By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000.

Developmental Biology. 6th edition.
Show detailsCleavage in Bird Eggs
Ever since Aristotle first followed its 3-week development, the domestic chicken has been a favorite organism for embryological studies. It is accessible all year and is easily raised. Moreover, at any particular temperature, its developmental stage can be accurately predicted. Thus, large numbers of embryos can be obtained at the same stage. The chick embryo can be surgically manipulated and, since it forms most of its organs in ways very similarly to those of mammals, it has often served as a surrogate for human embryos.
Fertilization of the chick egg occurs in the oviduct, before the albumen and the shell are secreted upon it. The egg is telolecithal (like that of the fish), with a small disc of cytoplasm sitting atop a large yolk. Like fish eggs, the yolky eggs of birds undergo discoidal meroblastic cleavage. Cleavage occurs only in the blastodisc, a small disc of cytoplasm 2–3 mm in diameter at the animal pole of the egg cell. The first cleavage furrow appears centrally in the blastodisc, and other cleavages follow to create a single-layered blastoderm (Figure 11.8). As in the fish embryo, these cleavages do not extend into the yolky cytoplasm, so the early-cleavage cells are continuous with each other and with the yolk at their bases (Figure 11.8E). Thereafter, equatorial and vertical cleavages divide the blastoderm into a tissue five to six cell layers thick. These cells become linked together by tight junctions (Bellairs et al. 1975; Eyal-Giladi 1991). Between the blastoderm and the yolk is a space called the subgerminal cavity (Figure 11.9A). This space is created when the blastoderm cells absorb fluid from the albumin (“egg white”) and secrete it between themselves and the yolk (New 1956). At this stage, the deep cells in the center of the blastoderm are shed and die, leaving behind a one-cell-thick area pellucida. This part of the blastoderm forms most of the actual embryo. The peripheral ring of blastoderm cells that have not shed their deep cells constitutes the area opaca. Between the area pellucida and the area opaca is a thin layer of cells called the marginal zone (or marginal belt) (Eyal-Giladi 1997; Arendt and Nübler-Jung 1999).* Some of the marginal zone cells become very important in determining cell fate during early chick development.
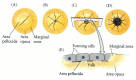
Figure 11.8
Discoidal meroblastic cleavage in a chick egg. (A-D) Four stages viewed from the animal pole (the future dorsal side of the embryo). (E) An early-cleavage embryo viewed from the side. (After Bellairs et al. 1978.)

Figure 11.9
Formation of the two-layered blastoderm of the chick embryo. (A, B) Primary hypoblast cells delaminate individually to form islands of cells beneath the epiblast. (C) Secondary hypoblast cells from the posterior margin (Koller's sickle and the posterior (more...)
Gastrulation of the Avian Embryo
The hypoblast
By the time a hen has laid an egg, the blastoderm contains some 20,000 cells. At this time, most of the cells of the area pellucida remain at the surface, forming the epiblast, while other area pellucida cells have delaminated and migrated individually into the subgerminal cavity to form the polyinvagination islands (primary hypoblast), an archipelago of disconnected clusters containing 5–20 cells each (Figure 11.9B). Shortly thereafter, a sheet of cells from the posterior margin of the blastoderm (distinguished from the other regions of the margin by Koller's sickle, a local thickening) migrates anteriorly to join the polyinvagination islands, thereby forming the secondary hypoblast (Eyal-Giladi et al. 1992). The two-layered blastoderm (epiblast and hypoblast) is joined together at the margin of the area opaca, and the space between the layers forms a blastocoel. Thus, although the shape and formation of the avian blastodisc differ from those of the amphibian, fish, or echinoderm blastula, the overall spatial relationships are retained.
The avian embryo comes entirely from the epiblast. The hypoblast does not contribute any cells to the developing embryo (Rosenquist 1966, 1972). Rather, the hypoblast cells form portions of the external membranes, especially the yolk sac and the stalk that links the yolk mass to the endodermal digestive tube. All three germ layers of the embryo proper (plus a considerable amount of extraembryonic membrane) are formed from the epiblastic cells. Fate maps of the chick epiblast are shown in Figures 11.10 and 1.8 (Schoenwolf 1991).
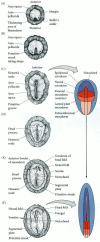
Figure 11.10
Cell movements of the primitive streak of the chick embryo. (A-C) Dorsal view of the formation and elongation of the primitive streak. The blastoderm is seen at (A) 3–4 hours, (B) 7–8 hours, and (C) 15–16 hours after fertilization. (more...)
The primitive streak
The major structural characteristic of avian, reptilian, and mammalian gastrulation is the primitive streak. This streak is first visible as a thickening of the epiblast at the posterior region of the embryo, just anterior to Koller's sickle (Figure 11.10A). This thickening is caused by the ingression of endodermal precursors from the epiblast into the blastocoel and by the migration of cells from the lateral region of the posterior epiblast toward the center (Figure 11.10B; Vakaet 1984; Bellairs 1986; Eyal-Giladi et al. 1992). As these cells enter the primitive streak, the streak elongates toward the future head region. At the same time, the secondary hypoblast cells continue to migrate anteriorly from the posterior margin of the blastoderm. The elongation of the primitive streak appears to be coextensive with the anterior migration of these secondary hypoblast cells. The streak eventually extends 60–75% of the length of the area pellucida.
The primitive streak defines the axes of the embryo. It extends from posterior to anterior; migrating cells enter through its dorsal side and move to its ventral side; and it separates the left portion of the embryo from the right. Those elements close to the streak will be the medial (central) structures, while those farther from it will be the distal (lateral) structures (Figure 11.10C-E).
As cells converge to form the primitive streak, a depression forms within the streak. This depression is called the primitive groove, and it serves as an opening through which migrating cells pass into the blastocoel. Thus, the primitive groove is analogous to the amphibian blastopore. At the anterior end of the primitive streak is a regional thickening of cells called the primitive knot or Hensen's node. The center of this node contains a funnel-shaped depression (sometimes called the primitive pit) through which cells can pass into the blastocoel. Hensen's node is the functional equivalent of the dorsal lip of the amphibian blastopore (i.e., the organizer) and the fish embryonic shield.
As soon as the primitive streak has formed, epiblast cells begin to migrate through it and into the blastocoel (Figure 11.11). The primitive streak has a continually changing cell population. Cells migrating through Hensen's node pass down into the blastocoel and migrate anteriorly, forming foregut, head mesoderm, and notochord; cells passing through the lateral portions of the primitive streak give rise to the majority of endodermal and mesodermal tissues (Schoenwolf et al. 1992). Unlike the Xenopus mesoderm, which migrates as sheets of cells into the blastocoel, cells entering the inside of the avian embryo ingress as individuals after undergoing an epithelial-to-mesenchymal transformation. At Hensen's node and throughout the primitive streak, the breakdown of the basal lamina and the release of these cells into the embryo is thought to be accomplished by scatter factor, a 190-kDa protein secreted by the cells as they enter the streak (Stern et al. 1990; DeLuca et al. 1999). Scatter factor can convert epithelial sheets into mesenchymal cells in several ways, and it is probably involved both in downregulating E-cadherin expression and in preventing E-cadherin from functioning
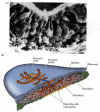
Figure 11.11
Migration of endodermal and mesodermal cells through the primitive streak. (A) Scanning electron micrograph shows epiblast cells passing into the blastocoel and extending their apical ends to become bottle cells. (B) Stereogram of a gastrulating chick (more...)
Migration through the primitive streak: formation of endoderm and mesoderm
The first cells to migrate through Hensen's node are those destined to become the pharyngial endoderm of the foregut. Once inside the blastocoel, these endodermal cells migrate anteriorly and eventually displace the hypoblast cells, causing the hypoblast cells to be confined to a region in the anterior portion of the area pellucida. This region, the germinal crescent, does not form any embryonic structures, but it does contain the precursors of the germ cells, which later migrate through the blood vessels to the gonads (see Chapter 19). The next cells entering the blastocoel through Hensen's node also move anteriorly, but they do not move as far ventrally as the presumptive foregut endodermal cells. Rather, they remain between the endoderm and the epiblast to form the head mesenchyme and the prechordal plate mesoderm (see Psychoyos and Stern 1996). These early-ingressing cells all move anteriorly, pushing up the anterior midline region of the epiblast to form the head process (Figure 11.12). Thus, the head of the avian embryo forms anterior (rostral) to Hensen's node. The next cells migrating through Hensen's node become chordamesoderm (notochord) cells. These cells extend up to the presumptive midbrain, where they meet the prechordal plate. The hindbrain and trunk form from the chordamesoderm at the level of Hensen's node and caudal to it.
Figure 11.12
Chick gastrulation from about 24 to about 28 hours. (A) The primitive streak at full extension (24 hours). The head process (anterior notochord) can be seen extending from Hensen's node. (B) Two-somite stage (25 hours). Pharyngeal endoderm is seen anteriorly, (more...)
Meanwhile, cells continue migrating inwardly through the lateral portion of the primitive streak. As they enter the blastocoel, these cells separate into two layers. The deep layer joins the hypoblast along its midline and displaces the hypoblast cells to the sides. These deep-moving cells give rise to all the endodermal organs of the embryo as well as to most of the extraembryonic membranes (the hypoblast forms the rest). The second migrating layer spreads between this endoderm and the epiblast, forming a loose layer of cells. These middle layer cells generate the mesodermal portions of the embryo and extraembryonic membranes. By 22 hours of incubation, most of the presumptive endodermal cells are in the interior of the embryo, although presumptive mesodermal cells continue to migrate inward for a longer time.
Regression of the primitive streak
Now a new phase of gastrulation begins. While mesodermal ingression continues, the primitive streak starts to regress, moving Hensen's node from near the center of the area pellucida to a more posterior position (see Figure 11.12). It leaves in its wake the dorsal axis of the embryo and the notochord. As the node moves posteriorly, the notochord is laid down, starting at the level of the future midbrain. While the anterior portion of the notochord is formed by the ingression of cells through Hensen's node, the posterior notochord (after somite 17 in the chick) forms from the condensation of mesodermal tissue that has ingressed through the primitive streak (i.e., not through Hensen's node). This portion of the notochord extends posteriorly to form the tail of the embryo (Le Douarin et al. 1996). Finally, Hensen's node regresses to its most posterior position, forming the anal region. At this time, all the presumptive endodermal and mesodermal cells have entered the embryo, and the epiblast is composed entirely of presumptive ectodermal cells.
As a consequence of the sequence by which the head mesoderm and notochord are established, avian (and mammalian) embryos exhibit a distinct anterior-to-posterior gradient of developmental maturity. While cells of the posterior portions of the embryo are undergoing gastrulation, cells at the anterior end are already starting to form organs (see Darnell et al. 1999). For the next several days, the anterior end of the embryo is more advanced in its development (having had a “head start,” if you will) than the posterior end.
Epiboly of the ectoderm
While the presumptive mesodermal and endodermal cells are moving inward, the ectodermal precursors proliferate. Moreover, the ectodermal cells migrate to surround the yolk by epiboly. The enclosure of the yolk by the ectoderm (again reminiscent of the epiboly of amphibian ectoderm) is a Herculean task that takes the greater part of 4 days to complete. It involves the continuous production of new cellular material and the migration of the presumptive ectodermal cells along the underside of the vitelline envelope (New 1959; Spratt 1963). Interestingly, only the cells of the outer edge of the area opaca attach firmly to the vitelline envelope. These cells are inherently different from the other blastoderm cells, as they can extend enormous (500 μm) cytoplasmic processes onto the vitelline envelope. These elongated filopodia are believed to be the locomotor apparatus of these marginal cells, by which they pull the other ectodermal cells around the yolk (Schlesinger 1958). The filopodia appear to bind to fibronectin, a laminar protein that is a component of the chick vitelline envelope. If the contact between the marginal cells and the fibronectin is experimentally broken (by adding a soluble polypeptide similar to fibronectin), the filopodia retract, and epidermal migration ceases (Lash et al. 1990).
Thus, as avian gastrulation draws to a close, the ectoderm has surrounded the yolk, the endoderm has replaced the hypoblast, and the mesoderm has positioned itself between these two regions. We have identified many of the processes involved in avian gastrulation, but we remain ignorant as to the mechanisms by which many of these processes are carried out.
WEBSITE
11.3 Epiblast cell heterogeneity. Although the early epiblast appears uniform, different cells have different molecules on their cell surfaces. This allows some of them to remain in the epiblast while others migrate into the embryo. http://www.devbio.com/chap11/link1103.shtml
VADE MECUM
Chick development.You can view movies of 3-D models of chick cleavage and gastrulation. [Click on Chick-Early]
Axis Formation in the Chick Embryo
While the formation of the body axes is accomplished during gastrulation, their specification occurs earlier, during the cleavage stage.
The role of pH in forming the dorsal-ventral axis
The dorsal-ventral (back-belly) axis is critical to the formation of the hypoblast and to the further development of the embryo. This axis is established when the cleaving cells of the blastoderm establish a barrier between the basic (pH 9.5) albumin above the blastodisc and the acidic (pH 6.5) subgerminal space below it. Water and sodium ions are transported from the albumin through the cells and into the subgerminal space, creating a membrane potential difference of 25 mV across the epiblast cell layer (positive at the ventral side of the cells). This process distinguishes two sides of the epiblast: a side facing the negative and basic albumin, which becomes the dorsal side, and a side facing the positive and acidic subgerminal space fluid, which becomes the ventral side. This axis can be reversed experimentally either by reversing the pH gradient or by inverting the potential difference across the cell layer (reviewed in Stern and Canning 1988).
The role of gravity in forming the anterior-posterior axis
The conversion of the radially symmetrical blastoderm into a bilaterally symmetrical structure is determined by gravity. As the ovum passes through the hen's reproductive tract, it is rotated for about 20 hours in the shell gland. This spinning, at a rate of 10 to 12 revolutions per hour, shifts the yolk such that its lighter components lie beneath one side of the blastoderm. This tips up that end of the blastoderm, and that end becomes the posterior portion of the embryo—the part where primitive streak formation begins (Figure 11.13; Kochav and Eyal-Giladi 1971; Eyal-Giladi and Fabian 1980).
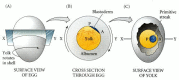
Figure 11.13
Specification of the chick anterior-posterior axis by gravity. Rotation in the shell gland (A) results in the lighter components of the yolk pushing up one side of the blastoderm (B). That more elevated region becomes the posterior of the embryo (C). (more...)
It is not known what interactions cause this portion of the blastoderm to become the posterior margin and to initiate gastrulation. The ability to initiate a primitive streak is found throughout the marginal zone, and if the blastoderm is separated into parts, each having its own marginal zone, each part will form its own primitive streak (Figure 11.14; Spratt and Haas 1960). However, once a posterior marginal zone (PMZ) has formed, it controls the other regions of the margin. Not only do these PMZ cells initiate gastrulation, but they also prevent other regions of the margin from forming their own primitive streaks† (Khaner and Eyal-Giladi 1989; Eyal-Giladi et al. 1992).
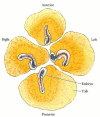
Figure 11.14
Regulation of the chick blastoderm. When the blastoderm is divided into four parts, each part can initiate gastrulation and give rise to an embryo. Usually, only the cells of the posterior marginal zone are able to form a primitive streak, and they inhibit (more...)
It now seems apparent that the PMZ contains cells that act as the equivalent of the amphibian Nieuwkoop center. When placed in the anterior region of the marginal zone, a graft of posterior marginal zone tissue (posterior to and not including Koller's sickle) is able to induce a primitive streak and Hensen's node without contributing cells to either structure (Bachvarova et al. 1998; Khaner 1998). Like the amphibian Nieuwkoop center, this region is thought to be the place where β-catenin localization in the nucleus and a TGF-β family signal coincide. The posterior marginal zone is the only region of the embryo secreting Vg1 (Mitrani et al. 1990; Hume and Dodd 1993; Seleiro et al. 1996).
The epiblast and middle layer cells in the anterior portion of Koller's sickle become Hensen's node (Bachvarova et al. 1998). The posterior portion of Koller's sickle contributes to the posterior portion of the primitive streak (Figure 11.15). Hensen's node has long been known to be the avian equivalent of the amphibian dorsal blastopore lip, since it is (1) the site where gastrulation begins, (2) the region whose cells become the chordamesoderm, and (3) the region whose cells can organize a second embryonic axis when transplanted into other locations of the gastrula (Figure 11.16; Waddington 1933; 1934; Diaz and Schoenwolf 1990). Moreover, the transplantation of Koller's sickle can also cause the formation of new axes, and the middle layer cells in Koller's sickle express goosecoid, just like the cells of Spemann's organizer (Izpisua-Belmonte et al. 1993; Callebaut and van Neuten 1994).

Figure 11.15
Formation of Hensen's node from Koller's sickle. (A) Diagram of the posterior end of an early (pre-streak) embryo, showing the cells labeled with fluorescent dyes in the photographs. (B) Just before gastrulation, cells in the anterior end of Koller's (more...)
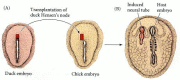
Figure 11.16
Induction of a new embryo by transplantation of Hensen's node. (A) A Hensen's node from a duck embryo is transplanted into the epiblast of a chick embryo. (B) A secondary embryo is induced (as is evident by the neural tube) from host tissues at the graft (more...)
As is the case in all vertebrates, the dorsal mesoderm is able to induce the formation of the central nervous system in the ectoderm overlying it. The cells of Hensen's node and its derivatives act like the amphibian organizer, and they secrete Chordin, Noggin, and Nodal proteins. These antagonize BMPs and dorsalize the ectoderm and mesoderm. The mechanisms for neural induction in the chick, however, probably differ from those of the frog, and the antagonism of the BMP signal does not appear to be sufficient for neural induction. In chick embryos, BMPs do not inhibit neural induction, nor does ectopic expression of chordin in the non-neural epiblast cause neural induction (see Streit and Stern 1999). Rather, fibroblast growth factors (FGFs) appear to generate neuronal phenotypes in the epiblast cells. Fibroblast growth factors are produced in Hensen's node and the primitive streak, and beads containing certain FGFs can induce trunk and hindbrain neuronal expression in the epiblast cells‡ (Alvarez et al. 1998; Storey et al. 1998). The factor or factors regulating anterior neuron production remain unknown, but recent evidence (Diaz and Schoenwolf 1990; Darnell et al. 1999) suggests that the anterior visceral endoderm is providing these signals.
Left-right axis formation
As we have seen, the vertebrate body is not symmetrical. Rather, it has distinct right and left sides. The heart and spleen, for instance, are generally on the left side of the body, while the liver is usually on the right side. The distinction between the right and left sides of vertebrates is regulated by two major proteins: the paracrine factor Nodal and the transcription factor Pitx2. However, the mechanism by which nodal gene expression is activated in the left side of the body differs among vertebrate classes. The ease with which chick embryos can be manipulated has allowed scientists to elucidate the pathways of left-right discrimination in birds more readily than in other vertebrates.
As the primitive streak reaches its maximum length, transcription of the sonic hedgehog gene ceases on the right side of the embryo, due to the expression on this side of activin and its receptor (Figure 11.17). Activin signaling blocks the expression of sonic hedgehog and activates the expression of fgf8. FGF8 prevents the transcription of the caronte gene. In the absence of Caronte, bone morphogenetic proteins (BMPs) are able to block the expression of nodal and lefty-2. This activates the snail gene (cSnR) that is characteristic of the right side of avian embryonic organs. On the left side of the body, the Lefty-1 protein blocks the expression of fgf8, while Sonic hedgehog activates caronte (Figure 11.18). Caronte is a paracrine factor that prevents BMPs from repressing the nodal and lefty-2 genes, and also inhibits BMPs from blocking the expression of lefty-1 on the ventral midline structures (Rodriguez-Esteban et al. 1999; Yokouchi et al. 1999). Nodal and Lefty-2 activate pitx2 and repress snail (cSnR). Lefty-1 in the ventral midline prevents the Caronte signal from passing to the right side of the embryo. As in Xenopus, pitx2 is crucial in directing the asymmetry of the embryonic structures. Experimentally induced expression of either nodal or pitx2 on the right side of the chick is able to reverse the asymmetry or cause randomization of the asymmetry on the right or left sides§ (Logan et al. 1998; Ryan et al. 1998).
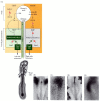
Figure 11.17
Pathway for left-right asymmetry in the chick embryo. (A) Left side: On the left side of Hensen's node, sonic hedgehog (shh) and lefty-1 are expressed. Lefty-1 blocks fgf8 expression, while Sonic hedgehog activates the expression of caronte (car). Meanwhile, (more...)
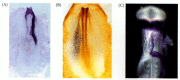
Figure 11.18
Transmission of the left-side signal from Hensen's node to the lateral plate mesoderm: the asymmetric expression of caronte, nodal, and pitx2 genes in the left side of the chick embryo. (A) Whole mount in situ hybridization of caronte mRNA. The view is (more...)
The course to either left- or right-sidedness can be interfered with at any point along the pathway. If activin is blocked by the experimental addition of follistatin to the embryo, the asymmetry of sonic hedgehog expression vanishes, and the heart has an equal chance of looping either way (Levin et al. 1995;1997). Conversely, when activin-soaked beads are placed to the left side of Hensen's node, the sonic hedgehog gene (usually expressed only on the left side) is repressed. This, in turn, suppresses the transcription of nodal. In this situation, too, the heart tube forms randomly, having an equal probability of being left or right. A similar condition can be produced by implanting cells secreting Sonic hedgehog into the right side of the node. In these cases, nodal is induced symmetrically in the lateral plate mesoderm, and the heart has a 50% chance of having a left-handed tube (Figure 11.19).
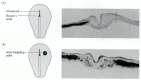
Figure 11.19
Ectopic expression of sonic hedgehog leads to symmetrical nodal expression and randomization of heart looping. (A) Wild-type expression of the chick nodal gene, showing expression on the left side (dark area). Nearly all hearts develop right-sided loops. (more...)
Footnotes
- *
Arendt and Nübler-Jung (1999) have argued that the region should be called the marginal belt to distinguish it from the marginal zone of amphibians. Here we will continue to use the earlier nomenclature.
- †
Earlier investigators (Waddington 1932) thought that the hypoblast induced the formation of the primitive streak and provided it with anterior-posterior polarity. However, Khaner (1995) rotated the epiblast with respect to the hypoblast at different stages of chick development and showed that the epiblast initiates primitive streak formation and maintains its polarity independently of the orientation of the hypoblast.
- ‡
A precondition for the BMP-chordin system to neuralize ectoderm is that dissociated (uninduced) presumptive ectoderm cells will become neural. In chick embryos, however, the dissociated epiblast cells develop into muscles (George-Weinstein et al. 1996).
- §
In humans, homozygous loss of PITX2 causes Rieger syndrome, a condition characterized by asymmetry anomalies. A similar condition is caused by knocking out this gene in mice (Fu et al. 1999; Lin et al. 1999).
- Early Development in Birds - Developmental BiologyEarly Development in Birds - Developmental Biology
Your browsing activity is empty.
Activity recording is turned off.
See more...