NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Yohimbine is an indole alkaloid derived from the bark of the Central African yohimbe tree (Pausinystalia yohimbe) that is widely used as therapy for erectile dysfunction. Yohimbine use has been associated with occasional severe adverse events, but has not been linked to serum enzyme elevations or clinically apparent acute liver injury.
Background
Yohimbine (yoe him' been) is a popular and widely used herbal which was traditionally used in Africa for multiple conditions including cough, fever, leprosy, heart disease and as an anesthetic, hallucinogen and aphrodisiac. In the West, yohimbe became popular as a sexual stimulant and used to treat erectile dysfunction. Yohimbe is derived from the bark of the African evergreen tree Pausinystalia yohimbe (synonym, P. johimbe). The bark extract has multiple constituents, but the focus of most interest has been yohimbine, an indole alkaloid which has been shown to be an alpha 2 adrenergic receptor antagonist. In animal models, yohimbine increases sexual activity and is likely to act by engagement and inhibition of the alpha 2 adrenergic receptors in the corpus cavernosum, causing sustained engorgement of the corporeal tissue of the penis. Yohimbine has been chemically synthesized and the synthetic form is what is currently marketed in the United States. The herbal bark extract may have other active components and is purported to be more potent and have more side effects. In clinical trials, synthetic yohimbine has had a consistent, although limited effect on erective dysfunction. Its effect on sexual desire is less well defined. The usual recommended dose of purified yohimbine is 5 to 10 mg three times a day. Drug tolerance or tachyphylaxis may occur. Side effects are usually mild and transient and are typical of alpha 2 adrenergic inhibition, including insomnia, anxiety, palpitations, chest pain, sweating, blurred vision and hypertension. Overdose can cause hypotension, tachycardia, seizures, paralysis and coma; deaths from overdose have been described.
Hepatotoxicity
In small clinical trials and case series, yohimbine therapy has not been linked to serum enzyme elevations or clinical liver disease. Although yohimbine is often found in weight loss and muscle building herbal combinations, it has not been associated with cases of clinically apparent acute liver injury.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Drug Class: Herbal and Dietary Supplements
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Yohimbine – Generic
DRUG CLASS
Herbal and Dietary Supplements
SUMMARY INFORMATION
Fact Sheet at National Center for Complementary and Integrative Health, NIH
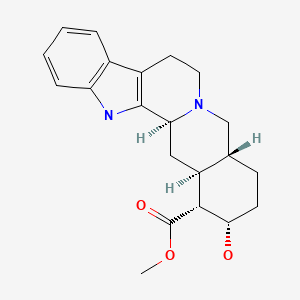
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Yohimbine | 146-48-5 | C21-H26-N2-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 05 April 2020
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott,1999: pp. 731-4.(Expert review of hepatotoxicity published in 1999; hepatotoxicity of herbal medications is discussed, but yohimbine is not mentioned).
- Seeff L, Stickel F, Navarro VJ. Hepatotoxicity of herbals and dietary supplements. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 631-58.(Review of hepatotoxicity of herbal and dietary supplements [HDS ]; yohimbine is not discussed).
- Yohimbe. In, PDR for Herbal Medicines. 4th ed. Montvale, New Jersey: Thomson Healthcare Inc. 2007: pp. 926-30.(Compilation of short monographs on herbal medications and dietary supplements).
- Carlsson C. Herbs and hepatitis. Lancet. 1990;336:1068. [PubMed: 1977040](Analysis of laboratory results from 395 patients found higher ALT levels among 53 patients taking herbals [55 U/L] than among those who did not [12 U/L]).
- Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246–52. [PubMed: 8418405](Among 1539 adults interviewed by telephone, 34% used an unconventional therapy during the previous 12 months, including 3% using herbal medicines).
- Sandler B, Aronson P. Yohimbine-induced cutaneous drug eruption, progressive renal failure, and lupus-like syndrome. Urology. 1993;41:343–5. [PubMed: 8470320](42 year old man developed skin rash one day after taking 3 tablets of yohimbine with fever, periorbital edema, erythroderma and desquamation, 19% eosinophils, progressive renal failure and polyserositis, requiring long term corticosteroids; no mention of hepatic involvement).
- De Smet PA, Smeets OS. Potential risks of health food products containing yohimbe extracts. BMJ. 1994;309:958. [PMC free article: PMC2541171] [PubMed: 7950687](Letter stressing the risks of yohimbine extracts and questioning the advisability of its general availability without a suitable warning labeling and lack of quality control, products often being mislabeled and the safe dosage not established, particularly in patients with autonomic dysfunction or heart disease).
- Vogt HJ, Brandl P, Kockott G, Schmitz JR, Wiegand MH, Schadrack J, Gierend M. Double-blind, placebo-controlled safety and efficacy trial with yohimbine hydrochloride in the treatment of nonorganic erectile dysfunction. Int J Impot Res. 1997;9:155–61. [PubMed: 9315493](Placebo controlled trial in 86 men with erectile dysfunction; side effects occurred in 30% of yohimbine recipients, but in only 10% of placebo recipients, but the nature and types of the side effects were not mentioned).
- Ernst E, Pittler MH. Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials. J Urol. 1998;159:433–6. [PubMed: 9649257](Systematic review of literature on yohimbine for erectile dysfunction identified 7 trials [419 patients], all of which showed evidence of efficacy; adverse events were uncommon; no mention of liver injury or ALT elevations).
- Morales A. Yohimbine in erectile dysfunction: the facts. Int J Impot Res. 2000;12 Suppl 1:S70–74. [PubMed: 10845767](Review of history, chemistry, mechanism of action, animal studies, clinical effects and safety of yohimbine, decrying the lack of scientifically rigorous clinical trials).
- Stedman C. Herbal hepatotoxicity. Semin Liver Dis. 2002;22:195–206. [PubMed: 12016550](Review and description of patterns of herbal induced liver injury, including discussion of potential risk factors, and herb-drug interactions; yohimbine is not mentioned).
- De Smet PAGM. Herbal remedies. N Engl J Med. 2002;347:2046–56. [PubMed: 12490687](Review of status and difficulties of herbal medications including lack of standardization, federal regulation, contamination, safety, hepatotoxicity and drug-herb interactions; specific discussion of 4 herbs with therapeutic promise: ginkgo, hawthorn, saw palmetto and St. John’s wort).
- Ernst E. Adulteration of Chinese herbal medicines with synthetic drugs: a systematic review. J Intern Med. 2002;252:107–13. [PubMed: 12190885](Systematic review of literature on adulteration of herbals with conventional medications, in 15 case reports and 2 cases series of 21 patients; included NSAIDs, corticosteroids, benzodiazepines, diuretics and antidiabetic medications, in up to 24% of products).
- Schiano TD. Hepatotoxicity and complementary and alternative medicines. Clin Liver Dis. 2003;7:453–73. [PubMed: 12879994](Comprehensive review of herbal associated hepatotoxicity, including common patterns of presentation; yohimbine not discussed).
- Pittler MH, Ernest E. Systematic review: hepatotoxic events associated with herbal medicinal products. Aliment Pharmacol Ther. 2003;18:451–71. [PubMed: 12950418](Systematic review of published cases of hepatotoxicity due to herbal medications listing 52 case reports or case series, most common agents being celandine [3], chaparral [3], germander [8], Jin Bu Huan [3], kava [1], Ma huang [3], pennyroyal [1], skullcap [2], Chinese herbs [9], valerian [1]; yohimbine not mentioned).
- Myers SP, Cheras PA. The other side of the coin: safety of complementary and alternative medicine. Med J Aust. 2004;181:222–5. [PubMed: 15310261](Discussion of the safety of complementary and alternative medicines).
- García-Cortés M, Borraz Y, Lucena MI, Peláez G, Salmerón J, Diago M, Martínez-Sierra MC, et al. Liver injury induced by “natural remedies”: an analysis of cases submitted to the Spanish Liver Toxicity Registry. Rev Esp Enferm Dig. 2008;100:688–95. [PubMed: 19159172](Among 521 cases of drug induced liver injury submitted to Spanish registry, 13 [2%] were due to herbals, none attributed to yohimbine).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 9% of cases were attributed to herbal medications; yohimbine was listed as present in some combinations implicated in cases, but not as the sole or main implicated agent).
- Haller C, Kearney T, Bent S, Ko R, Benowitz N, Olson K. Dietary supplement adverse events: report of a one-year poison center surveillance project. J Med Toxicol. 2008;4:84–92. [PMC free article: PMC3550135] [PubMed: 18570167](Surveillance of dietary supplement related poison control calls in California in 2006; identified 275 calls, yohimbine accounting for 10 cases with symptoms of anxiety, diaphoresis, hypertension, palpitations, headache and chest pain and one death).
- Giampreti A, Lonati D, Locatelli C, Rocchi L, Campailla MT. Acute neurotoxicity after yohimbine ingestion by a body builder. Clin Toxicol (Phila). 2009;47:827–9. [PubMed: 19640235](37 year old male bodybuilder developed fatigue, vomiting, seizures and coma, 2 hours after ingesting 5 grams of yohimbine [bilirubin normal; ALT 79 U/L, CPK 1042 U/L], resolving rapidly).
- Ho CC, Tan HM. Rise of herbal and traditional medicine in erectile dysfunction management. Curr Urol Rep. 2011;12:470–8. [PubMed: 21948222](Herbals are used increasingly as therapy of erectile dysfunction, often with little medical evidence of benefit; agents include ginseng, Epimedium, Tribulus terrestris, and yohimbine).
- Rebiere H, Guinot P, Civade C, Bonnet PA, Nicolas A. Detection of hazardous weight-loss substances in adulterated slimming formulations using ultra-high-pressure liquid chromatography with diode-array detection. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29:161–71. [PubMed: 22150438](Among 32 weight loss products available over-the-counter tested for contaminants, many contained caffeine and several had sibutramine, rimonabant, synephrine or yohimbine).
- Stickel F, Kessebohm K, Weimann R, Seitz HK. Review of liver injury associated with dietary supplements. Liver Int. 2011;31:595–605. [PubMed: 21457433](Review of current understanding of liver injury from herbals and dietary supplements focusing upon Herbalife and Hydroxycut products, green tea, usnic acid, Noni juice, Chinese herbs, vitamin A and anabolic steroids; no mention of yohimbine).
- Teschke R, Wolff A, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: a tabular compilation of reported cases. Liver Int. 2012;32:1543–56. [PubMed: 22928722](A systematic compilation of all publications on the hepatotoxicity of specific herbals identified 185 publications on 60 different herbs, herbal drugs and supplements but no publications implicated yohimbine).
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2013;37:3–17. [PubMed: 23121117](Systematic review of literature on HDS associated liver injury, but does not discuss or mention yohimbine).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 15 attributed to herbals or dietary supplements, but none to yohimbine).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to herbal supplements such as yohimbe).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, herbal and dietary supplements accounted for 146 cases [16%], but none were specifically linked to yohimbe).
- Seeff LB, Bonkovsky HL, Navarro VJ, Wang G. Herbal products and the liver: a review of adverse effects and mechanisms. Gastroenterology. 2015;148:517–532.e3. [PubMed: 25500423](Extensive review of possible beneficial as well as harmful effects of herbal products on the liver does not mention or discuss yohimbine).
- García-Cortés M, Robles-Díaz M, Ortega-Alonso A, Medina-Caliz I, Andrade RJ. Hepatotoxicity by dietary supplements: A tabular listing and clinical characteristics. Int J Mol Sci. 2016;17:537. [PMC free article: PMC4848993] [PubMed: 27070596](Listing of published cases of liver injury from HDS products, but does not list or mention yohimbine).
- Brown AC. Liver toxicity related to herbs and dietary supplements: Online table of case reports. Part 2 of 5 series. Food Chem Toxicol 2017; 107 (Pt A): 472-501. [PubMed: 27402097](Description of an online compendium of cases of liver toxicity attributed to HDS products, does not mention or list yohimbine).
- Tuerk PW, Wangelin BC, Powers MB, Smits JAJ, Acierno R, Myers US, Orr SP, et al. Augmenting treatment efficiency in exposure therapy for PTSD: a randomized double-blind placebo-controlled trial of yohimbine HCl. Cogn Behav Ther. 2018;47:351–71. [PubMed: 29448886](Among 26 combat veterans with post-traumatic stress disorder treated with prolonged exposure therapy and randomized to receive a single oral dose of yohimbine [21.6 mg] or placebo before an “imaginal exposure”, no adverse events were reported and the “overall side effect profile was not blatantly overt or prohibitively uncomfortable”).
- Meyerbröker K, Morina N, Emmelkamp PMG. Enhancement of exposure therapy in participants with specific phobia: A randomized controlled trial comparing yohimbine, propranolol and placebo. J Anxiety Disord. 2018;57:48–56. [PubMed: 29804894](Among 56 patients with phobias receiving visual reality exposure therapy [25 minutes twice, 3 times weekly] with yohimbine [15 mg], propranolol [40 mg] or placebo for 2 weeks, improvement in phobia was similar in all 3 groups and adverse events were not mentioned).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Microscopic and UPLC-UV-MS analyses of authentic and commercial yohimbe (Pausinystalia johimbe) bark samples.[J Nat Med. 2013]Microscopic and UPLC-UV-MS analyses of authentic and commercial yohimbe (Pausinystalia johimbe) bark samples.Raman V, Avula B, Galal AM, Wang YH, Khan IA. J Nat Med. 2013 Jan; 67(1):42-50. Epub 2012 Mar 9.
- Refractory priapism associated with ingestion of yohimbe extract.[J Med Toxicol. 2009]Refractory priapism associated with ingestion of yohimbe extract.Myers A, Barrueto F Jr. J Med Toxicol. 2009 Dec; 5(4):223-5.
- Determination of Yohimbine in Yohimbe Bark and Related Dietary Supplements Using UHPLC-UV/MS: Single-Laboratory Validation.[J AOAC Int. 2015]Determination of Yohimbine in Yohimbe Bark and Related Dietary Supplements Using UHPLC-UV/MS: Single-Laboratory Validation.Chen P, Bryden N. J AOAC Int. 2015 Jul-Aug; 98(4):896-901.
- Review Yohimbine in the treatment of erectile disorder.[Br J Clin Pract. 1994]Review Yohimbine in the treatment of erectile disorder.Riley AJ. Br J Clin Pract. 1994 May-Jun; 48(3):133-6.
- Review [Yohimbine in therapy of erectile dysfunction].[Fortschr Med. 1998]Review [Yohimbine in therapy of erectile dysfunction].Pittler MH. Fortschr Med. 1998 Jan 20; 116(1-2):32-3.
- Yohimbine - LiverToxYohimbine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...