NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Vericiguat is an orally available stimulator of soluble guanylate cyclase (sGC), a critical enzyme in the action of nitric oxide in inducing vascular relaxation, which is used in patients with chronic heart failure to reduce the risk of death and hospitalization. Vericiguat is associated with a low rate of serum aminotransferase elevations during therapy but has not been linked to instances of clinically apparent acute liver injury.
Background
Vericiguat (ver" i sig' ue at) is an orally available, small molecule stimulator of soluble guanylate cyclase which is used in patients with chronic heart failure to reduce the risk of death and hospitalizations for heart failure. Soluble guanylate cyclase (sGC) is responsible for the relaxation of smooth muscle cells and vasodilation mediated by nitric oxide, an important mediator of vascular tone. Patients with heart failure have a relative deficiency of nitric oxide, and guanylate cyclase activity is reduced. Stimulation of guanylate cyclase activity by vericiguat reduces blood pressure and improves cardiac efficiency. In large, randomized placebo controlled trials, therapy of patients with heart failure with vericiguat reduced cardiovascular mortality and decreased hospitalizations and chronic morbidity. Vericiguat was approved for use in the United States in 2021. Current formal indications are restricted to patients with symptomatic chronic heart failure with a left ventricular cardiac ejection fraction of less than 45% who have required hospitalization or outpatient treatment with intravenous diuretics for control of symptoms of heart failure. Vericiguat is available as tablets of 2.5, 5 and 10 mg under the brand name Verquvo. The recommended starting dose is 2.5 mg once daily (taken with food) with doubling of the dose every two weeks to a maintenance dose of 10 mg daily. Common side effects include symptomatic hypotension and anemia. Rare, but potential severe adverse reactions are embryo-fetal toxicity and a boxed warning cautions that women of child bearing potential should be on an effective means of birth control for the duration of treatment and at least one month thereafter.
Hepatotoxicity
In preregistration trials, serum aminotransferase elevations with mild bilirubin elevations were reported to occur in 2% of patients on vericiguat, but similar rates were reported with placebo therapy and the abnormalities resolved without dose modification or discontinuation of therapy. These abnormalities were considered the result of exacerbations of heart failure and congestive liver injury. There have been no published reports of clinically apparent liver injury with symptoms or jaundice attributed to vericiguat therapy, but the overall clinical experience with vericiguat has been limited.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which vericiguat might cause serum ALT elevations or liver injury is not known. Hepatic metabolism of vericiguat is via glucuronidation by UGT1A9 with minor involvement of the cytochrome P450 system. Vericiguat has not been associated with clinically significant drug-drug interactions.
Outcome and Management
The serum aminotransferase elevations that occur on vericiguat therapy are usually self-limited and rarely require dose modification or discontinuation. No instances of clinically apparent acute hepatitis, acute liver failure, chronic hepatitis or vanishing bile duct syndrome have been attributed to vericiguat. Cross sensitivity to liver related or other hypersensitivity reactions between vericiguat and other agents used in patients with heart failure (beta blockers, calcium channel blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers) has not been demonstrated, and is unlikely to occur.
Drug Class: Cardiovascular Agents, Heart Failure Agents
Other Drugs in the Subclass, Guanylate Cyclase Inhibitors: Riociguat
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Vericiguat – Verquvo®
DRUG CLASS
Cardiovascular Agents
Product labeling at DailyMed, National Library of Medicine, NIH
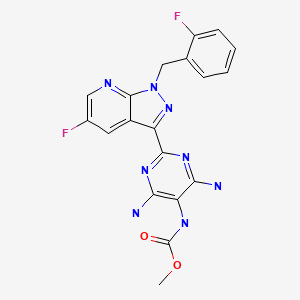
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Vericiguat | 1350653-20-1 | C19-H16-F2-N8-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 16 June 2023
Abbreviations used: sGC, soluble guanylate cyclase.
- Eschenhagen T. Therapy of heart failure. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 527-46.(Textbook of pharmacology and therapeutics).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2021/214377Orig1s000IntegratedR.pdf. (FDA website with product labels and the integrated review of the efficacy and safety of vericiguat that supported its approval for use in chronic heart failure mentions that liver injury arose in 25 of 2519 [1.0%] vericiguat-treated vs 12 of 2515 [0.5%] placebo-treated patients, but all cases had an alternative explanation, usually decompensated heart failure that improved despite continuing therapy; ALT elevations were listed as occur in 0.1% of treated patients and no treated patient died of liver failure [vs 3 placebo recipients]). - Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, et al. VICTORIA Study Group. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883–1893. [PubMed: 32222134](Among 5050 adults with symptomatic chronic heart failure and ejection fraction less than 45% who were treated with vericiguat [10 mg daily] or placebo, a composite endpoint of cardiovascular death or hospitalization for heart failure was less among vericiguat-treated subjects [35.5% vs 38.5%], and total and serious adverse event rates were similar, except for symptomatic hypotension [9.1% vs 7.9%] and anemia [7.6% vs 5.7%], while GGT elevations were less frequent with vericiguat [1.9% vs 2.6%]; no mention of ALT elevations or hepatotoxicity).
- Vericiguat (Verquvo) for heart failure. Med Lett Drugs Ther. 2021;63(1619):36–37. [PubMed: 33755653](Concise review of the mechanism of action, clinical efficacy, safety, and costs of vericiguat shortly after its approval for therapy of chronic heart failure in the US; mentions side effects of hypotension and anemia but does not mention ALT elevations or hepatotoxicity).
- Butler J, Zheng Y, Khan MS, Bonderman D, Lund LH, deFilippi CR, Blaustein RO, et al. VICTORIA Study Group. Ejection fraction, biomarkers, and outcomes and impact of vericiguat on outcomes across EF in VICTORIA. JACC Heart Fail. 2023;11:583–592. [PubMed: 37137660](In a secondary analysis of the VICTORIA trial of vericiguat for heart failure [Armstrong 2020], a lower baseline left ventricular ejection fraction was associated with a higher rate of clinical outcomes, but similar if not higher rates of response to vericiguat and similar adverse event rates; no mention of ALT elevations or hepatotoxicity).
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of the Efficacy and Safety of the Oral Soluble Guanylate Cyclase Stimulator: The VICTORIA Trial.[JACC Heart Fail. 2018]Review A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of the Efficacy and Safety of the Oral Soluble Guanylate Cyclase Stimulator: The VICTORIA Trial.Armstrong PW, Roessig L, Patel MJ, Anstrom KJ, Butler J, Voors AA, Lam CSP, Ponikowski P, Temple T, Pieske B, et al. JACC Heart Fail. 2018 Feb; 6(2):96-104. Epub 2017 Oct 11.
- Review Vericiguat: The First Soluble Guanylate Cyclase Stimulator for Reduction of Cardiovascular Death and Heart Failure Hospitalization in Patients With Heart Failure Reduced Ejection Fraction.[J Pharm Pract. 2023]Review Vericiguat: The First Soluble Guanylate Cyclase Stimulator for Reduction of Cardiovascular Death and Heart Failure Hospitalization in Patients With Heart Failure Reduced Ejection Fraction.Tran BA, Serag-Bolos ES, Fernandez J, Miranda AC. J Pharm Pract. 2023 Aug; 36(4):905-914. Epub 2022 Mar 31.
- Vericiguat for the treatment of heart failure: mechanism of action and pharmacological properties compared with other emerging therapeutic options.[Expert Opin Pharmacother. 2021]Vericiguat for the treatment of heart failure: mechanism of action and pharmacological properties compared with other emerging therapeutic options.Hulot JS, Trochu JN, Donal E, Galinier M, Logeart D, De Groote P, Juillière Y. Expert Opin Pharmacother. 2021 Oct; 22(14):1847-1855. Epub 2021 Jun 9.
- Review Vericiguat: A Review in Chronic Heart Failure with Reduced Ejection Fraction.[Am J Cardiovasc Drugs. 2022]Review Vericiguat: A Review in Chronic Heart Failure with Reduced Ejection Fraction.Kang C, Lamb YN. Am J Cardiovasc Drugs. 2022 Jul; 22(4):451-459. Epub 2022 May 28.
- Drug Treatment of Heart Failure with Reduced Ejection Fraction: Defining the Role of Vericiguat.[Drugs. 2021]Drug Treatment of Heart Failure with Reduced Ejection Fraction: Defining the Role of Vericiguat.Coats AJS, Tolppanen H. Drugs. 2021 Sep; 81(14):1599-1604. Epub 2021 Sep 3.
- Vericiguat - LiverToxVericiguat - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...