NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Colorectal Cancer: The Diagnosis and Management of Colorectal Cancer. Cardiff: National Collaborating Centre for Cancer (UK); 2011 Nov. (NICE Clinical Guidelines, No. 131.)
This publication is provided for historical reference only and the information may be out of date.
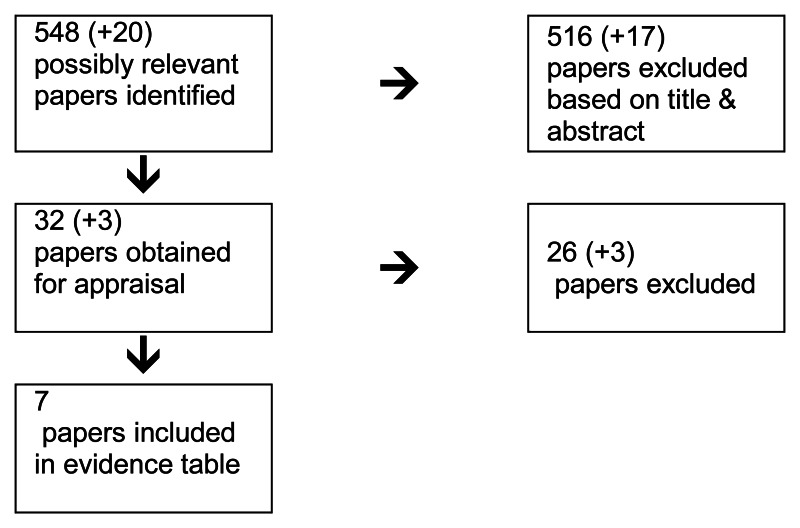
4.1. Management of Patients Presenting in Stage IV
4.1.1. In patients with colorectal cancer presenting with overt synchronous metastatic disease, what is the effectiveness of treating metastatic disease before, after or at the same time as treating the primary tumour?
Short Summary
There was very little evidence with which to address this topic and what was available consisted primarily of retrospective studies. There were 2 systematic reviews of retrospective studies (Hillingso et al, 2007 and Scheer et al, 2007), one randomised trial (Nordlinger et al, 2008) and 3 retrospective case series studies, two case matched (Moug et al, 2010 and Benoist et al, 2005) and one non-matched case series (Mentha et al, 2008).
Synchronous resection versus staged resection
A well conducted systematic review of which included 16 studies (Hillingso et al, 2007) and a more recent case series study (Moug et al, 2010) compared outcomes in patients undergoing synchronous resection and patients undergoing staged resection of primary tumour and liver metastases.
Length of Hospital Stay
A pooled estimate was possible from 8/11 studies reporting on length of hospital stay. The mean difference reported was −3.10 days (95% CI, −6.76–0.56) for patients undergoing synchronous resection indicating no significant difference between the two procedures in relation to the length of hospital stay. There was however significant statistical heterogeneity when pooling the studies (I2=92%; Χ2=82.85, p<0.00001) indicating that it may not be appropriate to conduct pooled analysis.
Morbidity
The results of the pooled analysis show that synchronous resection to be significantly better than staged resection in relation to post-operative morbidity (OR=0.68, 95% CI 0.49–0.81).
Mortality
On calculating the risk difference, there is no significant difference in the risk of mortality between the two groups (RD, 0.01, 95% CI −0.01–0.04).
5 year overall survival
There was no significant difference in 5 year survival for patients undergoing synchronous resection versus patients undergoing staged resection.
Preoperative Chemotherapy followed by surgery versus surgery alone
For chemotherapy followed by surgery versus immediate surgery, a single systematic review included only 7 studies (Scheer et al, 2007) deemed to be relevant and not all included studies were case matched meaning there was no comparison within the individual study. This, coupled with a non-matched case series study (Mentha et al, 2007) and a randomised trial investigating only progression free survival comprised the evidence base examining chemotherapy versus immediate surgery for patients with colorectal cancer and liver metastases.
Outcomes for which data were available included Length of hospital stay, tumour related complications in patients treated initially with chemotherapy, overall survival and progression free survival.
Length of Hospital Stay
One retrospective case series (Benoist et al, 2005) aimed at determining the best treatments strategy for patients with asymptomatic primary tumour and irresectable metastases reported mean hospital stay in the chemotherapy group was 11 days (SD=10 days, range=2–52 days) versus 22 days (SD=15 days, range=5–75 days) in the resection group (p=0.003).
Tumour Related Complications in patients receiving chemotherapy as initial treatment
The rate of intestinal obstruction reported in the included studies ranged from 5.6%–29%; the pooled proportion of patients developing bowel obstruction was 13.9% (95% CI 9.6% – 18.8%) (Scheer et al, 2007).
Haemorrhage due to primary tumour was reported in 4/7 studies included in the systematic review and ranged from 0%–3.7%; the pooled proportion of patients experiencing bleeding due to primary tumour was 3% (95% CI 0.95% – 6%) (Scheer et al, 2007).
Outcomes Related to Surgery
Postoperative mortality ranged from 0% to 4.6%; meta-analysis of the four studies showed a mortality of 2.7% (95% CI 1.1% – 5%) (Scheer et al, 2007).
Overall Survival
Scheer et al (2007) reported that for patients that underwent resection of the primary tumour median survival range from 14–23 months versus 8.2–22 months for patients treated with chemotherapy as first treatment.
Progression Free Survival
Hazard ratio for progression free survival was 0.79 (95.66% CI 0.62–1.02, p=0.058) which corresponds to a 7.3% increase in the rate of progression free survival at 3 years from 28.1% (21.3–35.3) to 35.4% (28.1–42.7) with chemotherapy and an increase in median progression free survival from 11.7 months to 18.7 months (Nordlinger et al, 2008).
Review Protocol
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
Patients presenting with operable primary colorectal tumour,with synchronous
| Surgery for primary Chemotherapy Surgery for metastases | Sequence of interventions synchronous versus staged surgery | Survival Quality of life Local Control Risks/Safety Complications |
Following a sy stematic search of relevant data sources (see appendix .1), the information specialist created a database of potentially relevant studies. All titles and abstracts were sifted by a single reviewer. Queries about inclusion were clarified by the GDG topic subgroup. The full studies were then obtained and reviewed and relevant studies were included in the final evidence review.
All update searches were sifted by a single reviewer and the list of potentially relevant studies was also checked for irrelevant studies by the GDG subgroup. Only studies which all subgroup members were in agreement were excluded. The remaining studies were obtained and reviewed with relevant studies included in the final evidence review.
In this topic, there is a need to consider if the synchronous metastatic disease is potentially operable (both at presentation and after chemotherapy).
In the event of inoperable metastatic disease, is there any role for surgery on primary or only in the case of obstruction?
Is there any evidence that lack of surgery results in worse prognosis (or increased morbidity)?
In the event of operable is there evidence of the optimum order of surgery (on primary or metastases first)?
There is a need to consider whether patients had pre-op chemo/radiotherapy.
| Reasons for Exclusions: | Quality of the included studies |
|---|---|
| Studies not relevant to PICO on full review | Systematic review of RCTs (n=0) |
| Studies included in a systematic review | Systematic review of combined study designs (n = 2) |
| Expert Review | Randomized controlled trial (n=1) |
| Quality of the study reporting meant that there was uncertainty surrounding the accuracy of the results contained within the study. | Prospective cross sectional study (n = 0) |
| Foreign Language with no translation | Case Series Studies (n = 3) |
| Guidelines (n=1) |
Volume of evidence
There was very little evidence with which to address this topic and what was available consisted primarily of retrospective studies. There were 2 systematic reviews of retrospective studies (Hillingso et al, 2007 and Scheer et al, 2007), one randomised trial (Nordlinger et al, 2008) and 3 retrospective case series studies, two case matched (Moug et al, 2010 and Benoist et al, 2005) and one non-matched case series (Mentha et al, 2008).
The body of evidence comparing synchronous resection to staged resection of primary tumour and operable liver metastases is greater than that comparing chemotherapy as intial treatment with surgery as initial treatment. A well conducted systematic review of which included 16 studies (Hillingso et al, 2007) and a more recent case series study (Moug et al, 2010) compared outcomes in patients undergoing synchronous resection and patients undergoing staged resection of primary tumour and liver metastases.
In contrast, for chemotherapy followed by surgery versus immediate surgery, despite appearing to comprise a similar volume of evidence, a single systematic review included only 7 studies (Scheer et al, 2007) deemed to be relevant and not all included studies were case matched meaning there was no comparison within the individual study. This, coupled with a non-matched case series study (Mentha et al, 2007) and a randomised trial investigating only progression free survival comprised the evidence base examining chemotherapy versus immediate surgery for patients with colorectal cancer and liver metastases.
Applicability
The available evidence is directly applicable to the population of interest, though in some cases there are studies included that were not case matched, for example studies evaluating chemotherapy as a first approach appear to more commonly be non-matched case series studies. Non comparator studies generally would not provide any evidence in favour of one or other treatment or course of treatments, though in this case, where the quality of evidence is generally very low and where a randomised controlled trial is not likely to be conducted it could be argued that these studies do add to the overall body of evidence and allow some indirect inferences to be made.
One set of evidence based guidelines (Bipat et al, 2007) made recommendations on the use if simultaneous surgery and also on the use of neoadjuvant chemotherapy for patients with liver metastases.
Consistency
There was a good degree of consistency in the results of the evidence reviewed, though the evidence base was quite limited, with some outcomes drawing on single studies for evidence; this appears to be particularly the case with studies examining chemotherapy as a first treatment option.
Evidence Statement
Synchronous resection versus staged resection
Length of hospital stay
The body of evidence for length of hospital stay for synchronous resection versus staged resection consists of a single systematic review of observational studies (Hillingso et al 2009) and 1 retrospective case matched study (Moug et al 2010) comparing length of hospital stay in patients undergoing a staged resection procedure with patients undergoing a simultaneous resection procedure (Table 4.1).
Table 4.1
Quality assessment of studies reporting length of hospital stay (days).
From the systematic review a pooled estimate was possible from 8/11 studies reporting on length of hospital stay. The mean difference reported was −3.10 days (95% CI, −6.76–0.56) for patients undergoing synchronous resection indicating no significant difference between the two procedures in relation to the length of hospital stay. There was however significant statistical heterogeneity when pooling the studies (I2=92%; Χ2=82.85, p<0.00001) indicating that it may not be appropriate to conduct pooled analysis (Figure 4.1).

Figure 4.1
Length of hospital stay (days).
The reason for the remaining three studies not being included in the pooled analysis appears to be that the individual studies did not report mean length of hospital stay, instead reporting median length of hospital stay. An additional study, not included in the systematic review also reported median length of hospital stay (Moug et al, 2010). From these 4 studies, the median length of hospital stay ranged from 7–18 days in the synchronous resection group and from 14–20 days in the staged resection group.
Morbidity
The body of evidence for morbidity for synchronous resection versus staged resection consists of a single systematic review of observational studies (Hillingso et al 2009) and 1 retrospective case matched study (Moug et al 2010). comparing post-operative morbidity in patients undergoing a staged resection procedure with patients undergoing a simultaneous resection procedure. Morbidity appears to relate to postoperative complications and immediate inhospital morbidity though neither the systematic review (Hillingso et al, 2009) nor the case series (Moug et al, 2010) clearly define what they mean by morbidity (Table 4.2).
Table 4.2
Quality assessment of studies reporting post-operative morbidity.
The results of the pooled analysis show that synchronous resection to be significantly better than staged resection in relation to post-operative morbidity (OR=0.68, 95% CI 0.49–0.81) (Figure 4.2).

Figure 4.2
Post-operative morbidity.
In the systematic review (Hillingso et al, 2009), no pooled analysis was undertaken as the authors felt that there was too much heterogeneity, however on pooled analysis the I2 was 0% and the Χ2 was insignificant (p=0.45) suggesting no significant statistical heterogeneity. It is possible that the clinical heterogeneity identified by the authors of the original systematic review was the reason that no pooled analysis was performed.
Mortality
The body of evidence for mortality for synchronous resection versus staged resection consists of a single systematic review of observational studies (Hillingso et al 2009) and 1 retrospective case matched study (Moug et al 2010) comparing mortality in patients undergoing a staged resection procedure with patients undergoing a simultaneous resection procedure. Mortality has not been clearly defined in either the systematic review (Hillingso et al, 2009) nor the case series (Moug et al, 2010) though as both studies also report on long term survival separately it is likely that mortality relates to deaths resulting from the surgical procedure and is limited to a certain time frame after surgery though this information is not provided (Table 4.3).
Table 4.3
Quality assessment of studies reporting post-operative mortality.
Of the 14 studies reporting mortality, only 6 studies recorded any events and the pooled analysis from these six studies indicates that mortality was significantly lower in the staged resection group, however this does not present the whole picture, as in many studies no mortality was recorded and as zero event data cannot be included, these results are not reflected in the pooled analysis (Figure 4.3) .
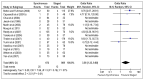
Figure 4.3
Mortality (Odds Ratio).
Calculating the risk difference instead of odds ratio allows the zero counts to be included in the analysis and indicates that there is no significant difference in the risk of mortality between the two groups (RD, 0.01, 95% CI −0.01–0.04). Risk difference is the comparson between the two groups in terms of the absolute difference (i.e. the risk in one group minus the risk in the other) and is calculated as risk in the experimental group minus risk in the control group. In this case, the risk difference indicates that there is a 1% increase in risk of mortality in the synchronous resection group, though this is not statistically significant (Figure 4.4).
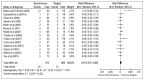
Figure 4.4
Mortality (Risk Difference).
5 year overall survival
The body of evidence for 5 year survival for synchronous resection versus staged resection consists of a single systematic review of observational studies (Hillingso et al 2009) and 1 retrospective case matched study (Moug et al 2010) comparing 5 year survival in patients undergoing a staged resection procedure with patients undergoing a simultaneous resection procedure (Table 4.4).
Table 4.4
5 year overall survival.
There was no significant difference in 5 year survival for patients undergoing synchronous resection versus patients undergoing staged resection (Figure 4.5).

Figure 4.5
5 year Overall Survival.
Preoperative Chemotherapy followed by surgery versus surgery alone
Length of hospital stay (days)
One retrospective case series (Benoist et al, 2005) aimed at determining the best treatments strategy for patients with asymptomatic primary tumour and irresectable metastases reported mean hospital stay in the chemotherapy group was 11 days (SD=10 days, range=2–52 days) versus 22 days (SD=15 days, range=5–75 days) in the resection group (p=0.003). The study states that the difference in mean hospital stay was related to hospital stay for primary tumour resection (Table 4.5).
Table 4.5
Quality assessment of studies reporting length of hospital stay (days).
Outcome Measures in Patients initially treated with Chemotherapy
Tumour related complications
The most important tumour related complication was intestinal obstruction, details of which were reported in 6/7 studies in the systematic review (Scheer et al, 2007); other complications reported included haemorrhage and peritonitis and fistula (Table 4.6).
Table 4.6
Quality Assessment for studies reporting tumour related complications.
The rate of intestinal obstruction reported in the included studies ranged from 5.6%–29%; the pooled proportion of patients developing bowel obstruction was 13.9% (95% CI 9.6% – 18.8%) (Scheer et al, 2007).
Haemorrhage due to primary tumour was reported in 4/7 studies included in the systematic review and ranged from 0%–3.7%; the pooled proportion of patients experiencing bleeding due to primary tumour was 3% (95% CI 0.95% – 6%) (Scheer et al, 2007).
A total of 2 studies included in the systematic review (Scheer et al, 2007) reported on peritonitis and fistula due to the unresected tumour; one study reported that 6.1% of patients developed peritonitis or fistulae. It appears that the second study reported that no patients developed fistulae or peritonitis thought this is somewhat unclear from the text.
Also from the systematic review (Scheer et al, 2007), a single study included reported that 37% of patients initially treated with chemotherapy experienced grade 3–4 toxicities.
Curative Resection
From one systematic review (Scheer et al, 2007), 3/7 studies reported on patients in whom curative resection of primary tumour and metastases was attempted as a result of downstaging by chemotherapy (Table 4.7).
Table 4.7
Quality Assessment for studies reporting curative resection rates.
One study reported that curative resection was successful in 6/13 patients with 3 undergoing one-stage resection and 3 undergoing staged resection. The success rate for resection was not reported in the second study and in the third study only as single patient underwent curative resection (Scheer et al, 2007).
From a single case series study (Mentha et al, 2008), 30 patients were treated with chemotherapy prior to liver surgery; primary tumour could be removed at the same time as the liver metastases in 7 patients (or at the same time as first liver resection for patients undergoing 2-step hepatectomies).
Outcome Measures or Resection of Primary Tumour as Initial Therapy
From 1 systematic review (Scheer et al, 2007), 5/7 studies described the results of primary tumour resection with postoperative morbidity described in 4 studies.
Postoperative morbidity ranged from 18.8% to 47% though these results included complications of variable severity; major complications included obstruction, haemorrhage and sepsis and pooled analysis resulted in 11.8% (95% CI 4.4% – 22%) of patients experiencing major complications after surgery.
A total of 3 studies reported minor complications with the most common complications being wound infection (5.5%–10.6%) and urinary tract infection (2.4%–6.1%); pooled analysis resulted in an overall 20.6% (95% CI 15.6%–26%) of patients who had minor complications following surgery.
Postoperative mortality ranged from 0% to 4.6%; meta-analysis of the four studies showed a mortality of 2.7% (95% CI 1.1% – 5%).
Overall Survival
From one systematic review (Scheer et al, 2008), median survival was addressed in 6/7 studies and for patients that underwent resection of the primary tumour median survival range from 14–23 months versus 8.2–22 months for patients treated with chemotherapy as first treatment (Table 4.8).
Table 4.8
Quality Assessment for studies reporting overall survival.
Two studies included in the review reported a statistically significant difference in survival between resected and unresected patients. One study described a median survival of 14 months for patients treated with resection versus 8.2 months in the group initially treated by chemotherapy though multivariate analysis revealed that performance status and a presence of peritoneal or omental metastases were significant factors affecting survival and that resection status of the primary tumour was not significantly associated with survival.
The second reported a median survival of 16 months for patients initially treated with resection versus 9 months for patients treated with chemotherapy, though again on univariate analysis, resection status was not significantly associated with survival while number of distant sites involved, metastatic disease confined to the liver and volume of hepatic replacement by the tumour were significant factors (Scheer et al, 2007).
From a single case series (Mentha et al, 2008) examining the effect of chemotherapy followed by liver surgery, the overall actuarial survival rates were 91% at 1 year, 82% at 2 years, 54% at 3 years, 41% at 4 years and 30% at 5 years from start of treatment in 35 patients (intent to treat).
Median survival was 44 months.
Progression free survival
One randomised trial (Nordlinger et al, 2008) compared perioperative chemotherapy and surgery versus surgery alone. Median follow up was 3.9 years and there were 254 recorded events of progression free survival in all patients (intent to treat) (Table 4.9).
Table 4.9
Quality Assessment for studies reporting progression free survival.
Hazard ratio for progression free survival was 0.79 (95.66% CI 0.62–1.02, p=0.058) which corresponds to a 7.3% increase in the rate of progression free survival at 3 years from 28.1% (21.3–35.3) to 35.4% (28.1–42.7) with chemotherapy and an increase in median progression free survival from 11.7 months to 18.7 months.
When applying the usual definition of progression free survival (those not operated or not resected were not penalised as events until further disease progression or death), the hazards ratio was 0.76 (0.59–0.98, p=0.023) corresponding to a 7.3% increase in the rate of progression free survival at 3 years from 28.6% (21.7–35.8) to 37.9% (30.5–45.3) with chemotherapy and adjustment of primary analysis for stratification factors did not change the results.
Conclusions
Hillingso et al, 2009 conclude that a randomised trial would be the best way to provide strong evidence on which to base recommendations, however their sample size calculations indicate that more than 1,000 patients would need to be treated in each group in order that a clinically relevant difference in post-operative morbidity be observed. It was felt that to achieve this, a large multi-centre trial would be required and it presented a possible ethical dilemma in that persuading patients, particularly those with the least disseminated disease to the staged arm would be difficult. It was therefore concluded that such a trial would never be performed.
On the basis of weak evidence (resulting from bias and apparent heterogeneity) Hillingso et al, 2010 recommended that combined resection be undertaken in selected patients provided surgeons specialised in colorectal and hepatobiliary surgery are available as the data suggest that this approach leads to shorter hospital stay and less post operative morbidity but there was no difference in 5 year survival for either procedure.
One set of evidence based guideline for Dutch patients (Bipat et al, 2007) recommended that the use of simultaneous resection of colorectal cancer and liver metastases should be avoided due to a high complication rate despite the fact that survival after simultaneous resection was comparable to that for staged resection. The recommendation was based on what the guideline classed as evidence level 3 (generally randomised trials of low quality or other non randomised comparative studies such as cohort and case control studies or poor quality descriptive studies).
The guideline also made recommendations on the use of neoadjuvant chemotherapy. Due to controversial data, the guideline recommends that neoadjuvant chemotherapy be used only in clinical research populations; again this was based on level 3 evidence.
Based on leverl 2 evidence, the guideline recommended that adjuvant chemotherapy should not be used routinely after curative surgery as it’s role is unclear. Level 2 evidence was described as being either low quality randomised trials or other non randomised comparative studies such as cohort and case control studies or a systematic review of these types of studies).
References
- Benoist S, Pautrat K, MItry E, Rougier P, Penna C, Nordlinger B. Treatment strategy for patients with colorectal cancer and synchronous irresectable liver metastases. British Journal of Surgery. 2005;92:1155–1160. [PubMed: 16035135]
- Bipat S, van Leeuwen MS, IJzermans JN, et al. Evidence based guideline on management of colorectal liver metastases in the Netherlands (Review). Netherlands Journal of Medicine. 2007;65(1):5–14. [PubMed: 17293634]
- Hillingso J, Wille-Jorgensen P. Staged or simultaneous resection of synchronous liver metastases from colorectal cancer – a systematic review. Colorectal Disease. 2009;11(1):3–10. [PubMed: 18637099]
- Mentha G, Roth A, Terraz S, et al. ‘Liver First’ Approach in the treatment of colorectal cancer with synchronous liver metastases. Digestive Surgery. 2008;25:430–435. [PubMed: 19212115]
- Moug SJ, Smith D, Leen E, Roxburgh C, Horgan PG. Evidence for a synchronous operative approach in the treatment of colorectal cancer with hepatic metastases: A case matched study. European Journal of Surgical Oncology. 2010;36(4):365–370. [PubMed: 20034757]
- Nordlinger B, Sorbye H, Glimlius B, Poston G, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. 2008. [PMC free article: PMC2277487] [PubMed: 18358928]
- Scheer MG, Sloots CE, van der Wilt GJ, Ruers TJM. Management of patients with asymptomatic colorectal cancer and synchronous irresectable metastases. Annals of Oncology. 2008;19(11):1829–1835. [PubMed: 18662955]
Evidence Tables
Download PDF (197K)
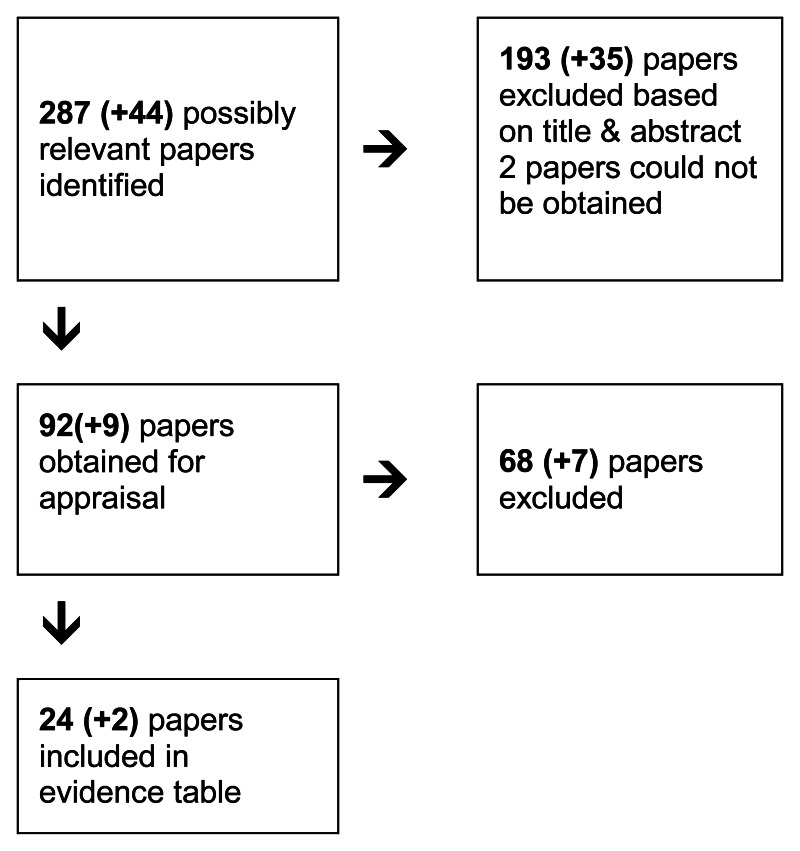
4.2. Imaging Hepatic Metastases
4.2.1. In a patient with colorectal cancer metastasised to the liver which imaging modality(s) most accurately determine the number and extent of metastases pre-operatively?
Short Summary
There were two meta-analyses available comparing PET to MRI and CT (Bipat et al 2005) and PET to CT (Wiering et al 2005). In both studies per patient analysis showed that PET has higher sensitivity than MRI and CT but this was not the case on a per lesion basis with sensitivities for al modalities comparable. Gadolinium contrast-enhanced MRI and SPIO-contrast enhanced MRI were better than non-enhanced MRI and CT and this was more manifest in the subgroup analysis that looked at specific sizes of lesions which showed that MRI had a better sensitivity in detecting the micrometastases of <1cm.
Since 2005 a number of studies have been carried out continuing comparing MRI and CT. In the recent 5 years PET has been fused with CT and there are now studies looking at the performance of PET/CT and comparing it to MRI, PET alone, and CT alone.
It appears that in a per-patient analysis PET/CT has consistently higher sensitivity in all the studies compared to MRI and CT with pooled analysis showing a summary sensitivity and accuracy for PET/CT of 94% for both compared with MRI (80% and 91% respectively) and CT (87% for both).
On per lesion analysis MRI appeared to be the modality showing higher sensitivities across individual studiescompared to CT and Pooled data shows comparable results with MRI having a combined sensitivity of 88% and accuracy of 87%, CT a sensitivity of 74% and accuracy of 78% and PET/CT a sensitivity of 79% and accuracy of 97%.
A number of studies carried out subgroup analyses looking at how the modalities diagnose lesions of particular sizes. Bartolozzi et al (2004), Bhattarajha et al (2004) and Wiering et al (2007) all found MRI has better sensitivity at picking up the smaller lesions <1cm compared to PET/CT and CT. The majority of lesions missed by PET/CT were micrometastases of <1cm.
Chua et al (2007) and Liu et al (2007) reported change in management as an outcome however both studies include the diagnosis of extrahepatic in their analysis. It was not possible to extract data for this relating to hepatic metastases only.
Updated Evidence
A systematic review and meta-analysis of data comparing the diagnostic accuracy of different imaging modalities for the diagnosis of colorectal liver metastases was available (Floriani et al, 2010). Pairwise comparisons suggested that MRI performed significantly better than CT for the detection of metastatic lesions (sensitivity OR: 0.66 (95%CI: 0.55–0.80) P<0.0001) but the data were highly heterogeneous. The superiority of MRI differed between the various CT techniques in per lesion analysis which probably accounts for the observed heterogeneity. MRI was also better than CT in a per patient analysis (sensitivity OR: 0.69 (95%CI: 0.47–0.99) P=0.05) which is a more reliable indicator. FDG-PET and ultrasound performed similarly to CT although significant between studies heterogeneity may well have confounded these results.
From a prospective case series of 34 patients (Mainenti et al, 2010) comparing MRI, PET/CT and CT, ROC analysis showed no significant difference between Gd and SPIO enhanced MRI and showed that both forms of MRI performed significantly better than all other modalities (p<0.05).
For lesions ≥10mm, the performance of PET/CT was significantly better than contrast enhanced CT (p<0.05).
No significant difference was observed between the modalities when considering the groups of lesion <10mm.
Review Protocol
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with colorectal cancer metastasised to the liver |
|
|
Following a systematic search of relevant data sources (see appendix .1), the information specialist created a database of potentially relevant studies. All titles and abstracts were sifted by a single reviewer. Queries about inclusion were clarified by the GDG topic subgroup. The full studies were then obtained and reviewed and relevant studies were included in the final evidence review.
All update searches were sifted by a single reviewer and the list of potentially relevant studies was also checked for irrelevant studies by the GDG subgroup. Only studies which all subgroup members were in agreement were excluded. The remaining studies were obtained and reviewed with relevant studies included in the final evidence review.
It was felt by GDG members that high level (randomised trials) should be considered in the first instance if not available then look to lower level (case series studies).
A number of date limits for searches were provided by GDG members in order to more efficiently target searches:
PET-CT – 2000 (this is when it came into use)
Contrast enhanced CT – 1995 (the advent of helical CT)
Contrast enhanced MRI – 1997 (marked an improvement in technology and contrast agents)
Other issues considered by the GDG included whether to look at determining operability form the imaging of the metastatic disease and whether consideration needed to be given to the incidence of metastatic disease at other sites, what would be the best method of detecting this e.g. with a PET scan or whether there are certain factors which imply a worse prognosis therefore needing additional scanning?
It was felt that this topic potentially posed two quite different questions: which investigation(s) offer the most accurate depiction or the anatomical relationships of known metastases for the surgeon or interventional radiologist to debate what is technically feasible - and secondly, which investigation(s) provide the most accurate assessment of the size/number of metastases?
These sound like similar questions, but there is an important distinction, with the answer to the first question likely to be determined by the inherent spatial resolution of the imaging techniques (ability to display small abnormalities) and the tissue contrast between metastases and normal liver. The two factors are interdependent - the human eye can only detect very small objects if they are presented with high contrast, while even quite large objects can escape detection if there is very little contrast with the background.
As a rule of thumb, both CT and US tend to suffer from a lack of liver lesion contrast but have high spatial resolution (1–2mm), while MR has much higher contrast but poorer (theoretical) spatial resolution (perhaps 5mm). The topic is interested in evidence which is right at the leading edge of imaging capabilities, therefore the searches can be restricted to spiral/helical CT; MR studies after about 1995 and US studies no earlier than 1990. US scanning augmented by microbubble contrast agents may also figure, though it was felt that the evidence will be thin and it’s not a widely used technique.
There was some brief discussion on intraoperative US at the last meeting (this is where the surgeon or a radiologist applies a high-frequency US probe directly to the liver during surgery to detect lesions which may not have been detected preoperatively and give details of the precise relationships of tumours to the major hepatic vessels). It was suggested that this practice is essentially obsolete, and if the thrust is to give guidance on preoperative assessment/patient selection, then clearly intraoperative US would be inappropriate to consider.
| Reasons for excluding studies: | Quality of the included studies |
|---|---|
| Expert Reviews | Systematic review of RCTs (n =0) |
| Foreign Language with no translation | Systematic review of combined study designs (n =3) |
| Guidelines not providing evidence base | Randomized controlled trial (n =2) |
| 2×2 tables not presented | Prospective cross sectional study (n =16) |
| Data unable to be extracted | Retrospective cohort study (n=5) |
| Studies not relevant to PICO | Case Series Studies (n = 0) |
| Studies published prior to 2005 | |
| Duplicate data |
Volume of evidence
There are 2 systematic reviews of cohort studies (Bipat 2005, Wiering 2005); both studies have been well designed and conducted according to the NICE quality checklist. Bipat et al (2005) applied the QUADAS checklist to assess the quality of included studies. Wiering et al (2005) applied a weighted quality assessment checklist which had been devised by the authors. Both reviews comment on the poor quality of the reporting of diagnostic accuracy studies as well as the flaws in study design.
There are 2 randomised controlled trials in the literature (Kim 2006 and Ruers 2009) both of good quality according to the NICE quality checklist.
There are 20 cohort studies available of which15 were prospective and 5 were retrospective with population ranging from 15 patients to 467.
For the purpose of this review the QUADAS checklist was used to extract the relevant study design characteristics and to perform quality assessment of the studies included. (as appears in the NICE guidelines manual p196–266).
The main QUADAS points where many studies were found to be sub optimal are as follows:
- 64% of the included studies did not report the period between the time the reference test was performed (histology or follow up imaging for those that did not have surgery) and the index test was carried out (14/22 studies scored ‘unclear’ to question 4 of the QUADAS). These studies may have incorporated disease progression bias.
- 50% of included studies did not give a description of the execution of the reference standard (11/22 studies scored ‘no’ to question 9 of the QUADAS). There may therefore be heterogeneity that has not been accounted for.
- 77% of included studies did not report on whether the interpretation of the reference standard results was carried out without knowing the result of the index test (blinding). An additional 14% of studies reported that they did not have blinding. (17/22 studies scored ‘unclear’ and 3/22 studies scored ‘no’ in response to question 11 of the QUADAS). There may be review bias as a result.
- In 100 % of the studies there were more than 1 reference tests. The participants received one of two reference tests depending on the result of their index test (22/22 studies scored no in response to question 6 of QUADAS). Patients that proceeded with hepatic resection have the lesion verified by histology. Patients that have a lesion thought to be benign do not go to surgery but are followed up with imaging 3 or 6 months later. The reference standards differ in their definition of liver metastasis. Histopathology has a precise definition and is the gold standard compared to repeat imaging, which bases definitions on the change in size of a lesion. This may lead to differential verification bias.
It is also important to note that the analysis of the data in the studies included both ‘per patient’ and ‘per lesion analyses’. Not all studies reported on both. Per lesion sensitivies are more impressive for MRI. Per lesion analysis on its own is potentially biased. Lesions in each patient are a cluster of observations. Each lesion is not always an independent observation from another lesion if a patient has multiple lesions. This introduces bias to the results.
Applicability
All included studies were directly applicable to the population of interest having looked at data relating to patients both females and male, with a confirmed diagnosis of colorectal cancer only, and either confirmed or lesions suspicious of liver metastases.
Studies that reported on diagnostic accuracy of the modalities of interest but did not distinguish between liver metastases from colorectal cancer and other cancers were excluded.
The age of the population, their co-morbidities, the referral patterns, the diagnostic setting are also similar between the studies and the population of interest.
None of the studies have excluded patients that have had prior chemotherapy but some have performed subgroup analysis.
Consistency
Studies which include patients who have received chemotherapy without performing subgroup analysis may introduce clinical heterogeneity and bias as lesions that are responding to chemotherapy treatment do not appear as well defined on PET scanning. The metabolism of the lesion is changed and this results in lesser or no appearance on the PET scan (Strauss 2007). This could lead to higher number of false negatives for PET-CT whereas chemotherapy does not affect CT or MRI. Some studies including patients receiving chemotherapy do subgroup analysis to investigate whether there is any effect, though this is not the case for all included studies.
The lesions patients present with are very heterogeneous; some are cystic others are solid, some are very small (micrometastases <1cm) and others are larger. Some studies report on accuracy of the modalities separately for two or three groups of different sized lesions.
The imaging modalities are heterogeneous in their technologies both in principle of how they make the diagnosis and in how they are developed over the years. Slice thickness, amount of contrast used, strength of magnetic field applied are some of the characteristics that have changes over the years. The two meta-analyses presented in the evidence have performed subgroup analyses looking at these features separately (Wiering et al, 2005 and Bipat et al, 2005).
The diagnosis is based on different radiologists across all the studies reading the images. They have different levels of experience and different abilities.
Other factors
Selection bias
For this review studies that were published prior to 2005 have been excluded as two high quality meta-analyses that summarise the data prior to 2005 were identified in the literature. This may introduce a selection bias to the review. However heterogeneity may be reduced looking at studies that compare modalities of more recent technological advancement.
Evidence Statement
Per patient analysis
12 studies reported CT data per patient, 9 studies reported MRI data per patient, 7 studies reported PET/CT data per patient (Figure 4.7).

Figure 4.7
PER PATIENT SUMMARY ANALYSIS.
CT data
The sensitivity of CT ranged from 47% to 100%. The PPV for CT ranged from 86%–100%. Specificity for CT ranged from 0 to 100%. The accuracy for CT ranged from 50% to 98%.
Though there has been no weighting to the following summary values the overall sensitivity and PPV for CT from the 12 studies as calculated from a summary 2×2 table is
- Total TP=770
- Total FP=41
- Total FN=112
- Total TN=266
- Total = 1189
- SUMMARY SENSITIVITY FOR CT = 770/882 = 87%
- SUMMARY PPV FOR CT = 770/770+41 = 770/811 = 95%
- SUMMARY ACCURACY = 770 + 266/1189 = 87%
MRI data
The sensitivity of MRI ranged from 50% to 100%. Specificity ranged from 0% to 100%. In a number of studies specificity estimates are not possible as there are no benign lesions identified at all in the population. PPV ranged from 91% to 100%. The accuracy for MRI ranged from 48% to 100%.
Though there has been no weighting to the following summary values the overall sensitivity and PPV for MRI from the 9 studies as calculated from a summary 2×2 table is
- Total TP=336
- Total FP=13
- Total FN=86
- Total TN=142
- Total = 577
- SUMMARY SENSITIVITY FOR MRI = 336/336 + 86 = 80%
- SUMMARY PPV FOR MRI = 336/336 +13 = 96%
- SUMMARY ACCURACY FOR MRI = 336+142/577 = 91%
PET/CT data
The sensitivity for PET/CT ranged from 91% to 100%. Specificity ranged from 60% to 100%. In a number of studies specificity estimates are not possible as there are no benign lesions identified at all in the population. The PPV tanged from 93% to 100%. Accuracy ranged from 91%–100%
Though there has been no weighting to the following summary values the overall sensitivity and PPV for PET/CT from the 6 studies as calculated from a summary 2×2 table is
- Total TP=273
- Total FP=8
- Total FN=19
- Total TN=153
- Total = 453
- SUMMARY SENSITIVITY FOR PET/CT = 273/273+19 = 94%
- SUMMARY PPV FOR PET/CT = 273/273+19 = 94%
- SUMMARY ACCURACY FOR PET/CT = 273+153/453 = 94%
Per lesion analysis
7 studies reported CT data per lesion, 12 studies reported MRI data per lesion, 6 studies reported PET/CT data per lesion (Figure 4.6).

Figure 4.6
PER LESION SUMMARY ANALYSIS.
CT data
The sensitivity of CT ranged from 67% to 97%. The PPV for CT ranged from 63%–100%. Specificity for CT ranged from 0 to 67%. In a number of studies specificity estimates are not possible as there are no benign lesions identified at all in the population. This is a possibility in a population that is so highly selective for suspicion of malignancy. The accuracy for CT ranged from 64% to 84%.
Though there has been no weighting to the following summary values the overall sensitivity and PPV for CT from the 7 studies as calculated from a summary 2×2 table is
- Total TP=704
- Total FP=78
- Total FN=252
- Total TN=114
- Total = 1048
- SUMMARY SENSITIVITY FOR CT = 704/956 = 74%
- SUMMARY PPV FOR CT = 704/792 = 90%
- SUMMARY ACCURACY FOR CT = 704+114/1048 = 78%
MRI data
The sensitivity of MRI ranged from 81% to 100%. Specificity ranged from 59% to 100%. In a number of studies specificity estimates are not possible as there are no benign lesions identified at all in the population. PPV ranged from 81% to 100%. The accuracy for MRI ranged from 71% to 100%.
Though there has been no weighting to the following summary values the overall sensitivity and PPV for MRI from the 12 studies as calculated from a summary 2×2 table is
- Total TP=1139
- Total FP=45
- Total FN=158
- Total TN=229
- Total = 1571
- SUMMARY SENSITIVITY FOR MRI = 1139/158 = 88%
- SUMMARY PPV FOR MRI = 704/792 = 96%
- SUMMARY ACCURACY FOR MRI = 1139+229/1571 = 87%
PET/CT data
The sensitivity for PET/CT ranged from 61% to 100%. Specificity ranged from 60% to 100%. In a number of studies specificity estimates are not possible as there are no benign lesions identified at all in the population. The PPV tanged from 94% to 100%. Accuracy ranged from 61%–100%
Though there has been no weighting to the following summary values the overall sensitivity and PPV for PET/CT from the 6 studies as calculated from a summary 2×2 table is
- Total TP=410
- Total FP=5
- Total FN=112
- Total TN=96
- Total = 523
- SUMMARY SENSITIVITY FOR PET/CT = 410/522 = 79%
- SUMMARY PPV FOR PET/CT = 410/415 = 99%
- SUMMARY ACCURACY FOR PET/CT = 410+96/523 = 97%
Updated Evidence
Floriani et al (2010) presented a systematic review and meta-analysis of data on the diagnostic accuracy of different imaging modalities for the diagnosis of colorectal liver metastases. The number of patients exceeded 1,774. The authors noted that high likelihood ratios indicated that all imaging modalities performed well. Pairwise comparisons suggested that MRI performed significantly better than CT for the detection of metastatic lesions (sensitivity OR: 0.66 (95%CI: 0.55–0.80) P<0.0001) but the data were highly heterogeneous. The superiority of MRI differed between the various CT techniques in per lesion analysis which probably accounts for the observed heterogeneity. MRI was also better than CT in a per patient analysis (sensitivity OR: 0.69 (95%CI: 0.47–0.99) P=0.05) which is a more reliable indicator. FDG-PET and ultrasound performed similarly to CT although significant between studies heterogeneity may well have confounded these results.
Mainenti et al (2010) conducted a prospective case series study which compared contrast enhanced ultrasound (CEUS), mulidetector CT (MDCT), 1.5T MRI with godlinium chelate and superparamagnetic iron oxide (SPIO) contrast agents and PET-CT in 34 patients.
ROC analysis showed no significant difference between Gd and SPIO enhanced MRI and showed that both forms of MRI performed significantly better than all other modalities (p<0.05).
For lesions ≥10mm, the performance of PET/CT was significantly better than contrast enhanced CT (p<0.05).
No significant difference was observed between the modalities when considering the groups of lesion <10mm.
On a per patient basis, no significant difference was observed between the modalities.
On a per patients basis, PET/CT correctly identified 100% of patients with liver metastasis as compared with 83% for all other modalities (5/6 patients).
Gd and SPIO enhanced MRI showed higher sensitivities than other modalities; both identified 81% of metastatic lesions (13/16) including all lesions ≥10mm and 5/8 lesions <10mm.
References
- Akiyoshi T, Oya M, Fujimoto Y, Kuroyanagi H, Ueno M, Yamaguchi T, Koyama M, Tanaka H, Matsueda K, Muto T. Comparison of preoperative whole-body positron emission tomography with MDCT in patients with primary colorectal cancer. Colorectal Disease. 2009;11:464–469. [PubMed: 18637927]
- Arulampalam THA. FDG-PET for the pre-operative evaluation of colorectal liver metastases. Eur J Surg Oncol. 2004;30:286–291. [PubMed: 15028310]
- Ashraf K. Colorectal carcinoma, preoperative evaluation by spiral computed tomography. Journal of the Pakistan Medical Association. 2006;56:149–153. [PubMed: 16711333]
- Bartolozzi C, Donati F, Cioni D, Procacci C, Morana G, Chiesa A, Grazioli L, Cittadini G, Cittadini G, Giovagnoni A, Gandini G, Maass J, Lencioni R. Detection of colorectal liver metastases: a prospective multicenter trial comparing unenhanced MRI, MnDPDP-enhanced MRI, and spiral CT. Eur Radiol. 2004;14:14–20. [PubMed: 14730384]
- Bhattaharjya SB. Prospective study of contrast-enhanced computed tomography, computed tomography during arterioportography, and magnetic resonance imaging for staging colorectal liver metastases for liver resection. Br J Surg. 2004;91:1361–1369. [PubMed: 15376205]
- Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, Stoker J. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis. Meta-analysis (DARE structured abstract). Radiology. 2005;237:123–131. [PubMed: 16100087]
- Cantwell CP, Setty BN, Holalkere N, Sahani DV, Fischman AJ, Blake MA. Liver Lesion Detection and Characterization in Patients With Colorectal Cancer: A Comparison of Low Radiation Dose Non-enhanced PET/CT, Contrast-enhanced PET/CT, and Liver MRI. J Comput Assist Tomogr. 2008;32:738–744. [PubMed: 18830103]
- Chua SC, Groves AM, Kayani I, Menezes L, Gacinovic S, Du Y, Bomanji JB, Ell PJ. The impact of F-18-FDG PET/CT in patients with liver metastases. European Journal of Nuclear Medicine and Molecular Imaging. 2007;34:1906–1914. [PubMed: 17713766]
- Coenegrachts K, De GF, ter BL, Walgraeve N, Bipat S, Stoker J, Rigauts H. Comparison of MRI (including SS SE-EPI and SPIO-enhanced MRI) and FDG-PET/CT for the detection of colorectal liver metastases. Eur Radiol. 2009;19:370–379. [PubMed: 18795299]
- Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, Giovagnoni A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J. Magn. Res. Imaging. 2010;31(1):19–31. [PubMed: 20027569]
- Kim HJ, Kim KW, Byun JH, Won HJ, Shin YM, Kim PN, Lee MS, Lee MG. Comparison of mangafodipir trisodium- and ferucarbotran-enhanced MRI for detection and characterization of hepatic metastases in colorectal cancer patients. AJR. American journal of roentgenology. 2006;186:1059–1066. [PubMed: 16554579]
- Koh DM, Brown G, Riddell AM, Scurr E, Collins DJ, Allen SD, Chau I, Cunningham D, Desouza NM, Leach MO, Husband JE. Detection of colorectal hepatic metastases using MnDPDP MR imaging and diffusion-weighted imaging (DWI) alone and in combination. Eur Radiol. 2008;18:903–910. [PubMed: 18193234]
- Kong G, Jackson C, Koh DM, Lewington V, Sharma B, Brown G, Cunningham D, Cook GJR. The use of F-18-FDG PET/CT in colorectal liver metastases-comparison with CT and liver MRI. European Journal of Nuclear Medicine and Molecular Imaging. 2008;35:1323–1329. [PubMed: 18347794]
- Liu YN, Huang MX, An Q, Wei JM. The Impact of PET/CT on Therapeutic Strategy of Patients with Colorectal Cancer Metastasis. Hepatogastroenterology. 2009;56:968–970. [PubMed: 19760922]
- Mainenti P, Mancini M, Mainolfi, et al. Detection of colorectal liver metastases: prospective comparison of contrast enhanced US, multidetector CT, PET/CT and 1.5 Tesla MR with extracellular and reticulo-endothelial cell specific contrast agents. Abdominal Imaging. 2010;35(5):511–521. [PubMed: 19562412]
- Nanashima A, Taheshita H, Sawai T, Sumida Y, Abo T, Tanaka K, Nonaka T, Sengyoku H, Hidaka S, Yasutake T, Nagayasu T. Preoperative Assessment of Liver Metastasis Originating from Colorectal Carcinoma: Is Super Paramagnetic Iron Oxide Particles-Magnetic Resonance Imaging (SPIO-MRI) Useful for Screening? Hepatogastroenterology. 2008;55:1750–1753. [PubMed: 19102384]
- Orlacchio A, Schillaci O, Fusco N, Broccoli P, Maurici M, Yamgoue M, Danieli R, D’Urso S, Simonetti G. Role of PET/CT in the detection of liver metastases from colorectal cancer. Radiol. Med.(Torino). 2009;114:571–585. [PubMed: 19444590]
- Rappeport ED, Loft A, Berthelsen AK, von der Recke P, Larsen PN, Mogensen AM, Wettergren A, Rasmussen A, Hillingsoe J, Kirkegaard P, Thomsen C. Contrast-enhanced FDG-PET/CT vs. SPIO-enhanced MRI vs. FDG-PET vs. CT in patients with liver metastases from colorectal cancer: A prospective study with intraoperative confirmation. Acta Radiol. 2007;48:369–378. [PubMed: 17453514]
- Regge D, Campanella D, Anselmetti GC, Cirillo S, Gallo TM, Muratore A, Capussotti L, Galatola G, Floriani I, Aglietta M. Diagnostic accuracy of portal-phase CT and MRI with mangafodipir trisodium in detecting liver metastases from colorectal carcinoma. Clin Radiol. 2006;61:338–347. [PubMed: 16546464]
- Ruers TJM. Improved selection of patients for hepatic surgery of colorectal liver metastases with 18F-FDG PET: A randomized study. J Nucl Med. 2009;50:1036–1041. [PubMed: 19525451]
- Schwartz L, Brody L, Brown K, Covey A, Tuorto S, Mazumdar M, Riedel E, Jarnagin W, Getrajdman G, Fong Y. Prospective, blinded comparison of helical CT and CT arterial portography in the assessment of hepatic metastasis from colorectal carcinoma. World J Surg. 2006;30:1892–1901. [PMC free article: PMC1578594] [PubMed: 16855806]
- Selzner MK, Hany TF, Wildbrett P, McCormack L, Kadry Z, Clavien PA. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg. 2004;240:1027–1036. [PMC free article: PMC1356518] [PubMed: 15570208]
- Strauss LG, mitrakopoulou-Strauss A. Can PET-CT with FDG replace contrast enhanced CT for imaging of liver metastases? European Journal of Nuclear Medicine and Molecular Imaging. 2007;34:1902–1905. [PubMed: 17938920]
- Truant S, Huglo D, Hebbar M, Ernst O, Steinling M, Pruvot FR. Prospective evaluation of the impact of 18Ffluoro 2 deoxy D glucose positron emission tomography of resectable colorectal liver metastases. The British journal of surgery. 2005;92:362–369. [PubMed: 15672427]
- Vidiri A, Carpanese L, D’Annibale M, Caterino M, Cosimelli M, Zeuli M, David V, Crecco M. Evaluation of hepatic metastases from colorectal carcinoma with MR-superparamagnetic iron oxide. Journal of Experimental & Clinical Cancer Research. 2004;23:53–60. [PubMed: 15149151]
- Wiering B, Ruers TJM, Krabbe PFM, Dekker HM, Oyen WJG. Comparison of multiphase CT, FDG-PET and intra-operative ultrasound in patients with colorectal liver metastases selected for surgery. Ann Surg Oncol. 2007;14:818–826. [PubMed: 17136470]
Evidence Tables
Download PDF (877K)
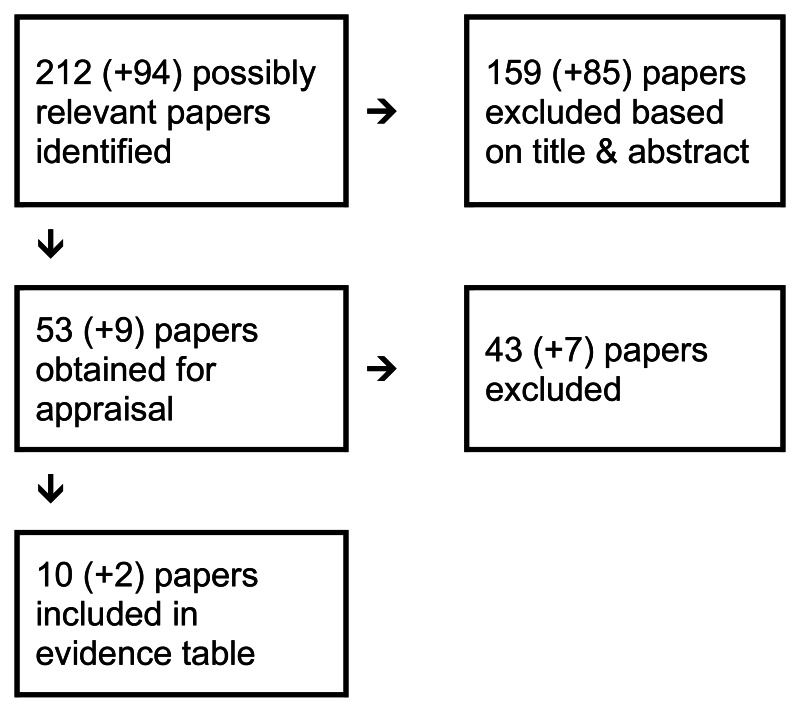
4.3. Imaging Extra-Hepatic Metastases
4.3.1. In a patient with colorectal cancer and extrahepatic metastases (e.g. lung, brain, peritoneum), which imaging modality most accurately determines the extent of metastases?
Short Summary
The evidence base for this question comprises one systematic review of observational studies (Wiering et al. 2005) and nine retrospective case series (Desai et al., (2003; Imdahl et al., 2000; Potter et al., 2009; Schmidt et al., 2009; Selzner et al., 2004; Squillaci et al., 2008; Tanaka et al., 2002; Valk et al., 1999, and Votrubova et al., 2006) None of the studies were designed to directly compare the effectiveness of the imaging techniques in detecting extra-hepatic metastases.
FDG-PET versus CT
Wiering et al. (2005) found that FDG-PET had a higher sensitivity and specificity (91.5% and 95.4%) than CT scan (60.9% and 91.1%) in detecting extra-hepatic metastases. Using only the highest weighted studies from the meta-analysis, the pooled sensitivity and specificity for FDG-PET were 91.2% and 98.4% respectively and for CT the sensitivity and specificity were 55.3% and 95.6%. Tanaka et al. (2002) reported that FDG-PET also had higher accuracy and sensitivity (78% and 88%) than CT (44% and 38%) in diagnosing peritoneal metastases, but the study numbers were very low (n=23). Valk et al. (1999) reported sensitivity and specificity for detecting extrahepatic metastases of 92% and 99% for FDG-PET compared with 61% and 96% for CT. The authors also added that FDG-PET had a significantly higher specificity than CT in detecting lung metastases.
Potter et al. (2009) found no significant difference in diagnostic accuracy between FDG-PET and CT/MRI but the study provided some information with regard to the role of the reader, since a significant difference in accuracy and sensitivity was found between the three individuals who interpreted the CT/MRI scans.
PET/CT versus MRI
Schmidt et al. (2009) found that PET/CT had higher sensitivity than whole body MRI in the detection of distant metastasis (80% versus 78%) but there was no difference in specificity (95%) and accuracy was similar (PET/CT: 87%, WB-MRI: 86%). Squillaci et al. (2008) did not report sensitivity or specificity but suggested that both modalities were equivalent in detecting extrahepatic metastases. Both studies concluded that PET/CT detected more lung metastases than WB-MRI.
PET/CT versus CT
Selzner et al. 2004 found no difference in the ability of PET/CT or ceCT to detect the presence of extrahepatic metastases but PET/CT was more sensitive than CT in the detection of lung metastases (100% versus 78%). PET/CT was also more sensitive than CT for portal and para-aortic lymph node metastasis (77% versus 46%) although these differences were not statistically significant.
Others
Votrubova et al. (2006) showed PET/CT was superior (sensitivity 95%, specificity 100%, accuracy 100%) to FDG uptake (sensitivity 74%, specificity 88%, diagnostic accuracy 88%) for the diagnosis of extra abdominal and/or hepatic recurrence of colorectal cancer and in the diagnosis of any form of colorectal cancer recurrence (p<0.05).
Desai et al. (2003) presented no data on the effect of PET on surgical decision making in patients with metastatic or recurrent colorectal cancer but observed that the information provided by PET complemented that provided by the CT scan. Imdahl et al. (2000) reported a higher sensitivity and specificity for PET (94% and 100%) compared with chest X-ray (64% and 98%) for the detection of pulmonary metastases.
Updated Evidence
Two studies (Metser et al., 2010 and Choi et al., 2010) were identified during updates as providing evidence for the topic though both studies were case series studies and neither were specifically designed to answer the question of which modality is best for identifying number and extent of extrahepatic metastases.
Choi et al (2010) evaluated the role of chest CT on preoperative staging of rectal cancer to assess the impact on treatment strategy though the study was of a low quality and it was difficult to draw any conclusions as to the effectiveness of chest CT on the preoperative staging of pulmonary metastases when compared with standard chest X-Ray.
Metser et al. (2010) compared the detection of tumour recurrence and metastases with FDG-PET/CT with contrast enhanced MDCT in patients with colorectal cancer and elevated CEA levels and reported that on event based analysis (number of lesions) PET/CT was significantly more sensitive that MDCT (p=0.002) but there was no difference in specificity (p=1.0) of the two modalities for detection or recurrence or metastases.
Tumour based analysis showed that PET/CT was significantly better than MDCT for the detection of recurrence and metastases (p<0.0001) though again there was no difference in specificity (p=0.56).
Review Protocol
| Population | Intervention | Comparison | Outcome |
|---|---|---|---|
| Patients with colorectal cancer and extrahepatic metastases (e.g. lung, brain, peritoneum, adrenal/spleen) |
|
|
|
Following a systematic search of relevant data sources (see appendix .1), the information specialist created a database of potentially relevant studies. All titles and abstracts were sifted by a single reviewer. Queries about inclusion were clarified by the GDG topic subgroup. The full studies were then obtained and reviewed and relevant studies were included in the final evidence review.
All update searches were sifted by a single reviewer and the list of potentially relevant studies was also checked for irrelevant studies by the GDG subgroup. Only studies which all subgroup members were in agreement were excluded. The remaining studies were obtained and reviewed with relevant studies included in the final evidence review.
High level evidence such as randomised controlled trials do not exist for this topic, therefore the evidence level accepted included lower level studies such as retrospective case series. A single systematic review was available for this topic however the evidence quality of the studies included in the review was low as this is all that is available.
A number of date limits were set by the GDG for more efficient and targeted searches:
PET-CT: 2000 onwards
PET: 1990 onwards
CT: 1993 onwards (data from spiral/helica CT era only)
MRI: 1990 onwards
The dates were selected by the GDG subgroup on the basis of improvements in available technology and likelihood that older methods are no longer used.
| Reasons for excluding studies: | Quality of the included studies |
|---|---|
| Studies did not report on extrahepatic metastases | Systematic review of RCTs (n = 0) |
| Studies were designed for follow-up rather than preoperative staging | Systematic review of combined study designs (n = 1) |
| Intervention modality not relevant to PICO | Randomized controlled trial (n = 0) |
| Comparison not relevant to PICO | Prospective cross sectional study (n = 0) |
| Foreign Language (no translation available) | Case Series Studies (n = 11) |
Volume of evidence
There was very little, poor quality evidence available to address this question. There was a single systematic review and meta-analysis of case-series studies, and the remainder of the evidence was drawn from retrospective case series’ in which the numbers of cases available to be reviewed is small with little detail provided with regards to factors such as inclusion/exclusion criteria, co-morbidities or other factors that may impact on the outcome of imaging.
Applicability
There is little direct evidence with which to answer this question. None of the studies identified were designed to address the question of which imaging modality provided the most accurate information on number and extent of extrahepatic metastases. The majority of studies identified were concerned with how effective imaging modalities were in detecting colorectal cancer recurrence (primary or metastastic) and how the results impact on management decisions. The accuracy of detecting extrahepatic metastases was a secondary outcome in the majority of studies, in many studies detecting metastases (liver and extrahepatic) was the focus and in such studies it was not possible to elucidate the results relating specifically to extrahepatic metastases, therefore such studies were not included.
Consistency
There appears to be some degree of consistency across the evidence base in relation to the effectiveness of the different imaging modalities in detecting extrahepatic metastases. There appears to be reasonable agreement that PET and PET/CT are more sensitive and specific than CT and/or MRI in the detection of extrahepatic metastases.
Other factors
Due to the poor evidence available with which to address this question, all study types were considered for inclusion, as well as any studies which reported potentially relevant or indirect information to answer the question. The majority of the included studies had very small numbers which meant that any meaningful statistical analysis was difficult to conduct and although accuracy, sensitivity and specificity were reported in many cases, in some cases this information was not available. Due to methodological differences across the studies it was not possible to combine the results of the different case series studies, though pooled estimates of sensitivity and specificity are provided as part of a systematic review and meta-analysis (Wiering et al. 2005).
The PICO listed MRI, CT, PET and PET-CT to be the interventions of choice and most studies compared two or more of these interventions, however in one case CT scanning was used to confirm PET diagnosis and therefore the results should be interpreted with caution (Imdahl et al. 2000) as from an initial look at the results it appears that CT scanning has 100% sensitivity and specificity.
Evidence Statement
There is a lack of good quality evidence available on which to base recommendations for the optimal imaging modality for determining the extent and number of extrahepatic metastases in patients with colorectal cancer. Much of the evidence has been drawn from studies which look at the contribution of such imaging modalities to the treatment plan for patients with recurrent colorectal cancer, including hepatic metastases. In patients with resectable liver metastases imaging is done to determine the presence or absence of extrahepatic metastases as the presence of any extrahepatic metastases is likely to preclude such patients from surgery. For this reason, the ability of imaging to determine the extent and number of extrahepatic metastasis is predominantly a secondary outcome in studies looking at whether patients with recurrence of either primary tumour or metastatic liver recurrence are candidates for surgery.
For the purposes of this evidence review, it was not possible to combine data as it was presented in any of the included studies due to inconsistencies and differences in study aims.
It is unlikely that it will be possible to conduct a randomised controlled trial to determine the best imaging modality.
Sensitivity and Specificity
There is evidence from a single systematic review and meta-analysis of case series studies that FDG-PET has a higher sensitivity and specificity (91.5% and 95.4% respectively) than does CT scan (60.9% and 91.1% respectively) (Wiering et al. 2005) for the detection of extra-hepatic metastases. When taking only the highest weighted studies included in the meta-analysis, the pooled sensitivity and specificity for FDG-PET were 91.2% and 98.4% respectively while for CT the sensitivity and specificity were 55.3% and 95.6% respectively.
FDG-PET versus CT
Two case series studies (Tanaka et al. 2002, Valk et al. 1999) with a combined patient population of 138, compared accuracy, sensitivity and specificity of FDG-PET and CT scanning. In both studies FDG-PET showed higher sensitivity and specificity than CT. Tanaka et al. found that FDG-PET was more accurate and sensitive (78% and 88% respectively) than CT (44% and 38% respectively) in the diagnosis of peritoneal metastases, though the numbers in this study were small (N=23). Valk et al. reported an overall sensitivity and specificity for extrahepatic metastases of 92% and 99% for FDG-PET compared with 61% and 96% for CT. In looking at specific sites of metastases, Valk et al. reported that FDG-PET was significantly more specific than CT for lung metastases.
PET/CT versus MRI
Two studies (Schmidt et al. 2009, Squillaci et al 2008) compared PET/CT to MRI. Schmidt et al. reported the PET/CT was more sensitive than whole body MRI in the detection of distant metastasis (80% compared with 78%), though there was no difference in specificity for either modality (95%) and accuracy was similar for both (PET/CT - 87%, WB-MRI - 86%). Squillaci et al. did not report sensitivity or specificity, but reported that PET/CT similar detection rates for both modalities in relation to extrahepatic metastases. Both studies reported that PET/CT revealed more lung metastases in patients than did WB-MRI.
PET/CT versus CT
From a single study (Selzner et al. 2004), the presence of extrahepatic metastases identified by ceCT and PET/CT were 31% and 45% respectively, though the difference was not statistically significant (p=0.13).
PET/CT was more sensitive than CT in the detection of lung metastases (100% and 78% respectively). PET/CT was also more sensitive than CT for portal and para-aortic lymph node metastasis (77% and 46% respectively) though these differences were not statistically significant.
In a study by Desai et al. (2003) the effect of PET on surgical decision making in patients with metastatic or recurrent colorectal cancer was the main focus. The study did not present any sensitivities or specifities, however it observed that the information provided by PET scans complements that which if provided by the CT scan.
A study by Potter et al. compared sensitivity and specificity of FDG-PET CT to CT and/or MRI serial review in colorectal cancer follow-up. There was no significant difference between FDG-PET and CT/MRI in relation to accuracy, sensitivity or specificity, though this is an overall result and does not distinguish between site of recurrence, therefore it is not possible comment on the accuracy, sensitivity and specificity in relation to extrahepatic metastases. The study may however provide some important information in relation to the role of the reader, as a significant difference in accuracy and sensitivity was found between the three individual readers of the CT/MRI scans.
Imdahl et al. (2000) reported a sensivity and specificity for PET of 94% and 100% respectively, compared with chest X-ray (64% and 98% respectively) for the detection of pulmonary metastases. In this study, CT was performed only in patients for whom PET scan or chest X-ray was indicative of pulmonary metastases and for this reason reported a sensitivity and specificity of 100%. It would however be misleading to say that CT was the better modality in this case however, as it was used for confirmatory purposes.
Votrubova et al. (2006) compared FDG uptake to PET/CT and reported a sensitivity of 74% and 95% respectively, a specificity of 88% and 100% respectively and an accuracy of 85% and 99% respectively. The specificity and accuracy of PET/CT was significantly higher for the diagnosis of extra abdominal and/or hepatic recurrence of colorectal cancer and in the diagnosis of any form of colorectal cancer recurrence (p<0.05).
Updated Evidence
Update searches identified 94 new studies of which the GDG members identified 9 as being potentially relevant for full review. On obtaining the full studies it was determined that only 2 studies were of relevance to the topic (Choi et al, 2010 and Metser et al, 2010).
Choi et al (2010) evaluated the role of chest CT on preoperative staging of rectal cancer to assess the impact on treatment strategy though the study was of a low quality and it was difficult to draw any conclusions as to the effectiveness of chest CT on the preoperative staging of pulmonary metastases when compared with standard chest X-Ray. The authors however, concluded that chest CT was an acceptable approach as it picked up pulmonary metastases which were not visualised on chest X-ray.
In total 9 patients with pulmonary metastases were identified on chest CT, 5/9 of whom were also identified on chest X-ray and in 3/4 patients whose metastases were missed, treatment strategy changed as a result of the findings of chest CT.
Metser et al. (2010) compared the detection of tumour recurrence and metastases with FDG-PET/CT with contrast enhanced MDCT in patients with colorectal cancer and elevated CEA levels
Event based analysis showed that for PET/CT and ceCT the sensitivities were 97.3% (95% CI, 85–99) and 70.3% (95% CI, 53–84) respectively (p=0.002) and the specificities were 94.4% (95% CI, 72–99) and 94.4% (72–99) respectively (p=1.0).
Tumour site based analysis showed that sensitivity for PET/CT and ceCT was 98.1% (95% CI, 52–78%) respectively (p<0.0001) and the specificities were 75% (95% CI, 34–96%) and 62.5% (95% CI, 24–91) respectively (p=0.56).
References
- Choi D, Kwak J, Kim J, et al. Preoperative Chest Computerised Tomography in Patients with Locally Advance Mid or Low Rectal Cancer: Its Role in Staging and Impact on Treatment Strategy. Journal of Surgical Oncology. 2010;102(6):588–592. [PubMed: 20607759]
- Desai D, Zervos E, Arnold M, Burak W, Mantil J, Martin E. Positron Emission Tomography Affects Surgical Management in Recurrent Colorectal Cancer Patients. Annals of Surgical Oncology. 2003;10(1):59–64. [PubMed: 12513962]
- Imdahl A, Reinhardt MJ, Nitzche EU, Mix M, Dingeldey A, Einert A, Baier P, Farthmann EH. Imapct of 18F-FDG-positron emission tomography for decision making in colorectal cancer recurrences. Langenbeck’s Archives of Surgery. 2000;385:129–134. [PubMed: 10796051]
- Metser U, You J, McSweeny S, et al. Assessment of Tumour Recurrence in Patients with colorectal cancer and elevated carcinoembryonic antigen level: FDG PET/CT versus contrast enhanced 64-MDCT of the chest and abdomen. AJR. 2010;194:766–771. [PubMed: 20173157]
- Potter KC, Husband JE, Houghton SL, Brown G. Diagnostic accuracy of serial CT/Magnetic resonance imaging review vs. positron emission tomography/CT in colorectal cancer patients with suspected and known recurrence. Diseases of the Colon and Rectum. 2009;52(2):253–259. [PubMed: 19279420]
- Schmidt GP, Baur-Melnyk A, Haug A, Utzschneider S, Becker CR, Tiling R, Reiser MF, Hermann KA. Whole-body MRI at 1.5 T and 3 T compared with FDG-PET-CT for the detection of tumour recurrence in patients with colorectal cancer. 2009. [PubMed: 19190917]
- Selzner M, Hany T, Wildbreet P, McCormack L, Zakiyah K, Clavien PA. Does the novel PET/CT imaging modality impact on the treatment of patients with metstatic colorectal cancer of the liver. Annals of Surgery. 2004;240(6):1027–1036. [PMC free article: PMC1356518] [PubMed: 15570208]
- Squillaci E, Maneti G, Mancino S, Ciccio C, Calabria F, Danieli R, Schillaci O, Simonetti G. Staging of colon cancer: whole body MRI vs. whole body PET-CT – initial clinical experience. Abdom Imaging. 2008;33:676–688. [PubMed: 18373114]
- Tanaka T, Kawai Y, Kanai M, Taki Y, Nakamoto Y, Takabayashi A. Usefulness of FDG-positron emission tomography in diagnosing peritoneal recurrence of colorectal cancer. The American Journal or Surgery. 2002;184:433–436. [PubMed: 12433608]
- Valk P, Abella-Culmna E, Haseman M, Pounds T, Tesar R, Myers R, Greiss H, Hofer G. Whole-body PET Imaging with [F18] Fluorodeoxyglucose in Management of Recurrent Colorectal Cancer. Arch. Surg. 1999;134:503–511. [PubMed: 10323422]
- Votrubova J, Belohlavek O, Jaruskova M, Oliverius M, Lohynska R, Trskova K, Sedlackova E, Lipska L, Stahalova V. The role of FDG-PET/CT in the detection of recurrent colorectal cancer. Eur J Nucl Med Mol Imaging. 2006;33:779–784. [PubMed: 16565845]
- Wiering B, Krabbe P, Jager G, Oyen W, Ruers T. The impact of fluor-18-deoxyglucose-poitron emission tomography in the management of colorectal liver metastases; a systematic review and meta-analysis. Cancer. 2005;104(12):2658–2670. [PubMed: 16315241]
Evidence Tables
Download PDF (237K)
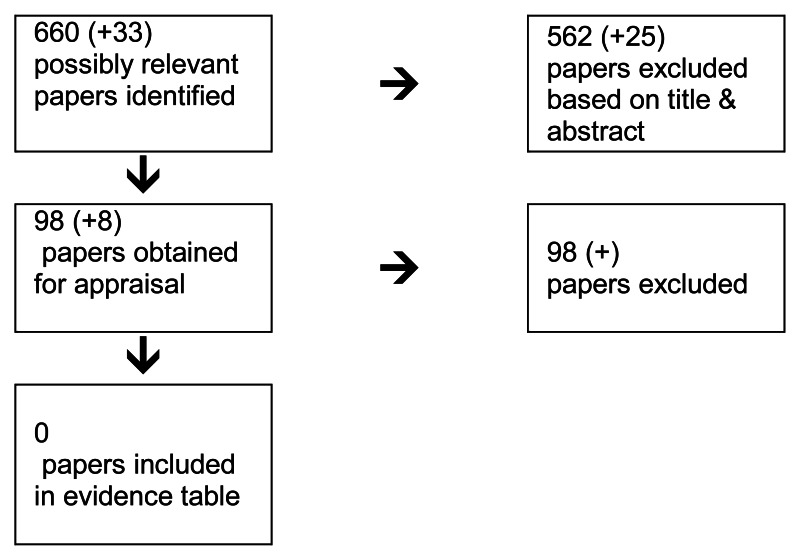
4.4. Chemotherapy in Metastatic Colorectal Cancer
4.4.1. What is the effectiveness of oxaliplatin and irinotecan-based chemotherapy regimens for patients with advanced and metastatic colorectal cancer?
Short Summary
The objective of this review and analysis was to identify and synthesise the evidence on the clinical and cost effectiveness of chemotherapy regimens containing irinotecan or oxaliplatin for the treatment of advanced colorectal cancer. Evidence on the use of irinotecan or oxaliplatin for the treatment of advanced colorectal cancer has been previously reviewed and appraised within the scope of NICE Technology Appraisal Guidance 93 (TA93). The current review includes both an update to identify new evidence that has become available after TA93 was issued (August 2005) and an expansion to the scope to address the following issues that were deemed by the GDG to be relevant to recent developments in clinical practice:
- the use of irinotecan or oxaliplatin in combination with the oral fluoropyrimidine capecitabine
- sequencing of combination chemotherapy (first and second line)
The current review does not address the use of targeted agents or the use of capecitabine as monotherapy for the treatment of advanced colorectal cancer. These topics are covered elsewhere in related NICE technology appraisal guidance.
The following chemotherapy regimens were considered relevant to this review:
- FOLFOX (oxaliplatin in combination with 5-flourouracil and folinic acid)
- FOLFIRI (irinotecan in combination with 5-flourouracil and folinic acid)
- XELOX (oxaliplatin in combination with capecitabine)
- XELIRI (irinotecan in combination with capecitabine)
- irinotecan as a single agent
The GDG identified ten sequences based on these chemotherapy regimens that were considered relevant to current clinical practice (Table 4.10). Sequences were limited to two lines of treatment.
Table 4.10
Summary of ten chemotherapy treatment sequences of interest.
The search for evidence included randomised controlled trials (RCTs) that reported on response, progression-free survival and overall survival for one or more of the chemotherapy regimens of interest as first-line treatment, second-line treatment or as part of a prospectively sequenced trial. Head-to-head RCTs were not available to inform all comparisons of interest. In addition, overall survival is likely to be influenced by the sequence of chemotherapy treatments; data on overall survival that was reported from studies conducted only in first line (with limited information about subsequent treatment) or only in second line (with limited information about prior treatment) was regarded with caution, thus further limiting the number of head-to-head comparisons available to inform this endpoint.
In order to facilitate a comparative analysis of all ten chemotherapy sequences, it was necessary to consider evidence that enabled indirect comparison of the treatments of interest. For example, if an RCT existed comparing two treatments A vs B, and another RCT existed comparing B vs C, however no RCT was identified comparing A vs C, then the evidence from the RCTs comparing A vs B and B vs C can be used to produce an indirect estimate of the relative effectiveness of A vs C. For the analysis of first-line treatment effects, both head-to-head trials (direct comparisons) as well as indirect comparisons were simultaneously considered as part of the evidence base to inform the estimate of effect size between 2 or more treatments of interest, therefore the analysis for first line is referred to as a mixed treatment comparison (MTC). To quantify second-line treatment effects and overall survival for sequences of chemotherapy, only a small number of relevant studies were identified as part of the evidence base. Each comparison was informed by using either direct evidence from a head-to-head trial or indirect evidence via a common comparator, but not by both types of evidence simultaneously. Therefore the second-line analysis is more accurately referred to as an indirect (rather than mixed) treatment comparison.
The motivations for applying mixed and indirect treatment comparison techniques to the present analysis include:
- Indirect comparisons allow estimation of treatment effects for comparisons that have not been trialled head-to-head, without breaking randomisation (Sutton et al. 2008)
- All ten treatment sequences of interest can be compared simultaneously, using one consistent evidence base (for each outcome of interest). Consideration of both direct and indirect comparisons provides an opportunity to formally assess the consistency of the evidence
- Results of the analysis are needed to inform a comparative cost-effectiveness analysis of all ten treatment sequences of interest
Mixed and indirect treatment comparisons were modelled to estimate relative effects to a common baseline for the outcomes response rate, progression-free survival and overall survival. Important assumptions and methods underpinning the analysis are described in detail below. The analysis was performed using the Bayesian WinBUGS 1.4.3 software.
Assessing each individual trial using the NICE methodology checklist for randomised trials showed that in almost all aspects the individual studies were of a high standard methodologically. The method of randomisation was adequate in most cases with only a small number of studies not providing details of the method used and in almost all cases, the groups were well balanced at baseline, primarily the result of stratification for key factors. It was not clear in any study however, whether there was adequate allocation concealment. It was therefore concluded that overall, there was a low risk of selection bias.
In all studies patients in both arms received the same care apart from the treatment of interest, however none of the patients or treatment administrators was blinded as it was not possible given the type of treatments administered and methods of administration. Despite this however, it is unlikely that there was a high risk of performance bias overall as the studies were all comparing very similar treatments in comparable patients.
In first line, there appears to be a small benefit in favour of FOLFOX with respect to response rate. XELIRI was associated with the second highest probability of being the best out of the four regimens, however as there was only one RCT to connect XELIRI to FOLFIRI in the evidence network, the estimate of effectiveness for XELIRI is associated with a high degree of uncertainty as seen by the width of the 95% credible interval.
Treatment with FOLFOX/XELOX in second line (following FOLFIRI/XELIRI in first line) was associated with significantly higher response rate than FOLFIRI/XELIRI in second line (following FOLFOX/XELOX in first line). Response rates for single agent irinotecan in second line were comparable to FOLFOX/XELOX in second line, however FOLFOX/XELOX were still the treatment options associated with the highest probability of being the most effective regimens in second line.
Review Protocol
| Population | Intervention (first line, second line) | Comparison | Outcomes |
|---|---|---|---|
| Patients with advanced and metastatic colorectal cancer | FOLFOX, FOLFIRI FOLFOX, XELIRI FOLFOX, irinotecan XELOX, FOLFIRI XELOX, XELIRI XELOX, irinotecan FOLFIRI, FOLFOX FOLFIRI, XELOX XELIRI, FOLFOX XELIRI, XELOX | Each other |
|
Following a systematic search of relevant data sources (see appendix .1), the information specialist created a database of potentially relevant studies. All titles and abstracts were sifted by a single reviewer. Queries about inclusion were clarified by the GDG topic subgroup. The full studies were then obtained and reviewed and relevant studies were included in the final evidence review.
All update searches were sifted by a single reviewer and the list of potentially relevant studies was also checked for irrelevant studies by the GDG subgroup. Only studies which all subgroup members were in agreement were excluded. The remaining studies were obtained and reviewed with relevant studies included in the final evidence review.
Evidence on the use of irinotecan or oxaliplatin for the treatment of advanced colorectal cancer has been previously reviewed and appraised within the scope of NICE Technology Appraisal Guidance 93 (TA93). The current review includes both an update to identify new evidence that has become available after TA93 was issued (August 2005) and an expansion to the scope to address the following issues that were deemed by the GDG to be relevant to recent developments in clinical practice:
- The use of irinotecan or oxaliplatin in combination with the oral fluoropyrimidine capecitabine
- Sequencing of combination chemotherapy (first and second line)
In this PICO, it was assumed that patients are eligible to receive either oxaliplatin or irinotecan treatment. This means separate consideration was not specifically given to patients who received oxaliplatin in the adjuvant setting (and who may therefore be less likely to benefit from further oxaliplatin as a first-line treatment for advanced disease).
The current review does not address the use of targeted agents or the use of capecitabine as monotherapy for the treatment of advanced colorectal cancer.
The following chemotherapy regimens were considered relevant to this review:
- FOLFOX (oxaliplatin in combination with 5-flourouracil and folinic acid)
- FOLFIRI (irinotecan in combination with 5-flourouracil and folinic acid)
- XELOX (oxaliplatin in combination with capecitabine)
- XELIRI (irinotecan in combination with capecitabine)
- irinotecan as a single agent
From the PICO, it is clear we are interested in looking at sequences of treatment. It is likely that some of these sequences will not have been studied prospectively in a controlled trial. In order to make use of the widest possible evidence base, it will be likely that there will be a need to apply methods to put together (synthesise) the available evidence for the analysis. The following approach is planned:
- All available evidence on any of the regimens identified in the line of treatment which have been specified will be reviewed. For those regimens that were reviewed as part of TA93, the body of evidence that was identified and included as part of TA93 will be combined with any new evidence that is identified through an updated search of the relevant literature published since TA93.
- Response/progression free survival data by line will be extracted where available
- Overall survival will be based on data available from sequenced trials
- If head-to-head evidence is not available for some regimens, there may be a need to use indirect treatment comparisons.
| Reasons for Exclusion: | Quality of the included studies |
|---|---|
| Not a relevant comparator | Systematic review of RCTs (n =0) |
| Population not relevant | Systematic review of combined study designs (n =0) |
| Not a randomised trial | Randomized controlled trial (n =23) |
| Foreign Language | Prospective cross sectional study (n =) |
| Expert Reviews | Retrospective cohort study (n=) |
| Abstracts Only | Case Series Studies (n = 0) |
| Data not reported in a format suitable for inclusion in mixed treatment analysis |
Head-to-head RCTs were not available to inform all comparisons of interest. In addition, overall survival is likely to be influenced by the sequence of chemotherapy treatments; data on overall survival that was reported from studies conducted only in first line (with limited information about subsequent treatment) or only in second line (with limited information about prior treatment) was regarded with caution, thus potentially further limiting the number of head-to-head comparisons available to inform this endpoint.
In order to facilitate this review, it was necessary to consider evidence that also enabled indirect comparison of the treatments of interest. For example, if an RCT existed comparing two treatments A vs B, and another RCT existed comparing B vs C, however no RCT was identified comparing A vs C, then the evidence from the RCTs comparing A vs B and B vs C can be used to produce an indirect estimate of the relative effectiveness of A vs C. For the present review, both head-to-head trials (direct comparisons) as well as indirect comparisons were considered as part of the evidence base, therefore the analysis presented here is referred to as a mixed treatment comparison (MTC).
Quality Assessment
The quality assessment for this topic cannot be produced in GRADE as the software cannot yet accommodate the issues surrounding indirect treatment comparisons. GRADE has been designed to assess the quality of the total body of evidence for a given outcome rather that the methodological quality of individual studies included in the analysis. While this is certainly a more informative and useful way in which to assess the quality of evidence, an indirect treatment comparison presents a particular problem in that the information used to inform the model includes, where possible, direct evidence, but in many cases will also include data from studies which do not directly assess the interventions of interest against each other and is so considered indirect evidence.
Assessing each individual trial using the NICE methodology checklist for randomised trials showed that in almost all aspects the individual studies were of a high standard methodologically. The method of randomisation was adequate in most cases with only a small number of studies not providing details of the method used and in almost all cases, the groups were well balanced at baseline, primarily the result of stratification for key factors. It was not clear in any study however, whether there was adequate allocation concealment. It was therefore concluded that overall, there was a low risk of selection bias.
In all studies patients in both arms received the same care apart from the treatment of interest, however none of the patients or treatment administrators was blinded as it was not possible given the type of treatments administered and methods of administration. Despite this however, it is unlikely that there was a high risk of performance bias overall as the studies were all comparing very similar treatments in comparable patients.
In the majority of studies, it was unclear how the individual arms were affected by patient drop outs or partial treatment administration. The median number of treatment cycles per arm was reported and in some studies a full study flow chart was provided which detailed the number of patients in each arm that received treatment, dropped out or were lost to follow-up. Median length (and in some cases, range) of follow up was reported in all studies and a number of studies also reported the length of time post recruitment that data were collected, however this information was for the whole patient group as opposed to each arm and it was not clear from any of the individual studies whether the length of follow-up was similar in both arms. There is a possibility that some studies might be affected by attrition bias, however, from the data that are reported, this seems unlikely.
Overall, the length of follow-up and outcomes reported were deemed to be appropriate. The primary outcomes of interest to the topic were response, progression-free survival and overall survival, with toxicity and quality of life also of interest where available.
- Response: a precise definition was provided as to what was considered a response and the data were clearly reported across the individual studies.
- Progression-free Survival: data on time to disease progression was reported as progression-free survival in most studies, however some trials reported time to progression instead
- Toxicity: toxicity was reported in some format in the majority of studies included in the review; most commonly reported were Grade 3–4 toxicities, though some studies also reported Grades 1–2 toxicities. Toxicities were reported primarily as a rate or an absolute value for each specific toxicity of interest.
- Quality of Life: the quality of reporting of quality of life was very poor across the individual studies, the majority of studies which included quality of life as an outcome did not provide any data, with only a small number of studies making any attempt to quantify the changes in QoL from baseline through the use of questionnaires.
Design
All studies included in the review were RCTs comparing treatments in first line or in a predetermined sequence of treatments and were all of high methodological quality.
Data for progression-free survival first line were taken from 22 randomised studies, including the prospectively sequenced studies; data for progression-free survival second line were taken from only prospectively sequenced studies and data to inform overall survival on second line treatment for a given sequence.
Limitations
The primary limitation was the lack of trials comparing predefined sequences of treatment with the majority of studies available comparing treatments of interest either in first line only or second line only trials. A second major limitation occurred in relation to the second line studies in that results of second line treatment, particularly overall survival, are dependent not only on second line treatment but what the patient received in the first line and this was a factor that was not adequately addressed in second line studies.
Consistency
There was a high degree of consistency across the individual trials in relation to populations included (irrespective of treatments under investigation); effect size for treatments was consistent across the individual studies. On reviewing the individual trials it appeared that there were some differences in the doses administered for some treatment regimens which may have precluded combining the data, however this did not appear to impact effect size and GDG members were satisfied that studies could be combined for meta-analysis.
Indirectness
There were three sequenced studies (Tournigand et al, 2004; Cunningham et al, 2009 and Koopman et al, 2007) one of which was directly relevant to the topic in question and two of which provided some indirect evidence for the comparisons of interest. The majority of the data were taken from studies which were not comparing treatments of interest and so under normal GRADE methodology the evidence body would be downgraded for indirectness, however this was not considered appropriate in the context of an MTC analysis.
Evidence Synthesis Methods
First-line treatment
A total of twenty-three studies reported the number of responders out of the total number of patients receiving each treatment as first-line therapy, corresponding to the network of evidence in Figure 4.8. A list of included studies is provided in Table 4.11.
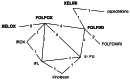
Figure 4.8
MTC network of evidence used to inform response rate and progression-free survival for first-line treatments. Treatments in bold text are of primary interest to the analysis. A line between two treatments indicates a head-to-head comparison (RCT) exists; (more...)
Table 4.11
Studies that informed the MTC for response rate and progression-free survival for first-line treatments.
First-line response rate relative effects
We assumed that for each trial j, the number of events in arm k, rjk, has a binomial likelihood rjk~Bin(pjk,njk) where pjk is the probability of an event (response) in arm k of trial j and njk are the total number of patients in arm k of trial j. A random effects model for pjk was fitted on the logit scale, such that for each trial logit(pj1)=μj in the control arm (k=1) and logit(pjk)=μj+δjk, for the treatment arms (k=2 or 3 for three arm trials) with δjk representing the trial-specific log-odds ratio of the treatment in arm k relative to the control treatment in trial j and μj representing the study-specific effects (baseline effects). We fit a random effects MTC model, with FOLFOX as the reference treatment, under the assumption of consistency and homogeneous variance of the random effects (Lu and Ades, 2004).
Defining tjk as the treatment in arm k of trial j, the trial-specific log-odds ratios, δjk, are drawn from one of the random effects distributions δjk~N(d(tjk)−d(tj1),σ2) where d(tjk) is the relative treatment effect of the treatment tjk vs FOLFOX, k=1,2,3 and σ2 is the between-study heterogeneity. A vague inverse-gamma prior on σ2 was used since it resulted in faster convergence and smoother posterior densities than the alternative Uniform prior on σ. Posterior mean and median results were largely unaffected by the choice of prior distribution, but the estimates of σ2 varied slightly.
First-line response rate baseline calculation for absolute effects
In order to obtain absolute effects, it is necessary to obtain a baseline treatment effect for the reference treatment (FOLFOX), on which the relative treatment effects are applied. Any of the four first-line treatments of interest could be used as the reference treatment, however FOLFOX was chosen as it was the most frequently studied treatment out of the twenty-three available head-to-head trials. A separate meta-analysis (on the logit scale) was performed on just the FOLFOX arms of the fifteen trials comparing FOLFOX to any other drug (in first line). The predictive distributions of the log-odds of FOLFOX in a future trial were assumed to be normal with posterior means mA=−0.1119 and standard deviations sdA=0.3071. These results were then used in the MTC model to generate a baseline treatment effect for FOLFOX, A~Normal(mA, sdA2) on the log-odds scale on which relative effects were added at each iteration, to deliver the posterior summaries of the absolute probability of response for each treatment.
First-line progression-free survival relative effects
All twenty-three studies listed in Table 4.11 that reported response rates also provided data on disease progression (reported as progression-free survival or time to progression). In twelve of these studies, median PFS was accompanied by a hazard ratio (HR) with associated confidence interval (CI). The HR should be preferred to the median for survival analysis as it incorporates information on censoring (Tierney et al., 2007), so when both were available, the analysis was carried out on the log-hazard ratio (LHR). The data were transformed from HR into LHR and the standard error of the LHR obtained from the transformed CI by assuming an underlying normal distribution (Parmar et al. 1998).
When only the median PFS and its CI were available (five studies), these were log-transformed and the standard error of ln(median) calculated by assuming an underlying normal distribution (Parmar et al., 1998). Checks were made to ensure that the CI were symmetric on the log-median scale.
Six studies presented only the median PFS with no measure of uncertainty. In five of these studies (Colucci et al. 2005, Seymour et al. 2007, de Gramont et al. 2000, Gennatas et al. 2006, Douillard et al. 2000, Souglakos et al. 2006) a p-value for the log-rank test of a difference in the Kaplan-Meier curves was available. This was used to obtain an approximate LHR and standard error assuming the test statistic referred to a standard normal distribution and no censoring. Since no information was available on the number of observed events it was assumed that all analysed patients had progressed (Tierney et al. 2007). Saltz et al. 2000 did not present a p-value for the comparisons of interest but the number of patients at risk at different time points was available. Survival probabilities at each of the time points were read off the survival curves and a LHR and variance estimated following Williamson et al. 2002.
Let yjk represent the log-hazard ratio of the treatment in arm k of study j, relative to the treatment in arm 1 of trial j, and Wjk represent the variance of the corresponding LHR. For the 17 trials for which the LHR and standard error were available (from the publications or imputed), the likelihood was defined as
where δjk are the trial-specific LHR for each study, assumed to come from the random effects distribution above. A random effects mixed treatment comparisons (MTC) model was fitted, with FOLFOX as the reference treatment, under the assumption of consistency and homogeneous variance of the random effects, as above (Lu et al. 2004).
Let Mjk represent the median PFS in arm k of study j and Vjk represent the variance of ln(Mjk). Then, for the 5 trials where the media PFS is used, the median PFS is assumed to follow a log-normal distribution such that Mjk ~ log-Normal(mjk, Vjk), and
Assuming the underlying PFS in arm k of trial i has an exponential distribution with rate λjk, the expected value of the median of an exponential distribution is ln(2)/λjk and the HR of arm k compared to arm 1 in trial j is λjk/λj1. Further, the expected value from a log-normal distribution is exp(mjk + Vjk/2), therefore we can model the log-rates by taking
and ln(λjk)= μj+δjk with δjk~N(d(tjk)−d(tj1),σ2), for the treatment arms (k=2 or 3 for three arm trials) with δjk representing the trial-specific log-hazard ratio of the treatment in arm k relative to the control treatment in trial j and μj representing the study-specific effects (baseline effects). Note that the trial-specific LHR, δ, are assumed to be coming from the same random effects distributions, whether they refer to a study with data on the LHR directly or through the link function for studies with data given as medians with uncertainty.
First-line progression-free survival baseline calculation for absolute effects
In order to obtain absolute effects, it is necessary to obtain a baseline median PFS for FOLFOX, on which the relative treatment effects are applied. Of the fifteen studies comparing FOLFOX to any other treatment (in first line), six did not report any uncertainty measure for the median in the FOLFOX arm. We have therefore used only the nine studies for which a variance for the log-median could be extracted (Comella et al. 2009, Martoni et al. 2006, Diaz-Rubio et al. 2007, Hochster et al. 2008, Ducreuc et al. 2010, Tournigand et al. 2004, Comella et al. 2005, Giacchetti et al. 2000, Cunningham et al. 2009) to calculate the baseline PFS on FOLFOX. A separate meta-analysis was performed on the FOLFOX arms of these nine trials. The predictive distributions of the log-hazard of PFS on FOLFOX in a future trial were approximately normal with posterior means mA= −2.467 and standard deviations sdA= 0.1569. These results were then used in the MTC model to generate a baseline A~Normal(mA, sdA2) on the log-hazard scale on which relative effects were added at each iteration, to deliver the posterior summaries on the absolute log-hazard and hazard PFS and time to progression for each treatment.
Second-line treatment and sequences
The search for RCTs identified four studies in which two treatments of interest had been compared specifically as second-line chemotherapy (Table 4.12). However upon examination of the inclusion criteria for these studies, it was noted that all patients in these trials had received either single agent irinotecan or singe agent 5-fluorouracil as first-line treatment for advanced colorectal cancer. Therefore, these studies did not reflect the specific treatment sequences of interest to the current review and were excluded from the indirect treatment comparison analysis.
Table 4.12
Second-line studies that included patients who received first-line treatment outside the treatment sequences of interest and were therefore excluded from the indirect treatment comparison analysis.
The only other source of data on second-line response rates and PFS for the treatment sequences of interest was from prospectively sequenced studies. Three prospectively sequenced trials were available (Tournigand et al. 2004, Koopman et al. 2007 and Seymour et al. 2007) and reported data on response rate and PFS after first and second line. However, Seymour et al. 2007 did not compare any sequences of interest or any sequences common to the other two trials, and was therefore excluded from the evidence space. The remaining trials provide evidence on only three of the ten sequences of interest and do not form a connected evidence network.
The endpoint overall survival was reported for all studies (first line, second line and prospectively sequenced). However, in the majority of the first-line studies, patients went on to receive a mix of second-line treatments. The second-line treatments offered were usually not pre-specified and rarely reported in sufficient detail. Furthermore, where some data was available on which second-line treatments were received by patients, the medians or HR for overall survival were not reported separately for the different treatments. Since we expect second-line treatment to influence overall survival (preliminary analyses, not shown, also suggested this was the case), it was not considered appropriate to use data on overall survival from first-line studies in which the patients who had second-line treatment received a mix of different chemotherapy to inform the analyses for specific treatment sequences. An exception to this was the Cunningham et al. 2009 trial that compared FOLFOX and 5-FU; although this was a first-line study, the protocol had pre-specified that patients who progressed on the first-line treatment should be offered irinotecan as second-line treatment. The trial further reported that a high proportion (over 75%) of patients received second-line irinotecan in both arms. It was therefore decided that this trial could be considered a ‘quasi-sequenced’ trial comparing the sequence FOLFOX followed by irinotecan to the sequence 5-FU followed by irinotecan. One other study (Porschen et al. 2007) also fulfilled these criteria. This was a first-line study of FOLFOX vs XELOX in which a high proportion of patients went on to receive irinotecan-based second-line treatment. This study was considered a ‘quasi-sequenced’ trial of FOLFOX followed by irinotecan vs XELOX followed by irinotecan. No other studies fulfilled the criteria for sequences of interest.
Even after inclusion of Cunningham et al. 2009 and Porschen et al. 2007 in the evidence base (Table 4.13), the network remains disconnected and still does not provide sufficient data to compare all sequences of interest. In discussion with members of the GDG, equivalence of the effectiveness of the oral and iv fluoropyrimidine formulations (capecitabine and 5-FU) was hypothesised. If data supported the assumption that the treatment effect of FOLFOX is the same as the treatment effect of XELOX, the treatment effect of FOLFIRI is the same as the treatment effect of XELIRI, and treatment effect of capecitabine is the same as the treatment effect of 5-FU in first and second line, this would allow the ten sequences of interest to reduce to only three sequences comprised of a fluoropyrimidine backbone combined with either oxaliplatin or irinotecan and irinotecan as a single agent in second line:
Table 4.13
Sequenced studies included in the MTC analysis to inform second-line response rate, progression-free survival and overall survival.
- FOLFOX or XELOX followed by FOLFIRI or XELIRI
- FOLFIRI or XELIRI followed by FOLFOX or XELOX
- FOLFOX or XELOX followed by single agent irinotecan
Exploratory analyses were conducted to confirm that this assumption was supported by the data on response and PFS. We checked if the 95% credible interval obtained from the first-line random effects MTC analysis for the HR of PFS included 1, which was the case for both XELOX vs FOLFOX and for XELIRI vs FOLFIRI. Similarly for response, the 95% credible interval for the OR for XELIRI vs FOLFIRI included 1, although for XELOX vs FOLFOX the upper limit did not (0.98). Although MTC analysis was not performed on studies that were only conducted in second line, data from Rothenberg et al. 2008 (comparing FOLFOX to XELOX) could still inform the equivalence of fluoropyrimidine-containing regimens. Analysis of this study showed that the 95% credible intervals for OR for response and HR for PFS both included 1.
Statistical models assuming equivalence of the effects of FOLFOX to XELOX, FOLFIRI to XELIRI and capecitabine to 5-FU were fitted for first -line response and PFS and were compared using the Deviance Information Criterion (DIC) to models that did not assume equivalence. These models were found to be similar in terms of model fit (DIC 83.2 for response and 54.4 for PFS, which were comparable to 83.6 and 56.1 respectively for the model not assuming equivalence).
Applying the above assumptions, this allowed us to form a connected evidence network shown in Figure 4.9. Since only one trial was available to inform each sequenced treatment comparison, a fixed effect model was fitted. It should be note that the assumption of equivalence in treatment effect between capecitabine and 5-FU was not extended to other aspects of treatment such as toxicity or cost. The latter parameters were not included in the indirect treatment comparison analysis and have been summarised elsewhere.

Figure 4.9
Network of sequenced studies to inform second-line response rate, progression-free survival and overall survival (assuming equivalent effect of capecitabine and 5-FU).
Second-line response rate and progression-free survival for sequences relative effects
Data on response rate and median PFS on second-line treatment for the sequences of interest were reported in Tournigand et al. 2004 and Koopman et al. 2007, but not in Cunningham et al. 2009 as the latter was a ‘quasi-sequenced’ study. However, Cunningham et al. 2009 did report that the median duration of second-line treatment was the same in both arms of this study. As patients usually continue treatment until disease progression (or unacceptable toxicity), we assumed that mean duration of treatment is highly correlated with PFS and imputed the HR of PFS on second-line treatment in the Cunningham et al. 2009 study as 1 (i.e. no difference in treatments). The standard error of the LHR was imputed as 0.1393 based on the relationship between the standard errors for all other LHRs and the study sample size, available from first and second- line studies both observed and imputed.
For the analysis of response rate on second-line treatment for a given sequence, rather than impute the number of patients responding to second-line treatment for the two arms of the trial, we imputed the LOR expected for this study, based on the relationship between all other observed LOR and the LHR for PFS in second line. The standard error for the LOR was imputed based on the relationship between all other available se(LHR) and the study sample size. The LOR of response on second line for the Cunningham et al. 2009 study was imputed as 0.03 with standard error=0.2492.
Overall survival for sequences relative effects
Two studies presented the HR and CI for overall survival. The analysis was carried out on the LHR for these studies with the standard error of the LHR obtained from the log-transformed CI by assuming an underlying normal distribution as above. One study reported only median overall survival and CI. These were log-transformed and the standard error of ln(median) calculated from the CI, as before.
The model used to combine the LHR and medians was the fixed effects version of the model used for first line data, so that for all trials for which the LHR and standard error were available, the likelihood was defined as
and for the trial in which median OS was reported, this was assumed to follow a log-normal distribution such that ln(Mjk)~ Normal(mjk, Vjk), j=1, k=1,2, mjk=ln(ln2) − ln(λjk)− Vjk/2 as before, and ln(λjk)= μj+d(tjk)−d(tj1).
Second-line response rate, progression-free survival and overall survival baseline calculation for absolute effects
Only one sequenced study provided information on the absolute effect of FOLFOX (XELOX) followed by FOLFIRI (XELIRI) (Tournigand et al. 2004). The baseline value calculated in the model for this study was taken to be the absolute effect of this sequence on second-line response rate, PFS and overall survival. A further element of uncertainty was added so that the absolute effects were calculated as the absolute effect of FOLFOX (XELOX) followed by FOLFIRI (XELIRI) plus a random term E with E ~ N(0,sE2) where sE was the predictive standard deviation for a future trial with FOLFOX as first-line treatment (obtained from all the first-line data, as above).
A baseline median OS for FOLFOX based on the first-line studies was obtained as follows: of the fourteen studies comparing FOLFOX to any other treatment in first line, data on OS was not extractable for the relevant comparisons for Seymour et al. 2007; Martoni at al. 2006 had no data on OS and a further 5 trials did not have any measure of uncertainty around the median OS in the FOLFOX arm. We therefore used the remaining eight trials (Comella et al. 2009, Diaz-Rubio et al. 2007, Hochster et al. 2008, Ducreux et al. 2010, Comella et al. 2005, Giacchetti et al. 2000, Cunningham et al. 2009, Tournigand et al. 2004) to calculate the baseline OS when receiving FOLFOX in first line. A separate meta-analysis was performed on the FOLFOX arms of these eight trials. The predictive distributions of the log-hazard of OS of FOLFOX in a future trial were approximately normal with posterior means mA= −3.218 and standard deviations sdA= 0.4690. Therefore sE=0.3071, 0.1606 and 0.4690 for response, PFS and OS respectively.
Model criticism
The posterior mean of the residual deviance (ResDev) will be used to assess whether the MTC model is satisfactory in terms of fit to the data. The residual deviance is the deviance for the fitted model minus the deviance for the saturated model. In an adequately fitting model, each data point should contribute about 1 to the posterior mean residual deviance (Spiegelhalter et al., 2002), so the posterior mean of the residual deviance will be compared to the number of data points used to inform each analysis. Inspection of each data point’s contribution to the residual deviance can help identify data points contributing to the model’s poor fit.
Estimation
All posterior summaries were obtained using Markov chain Monte Carlo (MCMC) simulation implemented in the WinBUGS 1.4.3 software. The study effects, μi, and all relative treatment effects have been given vague priors: N(0,10000). For all random effects MTC models, a vague prior is assumed for the common variances so that, 1/σ2~Gamma(0.001,0.001). Sensitivity of the results to Uniform(0,10) prior for σ was assessed and this did not change the posterior means of the treatment effects, but did make the results more unstable. Results using the Gamma priors are quoted throughout.
Three chains were run until convergence according to the Brooks-Gelman-Rubin diagnostic tool (Brooks et al. 1998) and through inspection of the history plots. These “burn-in” simulations were then discarded, and a further 100,000 iterations run for three independent chains in the models for first line data. In models for sequences 200,000 iterations were run post-convergence since there was moderate auto-correlation between the treatment effect estimates. All inference is based on the posterior summaries from these combined chains.
Mixed and Indirect Treatment Comparison Results
Results are presented below for the MTC for first-line treatment response rate and PFS and for the indirect treatment comparison for second-line sequenced treatment response rate, PFS and overall survival. Both relative effects and absolute estimates are reported for each outcome.
First-line treatment response rate
The results for first-line treatment response rate are shown in Tables 4.14 and 4.15.
Table 4.14
Posterior median of odds ratio (OR) for response rate for first-line treatment with 95% credible interval and probability that each treatment is best out of the four treatments of interest. OR < 1 favours the reference treatment.
Table 4.15
Posterior summaries of the absolute response rate for first-line treatment (median with 95% credible interval).
The residual deviance for the random effects model used for the analysis of first-line response rates was 48.7 which, compared to 49 data points, suggests a good model fit.
In first line, there appears to be a small benefit in favour of FOLFOX with respect to response rate. XELIRI was associated with the second highest probability of being the best out of the four regimens, however as there was only one RCT to connect XELIRI to FOLFIRI in the evidence network, the estimate of effectiveness for XELIRI is associated with a high degree of uncertainty as seen by the width of the 95% credible interval.
First-line treatment progression-free survival
The results for first-line treatment progression-free survival are shown in Tables 4.16 and 4.17.
Table 4.16
Posterior summaries (median with 95% credible interval) of hazard ratio (HR) for PFS for first-line treatment and probability that each treatment is best out of the 4 treatments of interest. HR > 1 favours the reference treatment.
Table 4.17
Posterior summaries (median with 95% credible interval) of mean and median PFS for first-line treatment. Baseline effects are based on all the available FOLFOX arms and assumed underlying exponential distribution.
The residual deviance for the random effects model used for the analysis of first-line PFS was 33.0 which, compared to 31 data points, suggests a good model fit.
FOLFOX was associated with a 66% probability of being the most effective of the four regimens with respect to PFS, however the 95% credible intervals for the hazard ratios of all other treatments included 1 (no difference between treatments). The uncertainty surrounding the effectiveness of XELIRI in terms of PFS is again evident by the width of the 95% credible interval. Estimates of median PFS for first-line treatment ranged from 5.7 months for XELIRI to 8,2 months for FOLFOX.
Second-line treatment response rates for sequences
The results for second-line treatment response rate are shown in Tables 4.18 and 4.19
Table 4.18
Posterior median of odds ratio (OR) for response rate for second-line treatment (in bold) as part of a sequence of treatments with 95% credible interval and probability that each second-line treatment is best out of the 3 regimens of interest, assuming (more...)
Table 4.19
Posterior summaries of the absolute response rate for second-line treatment (in bold) as part of a sequence of treatments (median with 95% credible interval).
The residual deviance for the fixed effects model used for the analysis of second-line response rates was 5.1 which, compared to 5 data points, suggests a good model fit.
Treatment with FOLFOX/XELOX in second line (following FOLFIRI/XELIRI in first line) was associated with significantly higher response rate than FOLFIRI/XELIRI in second line (following FOLFOX/XELOX in first line). Response rates for single agent irinotecan in second line were comparable to FOLFOX/XELOX in second line, however FOLFOX/XELOX were still the treatment options associated with the highest probability of being the most effective regimens in second line.
Second-line treatment progression-free survival for sequences
The results for second-line progression-free survival are shown in Tables 4.20 and 4.21.
Table 4.20
Posterior summaries (median with 95% credible interval) of hazard ratio (HR) for PFS for second-line treatment (in bold) as part of a sequences of treatments and probability that each second-line treatment is best out of the 3 regimens of interest, assuming (more...)
Table 4.21
Posterior summaries (median with 95% credible interval) of mean and median PFS for second-line treatment (in bold) as part of a sequence of treatments. Baseline effects are based on FOLFOX followed by FOLFIRI data with added uncertainty and assumed underlying (more...)
The residual deviance for the fixed effects model used for the analysis of second-line PFS was 5.0 which, compared to 5 data points, suggests a good model fit.
The reported hazard ratios favour FOLFIRI/XELIRI over FOLFOX/XELOX as a second-line treatment for the specified sequences. Estimates of median PFS for second-line treatment ranged from 2.5 months for FOLFOX/XELOX (when given after FOLFIRI/XELIRI in first line) to 4.2 months for FOLFIRI/XELIRI in second line (when given after FOLFOX/XELOX in first line).
Overall survival for sequences
The results for overall survival for sequences of treatment are shown in Tables 4.22 and 4.23.
Table 4.22
Posterior summaries (median with 95% credible interval) of hazard ratio (HR) for overall survival for sequences of treatment and probability that each sequence is best out of the 3 regimens of interest, assuming equivalence between the effect of capecitabine (more...)
Table 4.23
Posterior summaries (median with 95% credible interval) of mean and median OS for sequences of treatment, assuming equivalence between the effect of capecitabine and 5-FU. Baseline effects are based on FOLFOX followed by FOLFIRI data with added uncertainty (more...)
The residual deviance for the fixed effects model used for the analysis of overall survival was 4.0 which, compared to 4 data points, suggests a good model fit.
The estimate of median overall survival for all sequences in the indirect treatment comparison is approximately 21 months. There is a high degree of uncertainty in the estimates as seen by the wide 95% credible intervals, but nonetheless the analysis suggests with respect to overall survival, the effectiveness of all treatment sequences is comparable.
Cost-effectiveness analysis methods
A review of existing literature did not identify any published cost-effectiveness analyses that addressed all chemotherapy regimens and sequences of interest in the current guideline, therefore a new decision analytic model was developed alongside the MTC analysis.
A decision tree was constructed to reflect key events in the treatment pathway for advanced colorectal cancer patients in order to compare costs and health effects for the ten sequences of chemotherapy (Figure 4.10). In first line, patients receive one of four possible irinotecan or oxaliplatin-based combination chemotherapy regimens. Following disease progression on first-line treatment, the model allows for a proportion of patients to discontinue treatment. The remaining proportion of patients went on to receive one of five possible second-line treatments.
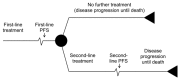
Figure 4.10
Basic structure of the cost-effectiveness model. The same structure was applied to all ten treatment sequences in the analysis.
Effectiveness was quantified in terms of quality-adjusted life years (QALYs). Survival time is partitioned in the model using the progression-free survival and overall survival results from the mixed and indirect treatment comparisons. While receiving chemotherapy, and prior to the onset of progressive disease, patients are assumed to be in a stable disease state. Following the point of progression in the model, patients are assumed to be in a progressive disease state with a lower overall quality of life. The model does not explore survival conditional on best response to treatment. This is because there was insufficient detail reported in the clinical literature to facilitate survival analysis dependent on tumour response.
The MTC analysis produced estimates of progression-free survival for each of the first-line treatments. Some assumptions (described in detail above) were made in order to create a connected evidence network to estimate second-line progression-free survival and overall survival for the treatment sequences of interest. Survival time was quality adjusted in the cost-effectiveness analysis using utility weights obtained from published sources.
For patients who only received one line of treatment, QALYs were calculated as follows:
For patients who received two lines of treatment, QALYs were calculated as follows:
where PFS1 = mean progression-free survival while on first-line treatment, PFS2 = mean progression-free survival while on second-line treatment and OS = mean overall survival for a given sequence of treatments for the combined population of patients receiving either one or two lines of treatment. The proportion of patients who went on to receive second-line treatment was reported in 15 studies (Colucci et al. 2005, Comella et al. 2005, Cunningham et al. 2009, Diaz-Rubio et al. 2007, Douillard et al. 2000, Goldberg et al. 2004, Goldberg et al. 2006, de Gramont et al. 2000, Kohne et al. 2005, Koopman et al. 2007, Martoni et al. 2006, Porschen et al. 2007, Seymour et al. 2007, Souglakos et al. 2006, Tournigand et al. 2004). This proportion was found to be approximately consistent (60%) across studies and also across different first-line treatments. As it was not possible to obtain separate overall survival curves for the subgroup of patients who only received one line of treatment and the subgroup of patients who received two lines of treatment, the QALY calculations above should be viewed as a weighted average of quality-adjusted survival across the combined patient population and not as separate absolute estimates of survival for each subgroup.
QALYs were further adjusted to take into account disutility associated with treatment-related toxicities. The toxicities included in the model were those that had considerable cost implications associated with management and/or measurable impact on patient well-being that could be quantified using disutility estimates available from published sources. Estimates of the rates of febrile neutropenia, Grade 3/4 diarrhoea and Grade 3/4 hand-foot syndrome were obtained from the clinical literature. It was not possible to conduct an MTC analysis using the available toxicity data, so mean rates of toxicity for each treatment were used to inform the cost-effectiveness model.
The model was developed from an NHS cost perspective. Costs in the model included drugs and drug administration, management of adverse events and supportive care. Given the relatively short time horizon of the model, discounting was not applied to either costs or health outcomes.
The model was made probabilistic to take into account the impact of parameter uncertainty on results. Probability distributions were created to reflect imprecision and Monte Carlo simulation was used to draw samples across all distributions. The decision tree was developed in TreeAge Pro 2009 software (TreeAge Software Inc, Williamstown, MA, USA).
Cost-effectiveness model inputs
Progression-free survival and overall survival
Details of the data sources, methods and results for estimating progression-free survival and overall survival using MTC techniques are presented above. For the cost-effectiveness analysis, a random sample of 30,000 simulations for first-line progression-free survival, second-line progression-free survival and overall survival estimates was obtained from the WinBUGS output. Rather than fitting a distribution to reflect uncertainty around the mean estimates for these parameters, simulations were inputted directly as chains into the cost-effectiveness model and sampled using Monte Carlo simulation.
Toxicity rates for febrile neutropenia, Grade 3/4 diarrhoea and Grade 3/4 hand-foot syndrome were obtained from the clinical literature that was identified during the systematic review for the MTC and are shown in Tables 4.24 and 4.25. Separate estimates were obtained for first-line treatment and second-line treatment. If there was insufficient data on second-line toxicity rates from prospectively sequenced studies, then studies conducted specifically in second line were included for the purpose of informing the cost-effectiveness analysis. Uncertainty in the estimates for toxicity rates was reflected by fitting beta distributions.
Table 4.24
First-line treatment toxicity rates used in the cost-effectiveness analysis.
Table 4.25
Second-line treatment toxicity rates used in the cost-effectiveness analysis.
Quality of Life
Quality of life was included as an outcome in a total of seven studies; 4 were first-line studies (Comella et al, 2009; Falcone et al, 2007; Douillard et al, 2000; DeGramont et al, 2000); 2 were second-line studies (Cunningham et al, 1999; Rougier et al, 1998) and 1 was a sequenced study (Koopman et al, 2007). Only 1 trial compared two treatments of interest and only in first line (Comella, 2009).
To compare quality of life in the FOLFOX and XELOX arms, baseline questionnaires were filled in by a total of 312 patients (97% of total patient population) and again at 8 weeks, 16 weeks and 24 weeks following treatment (Comella, 2009). The baseline single item and global health status/quality of life scores did not differ significantly between the two arms (Table 4.26).
Table 4.26
Patients showing significant change in the quality of life score during treatment.
No significant differences in the change of single scores were observed between the two arms apart from constipation (p=0.001) and financial item score (p=0.004).
At the predetermined time point for the comparison, a preservation of the quality of life was observed in 47% of patients in either arm.
A higher proportion of patients in the XELOX arm showed a deterioration of the global health status/quality of life score after 16 weeks and 24 weeks though the differences were not statistically significant.
Utility estimates
Utility estimates for stable (on treatment) and progressive disease were obtained from a published study of elicited preference values for health states associate with colon cancer (Best et al. 2010). The study was conducted using time trade-off techniques to elicit preferences from both patients and community members. The estimates for stable and progressive metastatic disease from the community sample only were applied in the cost-effectiveness model.
Disutility estimates to capture the impact of treatment-related toxicity on patient well-being for the specific regimens of interest in colorectal cancer were not available. Estimates obtained from a utility study conducted in metastatic breast cancer were used as a proxy (Lloyd et al. 2006). These estimates were applied in the cost-effectiveness model as utility decrements to the proportion of patients experiencing each of the toxicities.
Table 4.27 summarises the utility estimates used in the analysis.
Table 4.27
Utility values used in the cost-effectiveness analysis.
Drug costs
Information on drug doses for each treatment regimen was obtained from the literature. For some regimens, variations in dose or administration schedule were observed across studies. If inconsistency across studies was noted, then GDG input was obtained to confirm which doses were most reflective of current UK clinical practice (Table 4.28).
Table 4.28
Drug doses and administration schedule.
Drug cost per cycle
Drug cost per cycle was calculated based on cost data obtained from the British National Formulary assuming no wastage and an average body surface area of 1.75 m2 (NICE Developing Costing Tools Methods Guide January 2008). When available, the unit cost of non-proprietary formulations was used. An estimate of the cost of administration was obtained from NHS Reference Costs. Drug costs and drug administration costs per cycle are summarised in Tables 4.29 and 4.30.
Table 4.29
Drug cost per cycle.
Table 4.30
Drug administration cost per cycle.
Number of cycles
The duration of treatment in terms of number of cycles was extracted from the clinical literature (Table 4.31). For most first-line studies, the total number of cycles was reported and used to derive the mean number of cycles per patient. For second-line treatment and for XELIRI as first-line treatment, studies typically only reported the median number of cycles. For these estimates, uncertainty was reflected assuming a uniform distribution in the cost-effectiveness model.
Table 4.31
Number of treatment cycles.
Cost of adverse event management
Estimates of the cost of management of febrile neutropenia and severe diarrhoea were based on NHS reference costs (Table 4.32). The cost of management of hand-foot syndrome was not factored into the model as this is typically managed by interruption of treatment or dose-reduction (Gressett et al. 2006) so it was not possible to assess the impact on cost or effectiveness specifically attributable to this toxicity alone.
Table 4.32
Cost of management for febrile neutropenia and grade 3/4 diarrhoea.
Supportive care
Healthcare resource use associated with supportive care for advanced cancer patients was obtained from a UK study of the DIN-Link database (Guest et al. 2005). Estimates of resource use for GP visits, district nurse visits, outpatient visits and hospitalisations were obtained from this study while unit costs were based on more recent sources (Table 4.33). Supportive care costs were applied throughout the model during both active treatment and progressive disease.
Table 4.33
Supportive care costs.
Sensitivity analyisis
The cost-effectiveness model was analysed by performing Monte Carlo simulation, sampling 30,000 times from all available distributions and MTC chains. Mean costs and QALYs for each of the ten treatment sequences are reported, as well as the incremental cost-effectiveness ratio (ICER) for all treatment strategies that are not ruled out by dominance. Parameter uncertainty is propagated through the model using probabilistic sensitivity analysis and is reflected in the results shown in the cost-effectiveness acceptability curve (CEAC). The CEAC shows the probability that each treatment sequence is cost effective over a range of willingness to pay thresholds.
In addition to the base case analysis, a sensitivity analysis was run to assess the impact of drug discounts on the results of the cost-effectiveness model. Information on drug discounts was obtained from the NHS Commercial Medicines Unit (CMU) electronic Market Information Tool (eMIT), which provides suppliers with access pertaining to the generic pharmaceutical products that are covered within framework agreements (Table 4.34). The discounted prices are based on an estimate of NHS hospital-sector annual usage from English trusts for a given drug, the average (weighted arithmetic mean) price paid for that drug over the last four months of the period and a measure of the variance of that average (Department of Health, NHS Commercial Medicines Unit). At the time this modelling exercise was undertaken, discounted drug prices were available for all drugs included in the analysis except capecitabine.
Table 4.34
Comparison of list price and discounted drug cost per cycle.
Cost-effectiveness analysis results
Base case analysis
The total costs and total QALYs in the base case analysis for each of the ten sequences of chemotherapy are summarised in Table 4.35. Costs ranged from £16,285 for FOLFOX - irinotecan up to £18,568 for FOLFOX – XELIRI. Total QALYs ranged from 0.819 for XELIRI – XELOX up to 0.941 for FOLFOX – FOLFIRI.
Table 4.35
Total costs and effectiveness by treatment strategy (in order of increasing cost).
Taking FOLFOX – irinotecan as the reference (least expensive) strategy, all other strategies were shown to be less effective and also more costly (i.e. dominated) except the sequence FOLFOX – FOLFIRI (Table 4.36 and Figure 4.11). Compared to the reference strategy, the sequence FOLFOX – FOLFIRI produces 0.019 more QALYs (equivalent to approximately 7 days in ‘perfect’ health) and incurs £2,051 in additional costs. This yields an incremental cost-effectiveness ratio (ICER) of £109,604/QALY, suggesting that at a willingness to pay (WTP) threshold of £20,000/QALY, the sequential strategy of FOLFOX – FOLFIRI is not cost effective.
Table 4.36
Incremental cost effectiveness results.
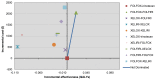
Figure 4.11
Cost-effectiveness plane showing all ten treatment sequences. The slope of the line connecting FOLFOX-irinotecan and FOLFOX-FOLFIRI indicates the incremental cost-effectiveness ratio (ICER).
Results presented above reflect the expected costs and effectiveness estimates for the treatment sequences of interest, however given uncertainty associated with many parameters in the model, we are also interested in the distribution over incremental costs, incremental effectiveness and the joint cost-effectiveness distribution (Briggs 2007). This is particularly relevant in the present analysis given that the differences in total QALYs between several strategies are small, with a number of data points lined up closely along the vertical axis of the cost-effectiveness plane which represents a difference in effectiveness of 0. Taking into account parameter uncertainty, probabilistic sensitivity analysis showed that simulation results for several sequences cross the vertical axis, suggesting there is a non-negligible probability that some sequences other than FOLFOX – FOLFIRI may also be equivalent or even more effective than the reference strategy. Cost-effectiveness acceptability curves (CEAC) can be used to show the probability of the various treatment options being cost effective over a range of WTP thresholds. The CEACs show that FOLFOX – irinotecan is consistently the strategy with the highest probability of being cost-effective, however as the WTP threshold increases, so does the probability that the sequences FOLFOX-FOLFIRI and XELOX-FOLFIRI are cost-effective (Figure 4.12).

Figure 4.12
Cost-effectiveness acceptability curves for the base case analysis.
Sensitivity analysis - drug discounts
If currently available data on the impact of price discounts for generic pharmaceutical products across the NHS are taken into account, FOLFOX-FOLFIRI remains the only non-dominated treatment strategy and the ICER falls to £47,801/QALY (Table 4.37).
Table 4.37
Cost-effectiveness results for non-dominated strategies taking into account price discounts for generic pharmaceutical products.
Probabilistic sensitivity analysis using discounted drug prices showed there is greater uncertainty about which strategy has the highest probability of being cost effective, as shown by the intersecting CEACs for FOLFOX-irinotecan, FOLFOX-FOLFIRI and XELOX-FOLFIRI over the range of WTP thresholds between approximately £20,000 and £40,000/QALY (Figure 4.13).
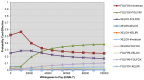
Figure 4.13
Cost-effectiveness acceptability curves using discounted drug prices.
Discussion
As the number of systemic treatment options for the management of colorectal cancer increases, and with more and more patients able to receive additional lines of chemotherapy, questions about the most effective way to use combinations and sequences of treatments have become relevant to current clinical practice. A systematic review was undertaken to identify new evidence that has become available since the publication of NICE Technology Appraisal 93 in 2005 on the clinical and cost-effectiveness of oxaliplatin and irinotecan-based chemotherapy. This evidence base was then used to conduct an integrated mixed treatment comparison and cost-effectiveness analysis to inform decision-making regarding optimal combinations and sequences of chemotherapy for the management of advanced colorectal cancer. Mixed treatment comparisons that draw on both direct and indirect evidence have become an important method to address decision problems that, often for feasibility reasons, cannot be practically answered by conducting further randomised controlled trials.
As a first-line treatment option, the mixed treatment comparison results suggest that FOLFOX was associated with a higher probability of being the most effective regimen with respect to both response rate and PFS. The small benefit in favour of FOLFOX was also evident when comparing second-line response rates, however was not the case with respect to second-line PFS. Perhaps most importantly, for the endpoint overall survival, the analysis showed no differences between the treatment sequences of interest.
The high level of uncertainty surrounding some of the results of the mixed treatment comparison are evident by the width of the 95% credible intervals. This is particularly evident in the estimates of effectiveness for XELIRI in first line where there was limited data available. To address the issue of sequencing of treatments, a decision was made to exclude evidence for which we could not be confident in determining that patients had received both first and second-line treatments that were of direct relevance to this analysis. The implication was that there were fewer studies to inform the second-line analysis of response rate, PFS and of overall survival. In order to connect the evidence network for sequences of treatment, a number of assumptions were required with respect to the equivalence of the effectiveness of the oral and iv fluoropyrimidine formulations. The validity of these assumptions were explored both by statistical methods and through discussion with GDG members.
The results of the mixed and indirect treatment comparisons were used as inputs to conduct a cost-effectiveness analysis. The cost-effectiveness analysis showed that when survival was quality-adjusted (taking into account both disease status and toxicities), the difference in total QALYs between the various sequential treatment strategies was in most cases modest. Taking FOLFOX-irinotecan as the reference (least costly) strategy, all other treatment sequences were found to be less effective (in terms of QALYs) and more costly except the sequence FOLFOX-FOLFIRI. The ICER comparing FOLFOX-FOLFIRI to FOLFOX-irinotecan was of £110K/QALY. When drug discounts were taken into account, the ICER for FOLFOX – FOLIRI vs FOLFOX-irinotecan fell to approximately £48K/QALY. Because of the small differences in total QALYs between strategies, it was important to consider how uncertainty may impact the results of the cost-effectiveness analysis. Taking parameter uncertainty and drug discounts into account, three strategies (FOLFOX-irinotecan, FOLFOX-FOLFIRI and XELOX-FOLFIRI) were associated with the highest probability of being cost effective.
References
- Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preference values associated with stage III colon cancer and adjuvant chemotherapy. Quality of Life Research. 2010;19:391–400. [PubMed: 20084462]
- Briggs A, Claxton K, Sculpher M. Decision modelling for healh economic evaluation. Oxford University Press; 2007.
- British National Formulary. [accessed 11 November 2010]. http://bnf
.org/bnf/index.htm. - Brooks S, Gelman A. General methods for monitoring convergence of iterative simulations. Journal of Computational and Graphical Statistics. 1998;7:434–55.
- Colucci G, Gebbia V, Paoletti G, Giuliani F, et al. Phase III Randomised Trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicentre study of the Gruppo Oncologico Dell’Italia Meridonale. Journal of Clinical Oncology. 2005;23(22):4866–4875. [PubMed: 15939922]
- Comella P, Massidda B, Filipelli G, Farris A, Natale D, et al. Randomised trial comparing biweekly oxaliplatin plus oral capecitabine versus oxaliplatin plus iv bolus fluorouracil/leucovorin in metastatic colorectal cancer patients: results of the Southern Italy Cooperative Oncology Study 0401. Journal of Cancer Research and Clinical Oncology. 2009;135:217–26. [PubMed: 18719941]
- Comella P, Massidda B, Filippelli G, Palmeri S, Natale D. Oxaliplating plus high dose folinic acid and 5 fluorouracil i.v. bolus (OXAFAFU) versus irinotecan plus high dose folinic acid and 5-fluorouracil i.v. bolus (IRIFAFU) in patients with metastatic colorectal carcinoma: a Southern Italy Cooperative Oncology Group phase III trial. Annals of Oncology. 2005;16(6):878–886. [PubMed: 15837702]
- Cunningham D, Sirohi B, Pluzanska A, et al. Two different first line 5 fluorouracil regimens with or without oxaliplatin in patients with metastatic colorectal cancer. Annals of Oncology. 2009;20:244–250. [PubMed: 18854549]
- Department of Health. Confirmation of Payment by Results (PbR) arrangements for 2010–11. http://www
.dh.gov.uk /en/Publicationsandstatistics /Publications /PublicationsPolicyAndGuidance /DH_112284. - Department of Health. National Reference Costs 2008–09. http://www
.dh.gov.uk /en/Publicationsandstatistics /Publications /PublicationsPolicyAndGuidance /DH_123459. - Department of Health, NHS Commercial Medicines Unit. Generic Pharmaceuticals electronic Market Information Tool (eMIT) WEB_VERSION_20100629. [accessed 21 December 2010]. http://www
.cmu.nhs.uk /Medicines/Generic/Pages/eMIT.aspx. - Diaz-Rubio E, Tabernero J, Gomez-Espana A, et al. Phase III study of Capecitabine plus oxaliplatin compared with continuous infusion fluorouracil as first line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the treatment if digestive tumours trial. Journal of Clinical Oncology. 2007;25(27):4224–4230. [PubMed: 17548839]
- Douillard J, Cunnigham D, Roth A, Navarro, James R, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. [PubMed: 10744089]
- Falcone A, Ricci S, Brunetti I, Pfanner E, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) as first line treatment for metastatic colorectal cance: the GRUPPO Ocologico Nord Ovest. Journal of Clinical Oncology. 2007;25:13. [PubMed: 17470860]
- Gennatas C, Papaxoninis G, Michalaki V, et al. A prospective randomised study of irinotecan (CPT-11), leucovorin (LV) and 5-fluorouracil (5FU) versus leucovorin and 5-fluorouracil in patients with advanced colorectal carcinoma. Journal of Chemotherapy. 2006;18(5):538–544. [PubMed: 17127232]
- Giacchetti S, Perpoint B, Zidani R, Le B, et al. Phase III multicenter randomised trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first line treatment of metastatic colorectal cancer. Journal of Clinical Oncology. 2000;18(1):136–147. [PubMed: 10623704]
- Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, et al. Randomised controlled trial of reduced dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: A North American intergroup trial. Journal of Clinical Oncology. 2006;24(21):3347–3353. [PubMed: 16849748]
- Goldberg R, Sargent D, Morton R, Fuchs C, et al. A randomised controlled trial of fluourouracil plus leucovorin, irinotecan and oxaliplation combinations in patients with previously untreated metastatic colorectal cancer. Journal of Clinical Oncology. 2004;22(1):23–30. [PubMed: 14665611]
- de Gramont A, Figer A, Seymour M, Homerin M, et al. Leucovorin and Flourouracil with or without oxaliplatin as first line treatment in advanced colorectal cancer. Journal of Clinical Oncology. 2000;18:2938–2947. [PubMed: 10944126]
- Gressett S, Stanford B, Hardwicke F. Management of hand-foot syndrome induced by capecitabine. Journal of Oncology Pharmacy Practice. 2006;12:131–141. [PubMed: 17022868]
- Guest J, Ruiz F, Greener M, Trotman I. Palliative care treatment patterns and associated costs of healthcare resource use for specific advanced cancer patients in the UK. European Journal of Cancer Care. 2006;15:65–73. [PubMed: 16441679]
- Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first line treatment of metastatic colorectal cancer: results of the TREE study. Journal of Clinical Oncology. 2008;26(21):3523–3529. [PubMed: 18640933]
- Kohne CH, De Greve J, Hartmann JT, Lang I, et al. Irinotecan combined with infusional 5-fluorouracil/folinic acid or Capecitabine plus celecoxib or placebo in the first line treatment of patients with metastatic colorectal cancer. EORTC study 40015. Annals of Oncology. 2008;19:920–926. [PubMed: 18065406]
- Kohne CH, van Cutsem E, Wils J, Bokemeyer C, et al. Phase III study of weekly high dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European organisation for research and treatment of cancer gastrointestinal group study. 2005. p. 40986. [PubMed: 15939923]
- Koopman M, Antonini NF, Douma J, Wals J, et al. Sequential versus combination chemotherapy with Capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet. 2007;370(9582):135–142. [PubMed: 17630036]
- Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. British Journal of Cancer. 2006;95:683–90. [PMC free article: PMC2360509] [PubMed: 16967055]
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine. 2004;23:3105–24. [PubMed: 15449338]
- Martoni AA, Pinto C, Di Fabio F, Lelli G, et al. Capecitabine plus oxaliplatin (XELOX) versus protracted 5-fluorouracil venous infusion plus oxaliplatin (PVIFOX) as first line treatment in advanced colorectal cancer: A GOAM phase II randomised study (FOCA trial). 2006. [PubMed: 17098421]
- Parmar M, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics In Medicine. 1998;17:2815–2834. [PubMed: 9921604]
- Porschen R, Arkenau HT, Kubica S, Greil R, et al. Phase III study of Capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: A final report of the AIO colorectal study group. Journal of Clinical Oncology. 2007;25(27):4217–4223. [PubMed: 17548840]
- Seymour MT, Maughan TS, Ledermann JA, Topham C, et al. Different strategies of sequential and combination chemotherapuy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet. 2007;370(9582):143–152. [PubMed: 17630037]
- Saltz L, Cox J, Blanke C, Rosen L, Fehrenbacher L, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. New England Journal of Medicine. 2000;343(13):905–915. [PubMed: 11006366]
- Souglakos J, Androulakis N, Sygrigos K, Polysos A. FOFLFOXIRI (folinic acid, 5 fluorouracil, oxaliplatin and irinotecan) versus (folinic acid, 5 fluorouracil and irinotecan) as first line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). British Journal of Cancer. 2006;94(6):798–805. [PMC free article: PMC2361370] [PubMed: 16508637]
- Spiegelhalter D, Best N, Carlin B, van der Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society (B). 2002;64:583–616.
- Sutton A, Ades A, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoecnomics. 2008;26:753–767. [PubMed: 18767896]
- Tierney J, Stewart L, Ghersi D, Burdett S, Sydes M. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;2007:8. [PMC free article: PMC1920534] [PubMed: 17555582]
- Tournigand C, Andre T, Achille R, Lledo G, Flesh M, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomised GERCOR study. Journal of Clinical Oncology. 2004;22(15):229–237. [PubMed: 14657227]
- Unit Costs of Health and Social Care. 2009. http://www
.pssru.ac.uk. - Williamson P, Smith C, Hutton J, Marson A. Aggregate data meta-analysis with time-to-event outcomes. Statistics In Medicine. 2002;21:3337–3351. [PubMed: 12407676]
Evidence Tables
Download PDF (2.0M)
4.4.2. What is the most effective treatment for advanced colorectal cancer patients when 5FU/FA based regimens are not tolerated or inappropriate?
Short Summary
There is no good quality evidence with which to address this question with the body of evidence comprising one randomised trial comparing raltitrexed to 5FU/LV from which the results of the raltitrexed arm will provide indirect evidence (Popov et al (2008)), one randomised phase II trial (Feliu et al (2005)) comparing raltitrexed + oxaliplatin with raltitrexed + irinotecan and a small number of non-randomised phase II trial (Aparicio et al (2005), Chiara et al (2005), Cortinovis et al (2004), Feliu et al (2004), Laudani et al (2004), Maroun et al (2006), Santini et al (2004), Vyzula et al (2006)).
For patients receiving treatment with raltitrexed, serious adverse events were reported in 16.3% of patients, deaths related to treatment were reported for 2.2% (n=20). The 5-year recurrence free survival rate was 47.8% (95% CI, 42.3% – 53%) for patients receiving raltitrexed. In the intention to treat population, the 5-year survival rate was 61.9% (95% CI 55.4% – 66.1%) (Popov, 2008).
Review Protocol
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with advanced or metastatic colorectal cancer | Raltitrexed (single agent or in combination with oxaliplatin or irinotecan) | No further Chemotherapy Irinotecan (single agent) | Response Progression Free survival Overall Survival Toxicity Quality of Life |
Following a systematic search of relevant data sources (see appendix .1), the information specialist created a database of potentially relevant studies. All titles and abstracts were sifted by a single reviewer. Queries about inclusion were clarified by the GDG topic subgroup. The full studies were then obtained and reviewed and relevant studies were included in the final evidence review.
All update searches were sifted by a single reviewer and the list of potentially relevant studies was also checked for irrelevant studies by the GDG subgroup. Only studies which all subgroup members were in agreement were excluded. The remaining studies were obtained and reviewed with relevant studies included in the final evidence review.
Include only studies published since 2004 as TA93 searches up to 2004
Include indirect evidence if necessary – studies comparing Raltitrexed to 5FU/FA based regimens are not directly applicable as they cannot randomise only patients intolerant to 5FU/FA, however the efficacy and toxicity of raltitrexed based treatments will not differ based on tolerability to 5FU/FA and therefore the raltitrexed arms of such trials will provide relevant but indirect evidence.
| Reasons for excluding studies: | Quality of the included studies |
|---|---|
| Expert Reviews | Systematic review of RCTs (n = 0) |
| Population not relevant to PICO | Systematic review of combined study designs (n =0) |
| Foreign language studies with no translations | Randomized controlled trial (n = 1) |
| Comparison not relevant to PICO | Non-randomised phase II trials (n=9) |
| Did not look at 5-FU intolerant patients | Prospective cross sectional study (n = 0) |
Volume of evidence
There was very little evidence available with which to address this topic, consisting primarily of single arm, non-comparator phase II studies. There was a single randomised trial comparing raltitrexed with 5FU/LV from which the data from the raltitrexed arm was deemed to provide indirect evidence.
Applicability
There are no studies which compare raltitrexed (single agent or in combination) to irinotecan (single agent) or to no further chemotherapy in patients that prove to be intolerant to 5FU/FA as such patients cannot currently be identified until they start 5FU/FA based treatment.
There are no studies comparing raltitrexed (single agent or in combination) to irinotecan (single agent) or to no further chemotherapy in any population.
There are a number of single arm, non-randomised, phase II trials which examine the efficacy and toxicities of raltitrexed in combination with oxaliplatin or irinotecan which provide indirect evidence.
There is a single randomised trial comparing raltitrexed to 5FU/LV from which the results of the raltitrexed arm will provide indirect evidence.
Evidence Statement
Raltitrexed Alone
From one randomised trial in which the risk of bias is not accurately assessable due to poor reporting (Popov, 2008), comparing raltitrexed with 5FU/LV, there is indirect evidence regarding the efficacy and toxicity of raltitrexed. The trial aimed to recruit a total of 2765 patients however early analysis of the first 647 patients showed a greater treatment completion rate in the 5FU/LV arm and more withdrawals due to serious adverse effects in the raltitrexed arm resulting in early closure of the trial with a total of 1921 patients recruited.
952 patients were randomised to the raltitrexed arm and received a median of 6 cycles of chemotherapy; the planned number of cycles was received by 42.4% (n=389) patients on the raltitrexed arm and when the study closed prematurely 28.5% (n=271) patients discontinued with raltitrexed treatment.
The median relative dose intensity of raltitrexed was 104% (range: 9–150%).
Adverse Events
From the raltitrexed arm of one randomised trial (Popov, 2008) serious adverse events were reported in 16.3% of patients, deaths related to treatment were reported for 2.2% (n=20) patients receiving raltitrexed of which 11 deaths were associated with a major protocol deviation and the majority of toxic deaths were reported from one cooperative group.
Recurrence
In the intention to treat population 38.9% of patients in the raltitrexed group relapsed or died while in the per protocol population, 43.1% of patients in the raltitrexed group relapsed or died. The 5-year recurrence free survival rate was 47.8% (95% CI, 42.3% – 53%) for patients receiving raltitrexed (Popov, 2008).
Survival
In the intention to treat population, 26.5% of patients in the raltitrexed group died during follow-up (median 49 months) and the 5-year survival rate was 61.9% (95% CI 55.4% – 66.1%).
In the per protocol population 29.5% of patients in the raltitrexed group and the 5-year survival rate was 62.6% (95% CI, 57.1% – 67.7%) in the raltitrexed group.
Raltitrexed plus Oxaliplatin
From a single phase II randomised trial comparing raltitrexed plus oxaliplatin to raltitrexed plus irinotecan (Feliu et al, 2005), overall response rate in the raltitrexed plus oxaliplatin arm was 46% (95% CI 29.5%–57.7%). Control of disease (CR, PR and SD) was achieved in 69% of patients and median time to progression was 8.2 months.
65% of patients experienced toxicity and there was one toxic death.
From three studies (Cortinovis et al (2004), Santini et al (2004), and Laudani et al (2004)) reported overall response rates ranging from 29%–45.5%.
From four studies (Cortinovis et al (2004), Vyzula et al (2006), Santini et al (2004), and Laudani et al (2004)) reported median time to progression ranged from 18 weeks – 7 months and reported median overall survival ranged from 54.4 weeks – 15 months.
Raltitrexed plus Irinotecan
From a single phase II randomised trial comparing raltitrexed plus oxaliplatin to raltitrexed plus irinotecan (Feliu et al, 2005), overall response rate in the raltitrexed plus irinotecan arm was 34% (95% CI 19.8%–48.4%). Control of disease (CR, PR, and SD) was achieved in 67% of patients median time to progression was 8.8 months.
70% of patients experienced toxicity and there were 3 toxic deaths.
From three studies (Feliu et al, 2004; Chiara et al, 2005 and Aparicio et al, 2005) the range of complete response was 27%–34%.
Feliu et al (2004) reported progression free survival of 11.1 months, Chiara et al (2005) reported a median progression free survival of 5 months and Aparicio et al (2005) reported a median time to progression of 6.3 months (95% CI 4–8.6 months).
Raltitrexed plus Oxaliplatin and Irinotecan
Maroun et al (2006), reported an overall response rate of 45% (95% CI, 31% – 68%), median time to progression of 7.3 months (95% CI 6.51–9.2 months) and median overall survival of 16.6 months (95% CI, 13.5 – 21.3).
References
- Aparicio J, Vincent JM, Maestu I, Bosch C, Galan A. First line treatment with Irinotecan and raltitrexed in metastatic colorectal cancer. Mature results of a multicentre phase II study. Oncology. 2005;68(1):58–63. [PubMed: 15809521]
- Chiara S, Nobile MT, Tomasello L, Acquati M. Phase II trial of irinotecan and raltitrexed in chemotherapy-naive advanced colorectal cancer. Anticancer Research. 2005;25(2B):1391–1396. [PubMed: 15865096]
- Cortinovis D, Bajetta E, Di Bartolomeo M, Dogini G. Raltitrexed plus oxaliplatin in the treatment of metastatic colorectal cancer. Tumori. 2004;90(2):186–191. [PubMed: 15237580]
- Feliu J, Salud A, Escudero P, Lopez-Gomez L. Irinotecan plus raltitrexed as first-line treatment in advanced colorectal cancer: a phase II study. British Journal of Cancer. 2004;90(8):1502–1507. [PMC free article: PMC2409728] [PubMed: 15083176]
- Feliu J, Castanon C, Salud A, Mel JR, Escudero P, Pelegrin A, Lopez-Gomez L, Ruiz M, Gonzalez E, Juarez F, Lizon J, Castro J, Gonzalez-Baron M. Phase II randomised trial of raltitrexed-oxaliplatin versus raltitrexed-irinotecan as first line treatment in advanced colorectal cancer. British Journal of Cancer. 2005;93:1230–1235. 2005. [PMC free article: PMC2361515] [PubMed: 16265344]
- Laudani A, Gebbia V, Leonardi V, Savio G. Activity and Toxicity of oxaliplatin plus raltitrexed in 5 fluorouracil refractory metastatic colorectal adenocarcinoma. Anti-Cancer Research. 2004;24(2C):1139–1142. [PubMed: 15154638]
- Maroun JA, Jonker D, Seymour L, Goel R, Vincent Phase I/II study of irinotecan (camptosar), oxaliplatin and raltitrexed (tomudex) (COT) in patients with advanced colorectal cancer. 2006. [PubMed: 16330204]
- Popov I, Carrato A, Sobrero A, Vincent M, Kerr D. Raltitrexed (Tomudex) versus standard leucovorin modulated bolus 5-fluorouracil: Results from the randomised phase III Pan-European Trial in adjuvant colon cancer 01 (PETACC-1). European Journal of Cancer. 2008;44(15):2204–2211. [PubMed: 18707870]
- Santini D, Massacesi C, D’Angelillo RM, Marcucci M. Raltitrexed plus weekly oxaliplation as first line chemotherapy in metastatic colorectal cancer: a multi centre non-randomised phase II study. Medical Oncology. 2004;21(1):59–66. [PubMed: 15034215]
- Vyzula R, Kocakova I, Demlova R, Kiss I. Raltitrexed plus oxaliplatin in the second line treatment of metastatic colorectal cancer. Neoplasma. 2006;53(2):119–127. [PubMed: 16575467]
Evidence Tables
Download PDF (252K)
4.5. Adjuncts to Chemotherapy in Unresectable Metastatic Disease
4.5.1. What is the most effective additional treatment to systemic chemotherapy to achieve cure or long-term survival in patients with apparently unresectable metastatic disease?
Short Summary
This topic aimed to determine whether patients originally identified as being incurable and with poor long term prognosis due to the presence of unresectable metastatic disease can achieve cure or long-term survival through treatment with systemic chemotherapy with or without additional treatments.
There was no comparative evidence with which to address this topic.
A systematic review of the literature identified no studies comparing any combination of the interventions of interest for this topic and although a small number of non comparative studies, investigating individual interventions were identified, it was considered that the evidenciary benefits of including such studies was low and would not inform any recommendations regarding the best form of treatment for this patient group.
Review Protocol
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Colorectal cancer patients with unresectable metastatic disease who have had systemic chemotherapy | Any combination of treatments including:
|
|
|
Following a systematic search of relevant data sources (see appendix .1), the information specialist created a database of potentially relevant studies. All titles and abstracts were sifted by a single reviewer. Queries about inclusion were clarified by the GDG topic subgroup. The full studies were then obtained and reviewed and relevant studies were included in the final evidence review.
All update searches were sifted by a single reviewer and the list of potentially relevant studies was also checked for irrelevant studies by the GDG subgroup. Only studies which all subgroup members were in agreement were excluded. The remaining studies were obtained and reviewed with relevant studies included in the final evidence review.
The GDG felt that with the number of interventions of interest, only high level should be reviewed in the first instance (randomised trials) and only if such evidence was not available should the searches be extended to include lower level studies (case series/observational studies).
Date limits set by the GDG were from 1996 onwards as this was when oxaliplatin and irinotecan became available for use and so anything prior to this would not be relevant to current UK practice.
Some other issues that were to be considered when reviewing the evidence included; surgical techniques (two-stage operations, portal vein embolisation), the addition of targeted therapies to chemotherapy, ablative techniques, and selective radiotherapy (Sirtex spheres and IMRT)
There was a need to consider sites of extrahepatic disease eg lung, nodal peritoneal and whether some have a better prognosis than others.
When reviewing evidence from clinical trials, specific issues to consider include:
- Level of staging at which treatment(s) applied
- Completion of planned care without alteration
- Patient response
- Symptom control and delay
- Adverse incidents
- Patient experience and quality of life
- Overall survival rate(s)
| Reasons for Exclusion of Individual Studies: |
|---|
| Study population not applicable to PICO |
| Interventions of interest not investigated |
| Outcomes of interest not investigated/reported |
| Foreign Language studies |
| No comparator to allow judgement on relative efficacy of treatments |
| Quality of the included studies |
| Systematic review of RCTs (n = 0) |
| Systematic review of combined study designs (n =0) |
| Randomized controlled trial (n = 0) |
| Prospective cross sectional study (n=0) |
| Case Series Studies (n=0) |
Volume of evidence
There was no evidence from randomised trials for any of the interventions under investigation and therefore the searches were extended to investigate whether there was any evidence from large cross sectional studies or case series. No comparative evidence relating to this topic could be found.
Applicability
There were no comparative studies identified as being relevant to the topic of interest and only a small number of non-comparative single arms studies which were considered to be of little use in the development of recommendations; the lack of a comparative study meant that there was no evidence available on which to base a decision on the relative efficacy and appropriateness of one intervention over another in this group of patients.
Consistency
It is not possible to comment on the consistency of the evidence as there are not enough data to compare.
Evidence Statement
There is topic represents a very specific, minority population of colorectal cancer patients. All patients in the population will be identified as having unresectable liver metastases and therefore there is little chance of curative surgery; however a small minority of patients will have their unresectable liver metastases converted to resectable liver metastases through the course of their treatment which, may lead to the patient undergoing curative surgery where it was initially deemed not to be a possible approach. There is no way to identify the patients who will ‘convert to being resectable’ and therefore there is a need to address whether it is appropriate to treat all initially unresectable metastasis as though they can be converted to being resectable through the course of normal treatment and whether any particular treatment approaches increase the chances of metastases becoming resectable.
A systematic review of the literature identified no studies comparing any combination of the interventions of interest for this topic and although a small number of non comparative studies, investigating individual interventions were identified, it was considered that the evidentiary benefits of including such studies was low and would not inform any recommendations regarding the best form of treatment for this patient group.
Updated Evidence
Update searches found a small number of low quality studies which were again non-comparative and investigating only single interventions.
- Management of Metastatic Disease - Colorectal CancerManagement of Metastatic Disease - Colorectal Cancer
Your browsing activity is empty.
Activity recording is turned off.
See more...