are then released from the MTOC and delivered to neurites [7].
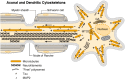
Figure 8-3
The axonal and dendritic cytoskeletons differ in both composition and organization. The major differences are illustrated diagramatically in this diagram. With one exception, all cytoskeletal proteins are synthesized on free polysomes in the cell body, (more...)
While MTs in neurons are composed of the same basic constituents as those in non-neuronal cells, they are strikingly more diverse (Table 8-1). Brain MTs contain tubulins of many different isotypes, with many different post-translational modifications and a variety of microtubule-associated proteins (MAPs). MT composition varies according to location, such as in axons or dendrites, suggesting that brain MTs exist in specialized forms to perform designated tasks in the unique environments of the neuron. For example, axonal MTs contain stable segments that are unusually resistant to treatments that depolymerize MTs in other cells. Such stable domains are preserved as short MT segments and may serve to nucleate or organize MTs in axons, particularly during regeneration [8]. This and other specializations of axonal MTs (see below) may reflect the unusual requirements of the neuronal cytoskeleton, where remarkably long MTs are maintained at considerable distances from sites of new protein synthesis in the cell body.
Table 8-1
Major Microtubule Cytoskeletal Proteins of the Nervous System.
Multiple genes exist for both α- and β-tubulin. Tubulin isotypes differ primarily at the carboxy terminus, the region where most post-translational modifications and MAP interactions occur. While most α- and β-tubulin isotypes are expressed in all tissues, some are expressed preferentially in different tissues. For example, class III and IVa β-tubulins are neuron-specific [9]. It is not known if such examples of tissue-specific expression imply that different isotypes are structurally suited to function in different tissues or merely that different tubulin genes are part of different tissue-specific developmental programs. Where they can be evaluated, all isotypes available in a given cell appear capable of coassembly and typically can be detected in all cellular MTs [9].
Brain MTs also contain a variety of post-translational modifications. When purified, mammalian tubulin is analyzed by isoelectric focusing and over 20 different isoforms can be seen [9]. These are explained by a combination of multiple genes and various post-translational modifications. The two best studied post-translational modifications of tubulin are α-tubulin detyrosination and acetylation [9]. Most α-tubulins are expressed with a carboxy-terminal tyrosine residue. This tyrosine can be removed by tubulin carboxypeptidase and then replaced by tubulin tyrosine ligase, in a rapid cycle that occurs on the majority of available tubulin. Since the carboxypeptidase acts only on assembled tubulin and the ligase acts on unassembled tubulin, this tyrosination/detyrosination cycle is linked to MT dynamics. Typically, newly assembled MTs will contain Tyr-tubulin. The longer an MT remains polymerized, the higher its content of de-Tyr-tubulin, or Glu-tubulin. A second prominent post-translational modification of neuronal α-tubulin is lysine ϵ—acetylation. The enzyme responsible for acetylation, tubulin acetyltransferase, like tubulin carboxypeptidase, acts only on assembled tubulin. Neuronal β-tubulin isotypes appear to be subject to fewer post-translational modifications [9]. The βIII isotype of brain is phosphorylated, and while the function of this modification is not clear, correlations with neurite outgrowth in neuroblastoma cells have been made. Another modification of β-tubulin is polyglutamylation, the addition of one to five glutamyl units to the γ-carboxyl group of a glutamate residue near the C terminus. Despite the plethora of neuronal tubulin post-translational modifications, to date, none is known to have a direct effect on MT properties.
MTs in vivo invariably include members of a heterogeneous set of proteins known as MAPs [10]. MAPs interact with MTs rather than with free tubulin and maintain constant stoichiometry with the tubulin in MTs through cycles of assembly and disassembly. Several categories of brain MAPs can be distinguished: the high-molecular-weight MAPs, which include MAPs 1A, 1B, 2A and 2B (>270 kDa); the tau proteins; MAPs of intermediate molecular weight, such as MAP3 and MAP4; and the molecular motors kinesin and dynein, which drive the intracellular transport of membrane-bound organelles along MT tracts (see Chap. 28). The other groups of MAPs are collectively known as “fibrous” or “structural” because they have been observed to form lateral extensions between adjacent MTs and between MTs and other cytoskeletal elements [10]. Such extensions do not generally represent stable cross-links but, rather, reflect transient interactions which facilitate MT function. One exception is the formation of stably cross-linked axonemal MTs of cilia and flagella, such as occur on ependymal cells in the CNS.
The high molar ratio of MAPs to tubulin in brain suggests that MAPs may play an important role in determining MT properties. Some MAPs are differentially distributed in neurons [10]. For example, MAP2 is found primarily in cell bodies and dendrites, and tau is enriched in axons. Additionally, changes in MAP expression and MAP phosphorylation during development suggest that they may play a role in modulating MT function in the developing brain [10]. For example, MAPs 1A and 1B occur in both axons and somatodendritic domains, but MAP1B is preferentially phosphorylated in axons and especially in developing axons. As many as six different tau proteins are derived by alternative splicing from a single tau gene [11]. Both the expression and the phosphorylation of the different tau isoforms are regulated throughout development. While MAPs affect nucleation and stability of MTs in vitro, these may not be their primary functions in vivo. Other likely functions include roles in MT spacing and organization, compartmentation and interaction with other cellular structures.
MTs serve multiple roles in neurons [6,8]. Besides acting as the substrate for the transport of membrane-bound organelles, MTs are necessary for the extension of neurites during development; they provide the scaffolding for maintaining neurites after extension, and they help maintain the definition and integrity of intracellular compartments. The diversity of these functions is reflected in differences in the biochemistry and metabolic stability of different MTs.
Neuronal and glial intermediate filaments provide support for neuronal and glial morphology
As a class of cytoskeletal structures, intermediate filaments (IFs) display an unusual degree of cell specificity and are often used as markers of cellular differentiation. They comprise a family of related genes that have been classified in five types. All five share homology in a core rod domain, which contains multiple α-helical domains that can form coiled coils. The sequence homology in this conserved domain is sufficient that some antibodies recognize all known IFs from mammals through a wide range of invertebrates. IFs are also ultrastructurally similar regardless of type, forming 8- to 10-nm rope-like filaments that may be several micrometers long. However, NFs differ from the other IFs because they have sidearms that project from the surface (Fig. 8-2). The result is that IFs in non-neuronal cells are often seen in densely packed bundles, while NFs are widely spaced (Fig. 8-4).
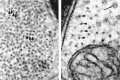
Figure 8-4
Glial filaments and neurofilaments are easily recognized in electron micrographs. The glial filaments lack side arms and often appear to be densely packed, with neighboring subunits almost touching (large arrows in left panel). In contrast, the spacing (more...)
IF types I and II are the keratins which are found in various combinations in epithelial cells throughout the body. Keratin IFs must include representative subunits of both type I and type II, with each tissue having a characteristic combination. In contrast, type III IF subunits typically form homopolymers. They include IFs that are characteristic of less differentiated cells like glial or neuronal precursors, as well as those seen in more specialized cell types like smooth muscle cells and mature astrocytes. These three types of IF not only share sequence homology within their rod domains, but their genes exhibit similar exon and intron structure. All type IV IFs are neuron-specific and have a common pattern of exons and introns that differs from that seen in types I—III. Finally, type V polypeptides are the nuclear lamins which form the walls of the nuclear envelope structure, rather than typical IF structures. While all eukaryotes express type V lamins, the other IF types are not found in plants, many unicellular organisms and the arthropods. Cytoplasmic IFs are, however, nearly ubiquitous in vertebrate cells, and some cells contain more than one type of IF in the cytoplasm. Curiously, oligodendrocyte precursors contain vimentin IFs, but these are lost during differentiation, making mature oligodendrocytes one of the few vertebrate cell types that lack cytoplasmic IFs.
The nervous system contains an unusually diverse set of IFs (Table 8-2) with distinctive cellular distributions and developmental expression [12,13]. Despite their molecular heterogeneity, all IFs appear as solid ropelike fibers, 8 to 12 nm in diameter. NFs can be hundreds of micrometers long and have characteristic sidearm projections, while filaments in glia or other non-neuronal cells are shorter and lack sidearms (Fig. 8-4). The existence of NFs was established long before much was known about their biochemistry or properties. As stable cytoskeletal structures, NFs were noted in early electron micrographs, and many traditional histological procedures that visualize neurons are based on a specific interaction of metal stains with NFs.
Table 8-2
Intermediate Filament (IF) Proteins of the Nervous System.
The primary type of IF in large myelinated axons is formed from three subunit proteins known as the NF triplet: NF high-molecular-weight subunit (NFH, 180 to 200 kDa), NF middle-molecular-weight subunit (NFM, 130 to 170 kDa) and NF low-molecular-weight subunit (NFL, 60 to 70 kDa) [12,13]. Each of the subunit proteins is coded for by a separate gene. The NF triplet proteins are type IV IF proteins which are expressed only in neurons and have a characteristic domain structure. The amino-terminal regions of all three subunits interact via α-helical coiled coils to form the core of the filament. NFM and NFH also have long carboxy-terminal regions, which project from the core filaments as sidearms. NFH and, to a lesser extent, NFM have a large number of consensus phosphorylation sites for proline-directed kinases in this carboxy-terminal extension (>50 on NFH and >10 on NFM in many species). In large myelinated axons, most, if not all, of these sites are phosphorylated [12,13]. This phosphorylation of NFH and NFM sidearms alters the charge density on the NF surface, repelling adjacent NFs with similar charge. Such mutual repulsion by the sidearms of NFs is thought to be a major determinant of axonal caliber [8].
Other IFs are also found in the nervous system [12,13]. Vimentin is the most widely expressed type III IF and is found in a variety of cell types, like fibroblasts, microglia and smooth muscle cells, as well as in embryonic cell types, including neuronal and glial precursors. Astrocytes and some Schwann cells contain the type III IF glial fibrillary acidic protein (GFAP). This distribution has led to the widespread use of GFAP immunoreactivity to identify astrocytes in culture and in tissue. In contrast to NFs, type III IFs like GFAP lack sidearms and often appear to be tightly bundled.
At least three other IFs occur in selected neurons or neuronal precursors: α-internexin, peripherin and nestin. All of these are most prominently expressed during development, then downregulated. Based on sequence and gene structure, peripherin is a type III IF, while α-internexin is a type IV IF. While the NF triplet proteins and α-internexin may be components of both CNS and PNS neurons, peripherin appears to be expressed preferentially in PNS neurons. Both can co-assemble with the NF triplet proteins in vitro and may do so in vivo but can also form homopolymeric filaments. Both are expressed at higher levels in a variety of developing neurons, and expression becomes more restricted as neurons mature. Many neurons cease to express α-internexin during maturation. However, a few neurons retain the expression of IFs containing α-internexin. Notably, IFs of parallel fibers in the cerebellar cortex contain only α-internexin subunits. Peripherin continues to be expressed in some peripheral neurons in maturity. Nestin is the most divergent of the IFs that form filaments and is sometimes considered to be a distinct IF type. Although nestin is specific to the nervous system, it is expressed in multipotent neuroectodermal precursors and suppressed during subsequent development. For this reason, it can be used as an early marker for differentiation of precursor cells.
Not all neurons have NFs. Indeed, one entire phylum in the animal kingdom, arthropods, expresses only type V nuclear lamins, so arthropod cells have no IF cytoskeletal structures at all. In addition, mature oligodendrocytes lack IFs, although their embryonic precursors contain vimentin. Clearly, the IFs are not essential for cell survival; yet in large myelinated fibers, NFs make up the bulk of axonal volume and represent a substantial fraction of total protein synthesis in the brain. In most organisms, IFs in both glia and neurons contribute to the distinctive morphologies of these cells. They are thought to provide mechanical strength and a stable cytoskeletal framework. In neurons, NFs play an important role in regulating cellular and axonal volumes and are a primary determinant of axonal caliber in large fibers. Finally, NFs exhibit an unusual degree of metabolic stability, which makes them well suited for a role in stabilizing and maintaining neuronal morphology [14].
Actin microfilaments and the membrane cytoskeleton play critical roles in neuronal growth and secretion
This major class of cytoskeletal elements is perhaps the oldest. Certainly, the actin cytoskeleton has the most diverse composition and organization. MFs are formed from 43-kDa actin monomers that are arranged like two strings of pearls intertwined into fibrils 4 to 6 nm in diameter (Fig. 8-2) [15]. A remarkable variety of proteins have been found to interact with actin MFs, ranging from myosin motors to cross-linkers, bundling proteins to anchoring proteins and sequestration proteins to small GTPase regulatory proteins [16,17].
Actin MFs are found throughout neurons and glia [18], but they are enriched in cortical regions near the plasmalemma and are particularly concentrated in presynaptic terminals, dendritic spines and growth cones. Under most circumstances in the nervous system, MFs are short oligomers organized into a meshwork most apparent near the plasma membrane and in the vicinity of axonal MTs. MFs are the main components of the membrane cytoskeleton [19,20], and this may be their primary role in mature neurons. The prominent actin bundles, termed stress fibers, seen in fibroblasts and many other non-neuronal cells in culture are not characteristic of neurons in vivo or in vitro. Most neuronal MFs are less than 1 μm in length. However, growth cones contain many longer MFs, with bundles of MFs in the filopodia and lamellipodia in addition to the typically more dispersed actin network (Fig. 8-5) [21]. The role of actin MFs in the growth cone will be considered in greater detail below.

Figure 8-5
The cytoskeletal elements of a growth cone are organized for motility. In this diagram of a growth cone, a typical distribution of major cytoskeletal structures is shown. The microfilaments are longer and more prominent in the growth cone than in other (more...)
Many MF-associated proteins [16] have been described in the nervous system (Table 8-3). In general, a good deal is known about their distribution and function in primary cultures of neurons and glia, but less is known about their role in the mature nervous system. Two that have been characterized more extensively are the major nonactin structural elements of the membrane cytoskeleton: spectrin and ankyrin. Spectrin is a flexible, rod-shaped molecule composed of homologous α and β subunits and was originally characterized as a component of the erythrocyte membrane cytoskeleton. Neurons were the first cell type, other than erythrocytes, that were shown to contain spectrins, and the brain form was initially called fodrin. Spectrin heterodimers align end to end to form tetramers, which are cross-linked by short actin MFs. This spectrin—actin meshwork is tightly coupled to the plasma membrane through direct binding to membrane proteins. Some of these interactions occur via the protein ankyrin, which has separate binding sites for specific membrane proteins and β-spectrin (see Fig. 2-5). In neurons, specific isoforms of spectrin and ankyrin are localized to axons, dendrites and paranodal regions. The spectrin and ankyrin isoforms in perikarya and dendrites tend to be highly homologous to the erythrocyte forms and distinct from the spectrin and ankyrin isoforms that occur in axons.
Table 8-3
Some Microfilament-Associated Proteins in the Nervous System.
A variety of additional MF bundling or linking proteins have been described in the nervous system. For example, fimbrin may play a role in the formation of MF bundles in growth cone filopodia. Still other actin-binding proteins may regulate MF assembly [22,23]. Gelsolin fragments MFs in a Ca2+-sensitive manner but also caps and nucleates MFs. In contrast, profilin and β-thymosins bind to actin monomers and may act in part by sequestering actin subunits, although this oversimplifies the effects of these proteins. The list of MF-associated proteins has become quite long, and this diversity reflects the many forms of MF-based cytoskeletal structures.
MFs in the nervous system appear to have a variety of functions. The neuronal membrane cytoskeleton plays a role in maintaining the distribution of plasma membrane proteins, establishing cell morphologies and segregating axonal and dendritic proteins into their respective compartments [20]. MFs and the membrane cytoskeleton also mediate the interactions between neurons and the external world, including extracellular matrix components and neighboring cells (see Chap. 7). In neurons and glia, cell adhesion sites, such as tight junctions and focal adhesion plaques, interact with the MF cytoskeleton either directly or indirectly. The cortical MF meshwork also restricts access of organelles to the plasma membrane and is involved in both regulated and constitutive secretion (see Chap. 9). Finally, MFs are the basis of filopodia and lamellipodia that are essential for cell migration, growth cone motility and myelination.
- The cytoskeleton is one of several biological elements that define eukaryotic cells
- Microtubules act as both dynamic structural elements and tracks for organelle traffic
- Neuronal and glial intermediate filaments provide support for neuronal and glial morphology
- Actin microfilaments and the membrane cytoskeleton play critical roles in neuronal growth and secretion
- Molecular Components of the Neuronal Cytoskeleton - Basic NeurochemistryMolecular Components of the Neuronal Cytoskeleton - Basic Neurochemistry
Your browsing activity is empty.
Activity recording is turned off.
See more...