By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003.

Holland-Frei Cancer Medicine. 6th edition.
Show detailsPrimary musculoskeletal neoplasms are relatively uncommon when compared with malignancies of other organ systems. Musculoskeletal involvement of malignancy is more often a result of metastatic disease from neoplasms of nonmusculoskeletal origins. It is these metastatic lesions that are most commonly evaluated by imaging. However, with the advent of computed tomography (CT), magnetic resonance (MR), and positron emission tomography (PET) imaging and the advances in technique and equipment for percutaneous interventions, radiographic examination has become a critical component of the preoperative work-up of primary musculoskeletal malignancies in almost all instances.
Radiographs, or “plain films,” continue to be the mainstay in the primary evaluation of osseous neoplasms. It is from the radiograph that the initial characterization of the abnormality is performed. The lesion is determined to be either “aggressive” or “nonaggressive” in appearance, based on its radiographic appearance. It is important that this terminology not be misinterpreted such that aggressive is thought of as equivalent to malignant, as this is not always the case. The same is true for nonaggressive lesions. Although they are often benign, some malignant bone lesions—metastatic disease or myeloma, for example—may have a deceptively nonaggressive appearance on radiographs. Regardless, it is from the radiograph that this initial assessment of primary osseous lesions is performed, and the differential diagnosis is determined. After this initial evaluation is performed, cross-sectional imaging may be used to further define the anatomic detail of a lesion. Is there a soft-tissue component to the lesion? If so, what is the extent of the soft-tissue component? What is the relationship to neurovascular structures? What anatomic compartments are involved? What is the extent of marrow involvement, and in special instances, are there “skip lesions” present? The answers to these questions can be gleaned from cross-sectional imaging and may help to narrow the differential diagnosis obtained from the radiograph, but should not be used in isolation to generate a differential diagnosis. The primary importance of the cross-sectional evaluation of these lesions is for treatment planning.
Primary neoplasms of the soft tissues of the musculoskeletal system may not be visible on radiographs. They often present as palpable masses or as pain or mass effect on adjacent structures. It remains important to obtain radiographs to obtain an initial assessment for any adjacent osseous involvement, and to determine the presence and/or extent of mineralization within the mass. These can be important clues in developing a differential diagnosis and some details may be visualized on radiographs that are more difficult to visualize with cross-sectional imaging. However, as many of the soft-tissue musculoskeletal neoplasms have no adjacent osseous involvement or mineralization, cross-sectional imaging plays a somewhat greater role in the development of a differential diagnosis than in primary osseous lesions.
Cross-sectional imaging is occasionally diagnostic, as in the pathognomonic appearance of benign lipomas (Figure 36e-1), or in cases where the number or distribution of the masses is diagnostic, such as in neurofibromatosis (Figure 36e-2). However, in many soft-tissue masses, the imaging characteristics are not diagnostic of the tumor type, and only a differential diagnosis can be provided based on probabilities. This differential diagnosis takes into account the signal characteristics of the mass, the anatomic location of the mass, the age of the patient and the clinical presentation, among other factors. There are some instances in which cross-sectional imaging will not determine whether a lesion is benign or malignant, but even in these cases it will provide valuable information on the extent of the lesion and anatomic relationships that will be important in the eventual sampling of the lesion and/or subsequent therapy.1

Figure 36e-1
T1-weighted (A) and T2-weighted (B) (without fat-suppression) axial MR images through the distal thigh reveal a high T1, high T2 signal mass anterior to the distal femur consistent with a benign lipoma. Note how the signal characteristics match those (more...)

Figure 36e-2
T1-weighted (A) and T2-weighted, fat-suppressed (B) coronal MR images through the sacrum reveal low to intermediate T1 signal, high T2 signal masses extending along the path of the sacral nerve roots and proximal sciatic nerve consistent with neurogenic (more...)
The appearance of musculoskeletal masses on MRI is relatively nonspecific in many instances, as most tumors have similar signal characteristics—high T2 signal and low or intermediate T1 signal (Figure 36e-3). However, MRI may provide a diagnosis, or at least a very limited differential, in some tumors based on signal characteristics, location, and distribution. Tumors in which MRI may provide a definitive diagnosis include benign lipomas, pigmented villonodular synovitis (PVNS), most hemangiomas (Figure 36e-4), and many neural tumors. Plantar fibromatosis (Figure 36e-5), Morton neuroma, accessory muscles, and myxomas are all masses that may be easily diagnosed with MR imaging. Some masses have characteristics, such as fluid-fluid levels in aneurysmal bone cysts and telangiectatic osteosarcomas, which may narrow the differential diagnosis considerably. MRI can also be very useful in differentiating an enchondroma from a chondrosarcoma by evaluating for the presence of a soft-tissue mass, which is virtually never present in an enchondroma.
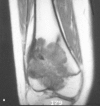
Figure 36e-3
T1-weighted (A) and T2-weighted, fat-suppressed (B) coronal MR images of an osteosarcoma in the distal femur. These signal characteristics are common in malignant and benign tumors; consequently, the signal alone is not diagnostic.
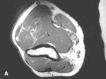
Figure 36e-4
T1-weighted (A) and T2-weighted, fat suppressed (B) axial MR images through the upper arm reveal a lobulated mass with interdigitating fat signal and extension through fascial planes. These characteristics are diagnostic of a soft tissue hemangioma.
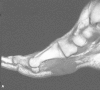
Figure 36e-5
T1-weighted (A) and T2-weighted, fat-suppressed (B) sagittal images of the foot reveal low T1, low T2 signal mass in the plantar aspect of the foot. This combination of signal characteristics and location is diagnostic of plantar fibromatosis.
Given the primary purposes of cross-sectional imaging—to determine the anatomic extent and delineate relationships to adjacent tissues and compartments—MRI, even though it may not provide a definitive diagnosis in all cases, is of primary importance in the evaluation of musculoskeletal neoplasms. This is a result of the superior ability of MRI to distinguish tissue types and to evaluate soft tissue edema and marrow abnormality, in comparison to CT. CT does have application in occasional cases in which characterization of mineralization is important (MRI is not as helpful in characterizing mineralization because of many factors, including the artifact caused by the paramagnetic effect of calcium). CT also has proven quite useful as a guide for percutaneous biopsy.2 Ultrasound is primarily useful as a guidance modality for percutaneous biopsy in select cases. It is occasionally used to provide information on the solid or cystic nature of a known mass, or to evaluate for flow within a lesion,3 but is not a primary diagnostic modality for musculoskeletal neoplasms. PET imaging has limited applications in musculoskeletal neoplasms currently, primarily being used for identification of distant soft-tissue metastases, although there is some research suggesting quantitative analysis of glucose metabolism might help to differentiate benign from malignant primary bone lesions.4, 5 However, this quantitative analysis is not standard practice. The standardized uptake value (SUV) is widely used in other malignancies, but there is considerable overlap of benign and malignant musculoskeletal lesions with regard to SUV.6
Dynamic contrast-enhanced MRI of musculoskeletal masses has been described as a potential tool for establishing a more accurate diagnosis.7 The pattern and degree of delayed enhancement, as well as patterns of dynamic enhancement, reportedly have potential utility in characterization of masses.8 However, the utility of contrast enhancement for musculoskeletal neoplasms in practice is relatively limited. The primary utility is in those few cases in which there is a need to determine whether a very bright T2 lesion is solid or cystic. A solid lesion will exhibit at least a small degree of enhancement throughout the mass, whereas a cystic or fluid-filled lesion will enhance only peripherally. Because of the reduced ability of CT imaging to distinguish between subtle tissue differences, contrast may have an increased role in CT evaluation of musculoskeletal malignancies in geographic locations where MRI is not easily accessible.
Once a lesion has been identified and characterized, often a biopsy of the lesion or resection of the lesion for histologic evaluation is necessary to make a more specific diagnosis prior to definitive therapy. Percutaneous sampling of musculoskeletal neoplasms has become increasingly important in this pretreatment evaluation. As the tools for performing these biopsies have improved, and as the experience level with the techniques has improved, this has become an alternative to open biopsy in many cases.9 Larger-gauge needles with acquisition of cores of tissue are necessary for characterization of most lesions, although experienced pathologists may make an accurate diagnosis of some neoplasms based on smaller-gauge fine-needle aspiration.10
Many musculoskeletal masses are palpable and therefore may be percutaneously sampled without imaging guidance by someone experienced in this type of procedure. In those cases that require imaging assistance, CT scanning provides an excellent way to visualize a path for needle placement. Although fluoroscopic guidance may be used to guide biopsies, the greater delineation of anatomic structures and the improved ability to evaluate for soft-tissue components of osseous masses and for thinning of the cortex make CT imaging the modality of choice in many, if not most cases. These advantages potentially decrease the required number of needle placements, allow better visualization of structures to be avoided, and therefore theoretically reduce the complication rate and improve the yield of biopsy. MRI may be used as a guide for percutaneous sampling, but requires special nonferromagnetic sampling equipment, and often requires an open MR scanner or an otherwise modified imaging system. This may translate into a more time-consuming approach to percutaneous sampling of musculoskeletal masses. Ultrasound may also be used as a guidance modality for sampling soft tissue masses, but is of limited utility in predominantly osseous lesions.
Regardless of the modality used for guidance of percutaneous sampling, it is critical for the physician performing the biopsy to be in communication with the surgeon who will be in charge of the definitive therapy. The compartmental anatomy of a lesion is vital to the surgeon’s eventual approach in many instances.11 Contamination of compartments not involved by the tumor by passing a needle through them on the biopsy approach can radically alter the surgical therapy in many sarcomas because of the need to resect the biopsy tract. In some cases, a lack of communication between the physician performing the biopsy and the surgeon may even result in an amputation having to be performed when a limb-sparing procedure might otherwise have been an option.
Involvement of the pathologist in the discussion of a lesion to be percutaneously sampled is also critical. The interpretation of histology of musculoskeletal neoplasms is best performed by a pathologist with particular experience in musculoskeletal oncology as the differences between malignant and benign histology may be subtle and can depend on characteristics of the lesion that cannot be obtained from a gross or microscopic sample alone. Histologic characteristics or patterns that would be interpreted as benign in one anatomic location may be interpreted as more ominous or aggressive in another location. Therefore, the radiologic assessment can play a critical role in the eventual histologic diagnosis in musculoskeletal neoplasms. Ultimately, a cooperative effort between the radiologist, the surgeon, the pathologist and the oncologist is optimal for a complete assessment and development of a treatment plan.
In summary, imaging evaluation of musculoskeletal neoplasms has become a critical part of the development of a treatment plan. Imaging has also provided an avenue for characterizing and even sampling lesions prior to institution of definitive therapy, thus reducing the risk of biopsy in many patients and providing a greater degree of information about the anatomy and extent of a tumor than has been available in the past. This also can help to avoid unnecessary interventions and can lead to a much more focused therapeutic plan in those patients in whom further intervention is required.
Contents
- References
- Imaging of Musculoskeletal Neoplasms - Holland-Frei Cancer MedicineImaging of Musculoskeletal Neoplasms - Holland-Frei Cancer Medicine
Your browsing activity is empty.
Activity recording is turned off.
See more...