NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Valproate or valproic acid is a branched chain organic acid that is used as therapy of epilepsy, bipolar disorders and migraine headaches and is a well known cause of several distinctive forms of acute and chronic liver injury.
Background
Valproate (val proe' ate) is a carboxylic acid derivative that appears to act by increasing brain levels of gamma aminobutyric acid (GABA), the major inhibitory neurotransmitter in the human brain. Valproate has been shown to be effective in several forms of seizures. Valproate was approved for therapy of epilepsy in adults and children in 1978 and is currently one of the major anticonvulsant medications used. Current approved uses include both monotherapy and in combination with other anticonvulsants for complex absence seizures, complex partial seizures and mixed seizure types. Valproate is also used for prevention of migraine headaches and for bipolar disorders. The recommended dose of valproate varies by indication. The initial recommended dose for therapy of seizures is 10 to 15 mg/kg/day, with increases of 5 to 10 mg/kg/day weekly to achieve an optimal clinical response. Monitoring of drug levels is often recommended. Valproate is available in multiple generic and brand formulations as capsules, tablets and syrups and in delayed release forms of 125, 250 and 500 mg. Oral forms also include divalproex, which dissociates into valproate in the gastrointestinal tract and is available in capsules of 125 mg under the brand name Depakote. Valproate sodium is also available in parenteral formulations. Valproate has multiple side effects and clinically significant drug interactions; common side effects include headache, insomnia, nervousness, somnolence, tremor, blurred vision, nausea, weight gain and rash. Uncommon but potentially severe adverse reactions include pancreatitis, hepatotoxicity, embryo-fetal toxicity, birth defects, suicidal ideation and behaviors, thrombocytopenia, hyperthermia, hyperammonemia and severe drug-drug interactions.
Hepatotoxicity
Prospective studies suggest that 5% to 10% of persons develop ALT elevations during long term valproate therapy, but these abnormalities are usually asymptomatic and can resolve even with continuation of drug. Unlike phenytoin and carbamazepine, valproate does not induce elevations in serum GGT levels. More importantly and not uncommonly, valproate can cause several forms of clinically apparent hepatotoxicity. Indeed, more than 100 fatal cases of acute or chronic liver injury due to valproate have been reported in the literature. Three clinically distinguishable forms of hepatotoxicity (besides simple aminotransferase elevations) can occur with valproate.
The first syndrome is hyperammonemia with minimal or no evidence of hepatic injury. This syndrome typically presents with progressive and episodic confusion followed by obtundation and coma. The time to onset is often within a few weeks of starting valproate or increasing the dose, but it can present months or even years after starting the medication (Case 1). The diagnosis is made by the finding of elevations in serum ammonia with normal (or near normal) serum aminotransferase and bilirubin levels. Valproate levels are usually normal or minimally high. The syndrome resolves within a few days of stopping valproate, but may reverse more rapidly with carnitine supplementation or renal hemodialysis.
The second form of injury from valproate is an acute hepatocellular injury with jaundice, typically accompanied by hepatocellular or mixed pattern of enzyme elevations (Case 2). This acute liver injury pattern usually has its onset within 1 to 6 months of starting valproate. The pattern of serum enzyme elevations can be hepatocellular or mixed; sometimes the serum aminotransferase levels are not markedly elevated, despite the severity of injury.
Immunoallergic features (fever, rash, eosinophilia) are usually absent, but rare cases with prominent features of hypersensitivity have been reported (Case 3). Multiple instances of fatal acute hepatic failure due to valproate have been published and valproate is regularly listed as a cause of drug induced acute liver failure. Liver histology is distinctive and reveals a microvesicular steatosis with central lobular necrosis, mild to moderate inflammation and cholestasis. In cases with a prolonged course, fibrosis, bile duct proliferation and regenerative nodules may be present. Prospective studies using historical controls suggest that carnitine (particularly intravenously) may be beneficial if given soon after presentation.
The third form of hepatic injury due to valproate is a Reye-like syndrome described in children on valproate who develop fever and lethargy (suggestive of a viral infection) followed by confusion, stupor and coma, with raised ammonia levels and marked ALT elevations but normal or minimally elevated bilirubin levels. Metabolic acidosis is also common and the syndrome can be rapidly fatal. Valproate may simply be an aspirin-like agent capable of triggering Reye syndrome if it is being taken when the child develops either influenza or varicella infection.
All three forms of valproate hepatotoxicity have features of mitochondrial injury, and liver histology usually demonstrates microvesicular steatosis with variable amounts of inflammation and cholestasis. Young age (<2 years), presence of other neurological conditions and concurrent use of other anticonvulsants appear to be important risk factors for acute liver failure due to valproate. Valproate has been rarely associated with anticonvulsant hypersensitivity syndrome and generally is a safe alternative in patients with that syndrome caused by the aromatic anticonvulsants.
Likelihood score: A (well known cause of several forms of clinically apparent liver injury).
Mechanism of Injury
The mechanism of valproate hepatotoxicity is thought to be due to mitochondrial toxicity, perhaps from inhibition of beta oxidation and subsequent loss of mitochondrial function. Valproate is extensively metabolized by the liver and excreted in urine. Valproate therapy lowers tissue carnitine levels which may affect mitochondrial function and cause the hyperammonemia and microvesicular steatosis. Genetic factors also appear to be important, as valproate hepatotoxicity is more common in patients who are heterozygous for mutations in gamma polymerase, the predominant DNA polymerase found in mitochondria. Children with the Alpers-Huttenlocher syndrome who are homozygous for this mutation are at very high risk of developing valproate hepatotoxicity which is often fatal and responds poorly to liver transplantation. Alpers syndrome is characterized by progressive cerebral degeneration, seizures and cirrhosis and has been shown to be due to mutations in the gene for polymerase gamma, the enzyme responsible for mitochondrial DNA replication. Indeed, valproate is considered contraindicated in children with known or suspected Alpers-Huttenlocher syndrome.
Outcome and Management
Valproate hepatotoxicity varies in severity from minimal and asymptomatic ALT elevations to severe liver injury with progressive jaundice, hepatic synthetic dysfunction, coma and death. Monitoring of symptoms and serum aminotransferase levels is recommended for children for the first 6 months of valproate therapy. Valproate has not been associated with vanishing bile duct syndrome, but cases of cirrhosis with severe fibrosis have been attributed to chronic indolent hepatotoxicity from valproate. Importantly, carnitine appears to be a specific antidote for valproate hepatotoxicity and studies in animal models and human studies with historical controls suggest that prompt administration of carnitine (particularly when given intravenously) improves survival in acute valproate hepatotoxicity. Oral carnitine was approved for use in valproate toxicity in 1992 and an intravenous formulation in 1996. The typically recommended dose is 100 mg/kg intravenously over 30 minutes (but less than 6 grams), followed by 15 mg/kg every four hours until clinical improvement.
Drug Class: Anticonvulsants
CASE REPORTS
Case 1. Hyperammonemia due to valproate.(1)
A 47 year old woman with left frontal astrocytoma was admitted to the hospital for a syncopal episode and control of seizures. She was being treated with phenobarbital, valproate and levetiracetam. While hospitalized she was started on irradiation for her brain tumor. After a month in the hospital, her seizures were reasonably well controlled, but she developed unexplained somnolence and confusion. Liver tests were not abnormal, but serum ammonia levels were markedly elevated. Valproate was stopped and her mental status improved rapidly, ammonia levels decreasing into the normal range within 3 days. She subsequently improved and was discharged two weeks later.
Key Points
| Medication: | Valproate (450 mg/day) |
|---|---|
| Pattern: | (R=not applicable) |
| Severity: | 1+ (no jaundice) |
| Latency: | 3 months |
| Recovery: | 4 days |
| Other medications: | Phenobarbital, levetiracetam, phenergan (all were continued) |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Ammonia (µmol/L) | Comments |
|---|---|---|---|---|---|---|
| 0 | Valproate started: 500 mg daily | |||||
| 60 days | 15 | 50 | 0.4 | Hospital admission | ||
| 80 days | 15 | 58 | 0.2 | |||
| 90 days | 0 | 28 | 59 | 0.1 | 286 | |
| 91 days | 1 days | 362 | Valproate stopped | |||
| 92 days | 2 days | 70 | ||||
| 93 days | 3 days | 36 | ||||
| 96 days | 6 days | 74 | 74 | 0.1 | 20 | |
| 103 days | 13 days | 90 | 155 | 0.3 | 37 | |
| Normal Values | <42 | <115 | <1.2 | |||
Comment
A woman on multiple anticonvulsants developed somnolence and confusion during a prolonged hospitalization during which she was receiving radiation for a brain astrocytoma. While there were other possible reasons for her change in mental status, astute physicians tested her blood ammonia level, which was markedly elevated. Withdrawal of valproate led to a prompt improvement. This case represents the hyperammonemia of valproate that typically occurs without other evidence of hepatitis injury, although hyperammonemia is believed to be caused by the effects of valproate on hepatic mitochondrial function.
Case 2. Acute liver failure due to valproate.(1)
A 15 year old adolescent male with seizures being treated with valproate was admitted to the hospital having developed jaundice after a three week history of fatigue and body aches. He had developed seizures at age 9 and was treated with phenytoin for several years, but was switched to valproate (750 mg/day) because of reappearance of seizures approximately 4 months before admission. Three weeks before admission, he developed an influenza-like illness with fever, fatigue, body aches and diarrhea. The diarrhea resolved, but fatigue persisted. He was seen for evaluation and found to have elevations in liver tests. He was treated with azithromycin, but continued to do poorly developing behavioral changes and then jaundice. Valproate was stopped, and he was admitted to the hospital 4 days later because of worsening jaundice and confusion. On examination, he was disoriented and jaundiced but without signs of chronic liver disease, rash or fever. Serum bilirubin was 11.5 and INR 4.1 (Table). He was placed in intensive care. Tests for hepatitis A, B and C and autoantibody tests were negative, and serum ceruloplasmin and copper were normal. An abdominal ultrasound showed no evidence of biliary obstruction. His hepatic failure worsened and he was transferred to a liver transplant center, undergoing liver transplantation ~3 weeks after his initial admission.
Key Points
| Medication: | Valproate (250→750 mg/day) |
|---|---|
| Pattern: | Mixed (R=4.3) |
| Severity: | 5+ (acute liver failure requiring liver transplantation) |
| Latency: | 3 months |
| Recovery: | No |
| Other medications: | Azithromycin |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments | |
|---|---|---|---|---|---|---|
| 0 | Valproate started: 250 mg raised to 750 mg daily | |||||
| 3.5 months | -3 weeks | 287 | 441 | 1.4 | Given azithromycin | |
| 0 | 248 | 423 | 3.1 | |||
| 4 months | 5 days | 240 | 401 | 15.2 | Admission: INR 4.1 | |
| 8 days | 147 | 267 | 15.5 | |||
| 15 days | 89 | 161 | 23.8 | |||
| 5 months | 22 days | 51 | 237 | 26.7 | Liver transplantation | |
| Normal Values | <42 | <115 | <1.2 | |||
Comment
Acute liver failure due to valproate hepatotoxicity. The latency period of 3 months and only moderate elevation in serum aminotransferase levels are typical of the acute hepatotoxicity from valproate. The explant showed massive hepatic necrosis with regenerative areas and mild fibrosis. The incubation period to onset of valproate hepatotoxicity is usually 1 to 3 months. Symptoms developing during this period should lead to a prompt discontinuation of valproate and administration of carnitine. In this instance, the history of a viral-like illness immediately before the onset of liver injury suggests a Reye-like syndrome. However, the clinical course and outcome were more typical of the acute hepatitis-like syndrome caused by valproate.
Case 3. Drug rash with eosinophilia and acute liver failure due to valproate.(2)
A 26 year old man developed fever, rash and lymphadenopathy 1.5 months after starting valproic acid (500 mg twice daily) because of seizures arising after an intracerebral bleed. He had no history of liver disease, alcohol abuse, drug allergies or risk factors for viral hepatitis. His other medications included baclofen (500 mg three times daily), clemastine (2 mg twice daily) and acetaminophen (1 gram four times daily). Upon presentation, physical examination revealed a generalized papular-pustular rash, facial edema, lymphadenopathy and scleral icterus. Laboratory testing showed a total serum bilirubin of 10.5 mg/dL (direct 6.7 mg/dL), ALT 2800 U/L, AST 1219 U/L, alkaline phosphatase 456 U/L, GGT 216 U/L and prothrombin time 21.6 seconds. Acetaminophen levels were undetectable and valproate levels were "subtherapeutic". Tests for viral hepatitis and autoimmune conditions were unrevealing. Abdominal ultrasound demonstrated enlargement of the liver and spleen but no evidence of biliary obstruction. A skin biopsy was compatible with a drug-induced hypersensitivity rash. Valproate and acetaminophen were stopped upon admission and the seizure disorder was managed with levetiracetam. Prednisone was started and the skin rash, lymphadenopathy and facial edema resolved rapidly. Symptoms resolved within 6 weeks and laboratory tests returned to normal by 15 weeks, at which point prednisolone was stopped.
Key Points
| Medication: | Valproic acid (500 mg, twice daily) |
|---|---|
| Pattern: | Hepatocellular (R=17) |
| Severity: | 4+ (jaundice and prolonged prothrombin time) |
| Latency: | 1.5 months |
| Recovery: | 15 weeks |
| Other medications: | Acetaminophen, baclofen, clemastine |
Comment
A man with recent onset of seizures developed the syndrome of drug-rash with eosinophilia and systemic symptoms (DRESS) within 6 weeks of starting valproic acid. He had fairly severe liver injury with jaundice and prolongation of the prothrombin time, but without other evidence of hepatic failure (encephalopathy or ascites). He responded rapidly upon discontinuation of the valproate and institution of prednisolone. DRESS syndrome is well known to occur with aromatic anticonvulsant therapy (phenytoin, phenobarbital, carbamazepine, lamotrigine), but is rare with valproate therapy alone. DRESS and Stevens-Johnson syndrome have also been linked to acetaminophen therapy, but quite rarely. In this instance, acetaminophen may have contributed to the severity of liver injury but was probably not primarily responsible. While this case report lacked some necessary information (eosinophil count, hepatitis virus serology, autoimmune marker testing), it is fairly convincing and demonstrates the wide variability in clinical presentation of valproate induced liver injury.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Valproate – Generic, Depakene®, Depakote®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
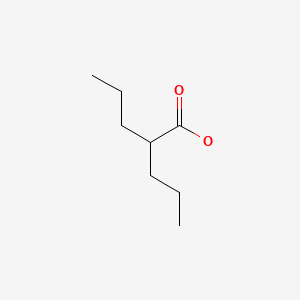
| Valproate | 99-66-1 | C8-H16-O2 |
|
CITED REFERENCES
- 1.
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159]
- 2.
- van Zoelen MA, de Graaf M, van Dijk MR, Bogte A, van Erpecum KJ, Rockmann H, Maarschalk-Ellerbroek LJ. Valproic acid-induced DRESS syndrome with acute liver failure. Neth J Med. 2012;70:155. [PubMed: 22516584]
ANNOTATED BIBLIOGRAPHY
Selected References updated: 31 July 2020
Abbreviations used: DRESS, drug rash with eosinophilia and systemic symptoms; SJS/TEN, Stevens-Johnson syndrome and toxic epidermal necrolysis.
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of valproate and liver injury published in 1999, estimated to occur in ~1:5,000 patients, more frequent in infants <2 years, onset insidious after 1-4 months of treatment [range 3 days-2 years], typically with ALT 3-25 times ULN, and with microvesicular steatosis and centrolobular necrosis on biopsy; high fatality rate).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-41.(Review of anticonvulsant induced liver injury mentions that the onset of liver injury from valproate is typically after 1 to 3 months, but is variable and instances of onset within days and after years of use have been published).
- Smith MD, Metcalf CS, Wilcox KS. Pharmacology of the epilepsies. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 303-26.(Textbook of pharmacology and therapeutics).
- Huttenlocher PR, Solitare GB, Adams G. Infantile diffuse cerebral degeneration with hepatic cirrhosis. Arch Neurol. 1976;33:186–92. [PubMed: 1252162](Description of 4 children [2 pair of siblings] who developed developmental delay and seizures starting between 1 and 3 years of age and progressing to disability and death in infancy, often with evidence of steatosis and cirrhosis, which is now often called Alpers-Huttenlocher syndrome [AHS]).
- Sackellares JC, Lee SI, Dreifuss FE. Stupor following administration of valproic acid to patients receiving other antiepileptic drugs. Epilepsia. 1979;20:697–703. [PubMed: 115681](4 cases of stupor due to valproate; 2 men and 2 women, ages 8 to 37 years, with onset 2-30 days after valproate added to other anticonvulsants, resolving in 3-7 days after stopping; normal valproate levels, ammonia levels not tested).
- Suchy FJ, Balistreri WF, Buchino JJ, Sondheimer JM, Bates SR, Kearns GL, Stull JD, Bove KE. Acute hepatic failure associated with the use of sodium valproate. Report of two fatal cases. N Engl J Med. 1979;300:962–6. [PubMed: 372803](Two fatal cases of valproate induced liver injury arising 6 and 8 weeks after starting valproate in 5 and 11 year olds [bilirubin 7.0 rising to 31 and 2.6 to 22 mg/dL, ALT 58 and 280 U/L, Alk P 15.7 and 500 U/L], autopsies showing submassive necrosis and microvesicular steatosis).
- Donat JF, Bocchini JA Jr, Gonzalez E, Schwendimann RN. Valproic acid and fatal hepatitis. Neurology. 1979;29:273–4. [PubMed: 372842](17 month old child with seizures developed jaundice one month after starting valproate [bilirubin 4.4 mg/dL, AST 130 U/L, ammonia 266 IU/L], progressing to hepatic failure and death one week later, autopsy showing changes suggestive of "viral or drug-induced hepatitis").
- Mathis RK, Hanson RF, Sharp HL. Hepatic failure from valproic acid. N Engl J Med. 1979;301:436. [PubMed: 379647](Letter in response to Suchy [1979] argues for mitochondrial injury as the cause of valproate hepatotoxicity, in that electron microscopy of liver biopsies from two children showed enlarged mitochondria with decreased matrix density, altered cristae, and increase in dense bodies).
- Gerber N, Dickinson RG, Harland RC, Lynn RK, Houghton LD, Antonias JI, Schimschock JC. Reye-like syndrome associated with valproic acid therapy. J Pediatr. 1979;95:142–4. [PubMed: 383927](12 year old girl developed fatigue and fever followed by progressive coma 2 months after starting valproate [bilirubin 1.3 mg/dL, ALT 78 U/L, prothrombin time 17 sec, ammonia 204 μmol/L], with acidosis, progressive coma and death; autopsy showing microvesicular fat in hepatocytes and renal tubular cells and centrolobular necrosis indicative of Reye syndrome).
- Jacobi G, Thorbeck R, Ritz A, Janssen W, Schmidts HL. Fatal hepatotoxicity in child on phenobarbitone and sodium valproate. Lancet. 1980;1(8170):712–3. [PubMed: 6103126](7 year old boy developed lethargy followed by coma 3 months after starting valproate [bilirubin rising to 17.5 mg/dL, ALT 60 U/L, ammonia 455 μmol/L], with acidosis and death, autopsy showing hepatic steatosis, centrolobular necrosis and cholestasis).
- Ware S, Millward-Sadler GH. Acute liver disease associated with sodium valproate. Lancet. 1980;2(8204):1110–3. [PubMed: 6107726](Five patients with valproate hepatotoxicity; 4 children, ages 2 to 9 years, treated with valproate for 3-5 months, developed acute liver injury, autopsies showing acute hepatic necrosis and microvesicular steatosis).
- Sodium valproate and the liver. Lancet. 1980;2(8204):1119–20. [PubMed: 6107730](Editorial in response to Ware [1980] commenting on the many reports of fatal valproate hepatotoxicity in young children with features of Reye syndrome).
- Le Bihan G, Bourreille J, Sampson M, Leroy J, Szekely AM, Coquerel A. Fatal hepatic failure and sodium valproate. Lancet. 1980;2(8207):1298–9. [PubMed: 6108469](17 year old girl developed fatigue 3 months after starting valproate [bilirubin 1.9 mg/dL, ALT 161 U/L, Alk P 190 U/L, prothrombin time 20 sec], with progressive hepatic failure, ascites, encephalopathy and liver transplant 1 month later).
- Young RS, Bergman I, Gang DL, Richardson EP Jr. Fatal Reye-like syndrome associated with valproic acid. Ann Neurol. 1980;7:389. [PubMed: 6769385](8 year old boy developed worsening seizures, fever and stupor 3 weeks after starting valproate [bilirubin not given, AST 564 U/L, ammonia 354 ug/dL], with hypoglycemia, acidosis and death within days, autopsy showing microvesicular fat in liver and renal tubules).
- Coulter DL, Allen RJ. Hyperammonemia with valproic acid therapy. J Pediatr. 1981;99:317–9. [PubMed: 6788925](Among "several hundred" children being treated with valproate, 11 developed stupor or coma, 8 of whom had elevated ammonia levels [mean 118.2 μmol/L], six of whom had normal liver tests and recovered within 1-3 days of stopping valproate, some tolerating lower doses).
- Gastaut H, Noel P. A case of fatal toxic hepatitis: recommendations for the administration of sodium valproate. Epilepsia. 1981;22:711–3. [PubMed: 6796404](2 year old boy developed stupor and coma 5 months after starting valproate [bilirubin 3 mg/dL, ALT 1975 U/L, ammonia 132 μg/dL], with progressive liver failure and death 5 days later, autopsy showing "ballooned hepatocytes with a clear cytoplasm but no steatosis").
- Zimmerman HJ, Ishak KG. Valproate-induced hepatic injury: analyses of 23 fatal cases. Hepatology. 1982;2:591–7. [PubMed: 6811394](Review of 23 fatal cases of valproate liver injury: ages 1 to 28 years, 56% male, onset after <1-4 months of 13-71 mg/kg/day; most patients were taking other anticonvulsants; symptoms were fatigue followed by jaundice, ascites and coma; no rash or eosinophilia; ALT levels were usually <300 U/L, AST>ALT, bilirubin 1.0-36 mg/dL, protime and ammonia usually elevated. Histology showed microvesicular steatosis, centrizonal and ballooning necrosis; some patients already had cirrhosis).
- Zafrani ES, Berthelot P. Sodium valproate in the induction of unusual hepatotoxicity. Hepatology. 1982;2:648–9. [PubMed: 6811395](Editorial in response to Zimmerman and Ishak [1982] describing 31 fatal cases from Europe, ages 9 months to 29 years, twice as common in children than adults, no rash or eosinophilia, onset with vomiting and weakness, jaundice late, ALT not very high, histology showing fat and centrilobular necrosis).
- Stricker BH. Ned Tijdschr Geneeskd. 1982;126:2111–3. [Liver damage caused by valproic acid] Dutch. [PubMed: 6817146](Review of valproate hepatotoxicity, mentioning that there have been at least 50 fatal cases reported in the literature, clinical features can resemble Reye syndrome and liver histology reveals microvesicular steatosis and centrolobular necrosis).
- Ohtani Y, Endo F, Matsuda I. Carnitine deficiency and hyperammonemia associated with valproic acid therapy. J Pediatr. 1982;101:782–5. [PubMed: 6813444](Testing of 14 patients on valproate and 11 on other anticonvulsants for carnitine levels reported low levels in those on valproate [33 vs 49 nM], correlating with dose and with ammonia levels; carnitine supplementation was associated with rapid decrease in ammonia levels, from 144 to 71 μg/dL).
- Itoh S, Yamaba Y, Matsuo S, Saka M, Ichinoe A. Sodium valproate-induced liver injury. Am J Gastroenterol. 1982;77:875–9. [PubMed: 6814241](37 year old woman developed fever and abdominal pain 1 month after starting valproate [bilirubin 2.2 mg/dL, ALT 1550 U/L, Alk P 206 U/L], with rapid improvement upon stopping and sharp rise in rise in bilirubin and ALT within one day of rechallenge; liver biopsy showed microvesicular steatosis, crystalline inclusions in mitochondria and marked centrilobular necrosis).
- Zaret BS, Beckner RR, Marini AM, Wagle W, Passarelli C. Sodium valproate-induced hyperammonemia without clinical hepatic dysfunction. Neurology. 1982;32:206–8. [PubMed: 6798491](3 cases of hyperammonemia in adults taking valproate, ages 20 to 51 years, onset days to years after starting, ammonia 56-146 μM, rapid reversal with stopping).
- Böhles H, Richter K, Wagner-Thiessen E, Schafer H. Decreased serum carnitine in valproate induced Reye syndrome. Eur J Pediatr. 1982;139:185–6. [PubMed: 6819143](3 year old girl developed fatal liver injury ~3 months after starting valproate [bilirubin 4.3 rising to 23 mg/dL, ALT 34 to 82 U/L, Alk P 350 U/L, ammonia 89 μg/dL, glucose 23 mg/dL, low carnitine levels and abnormal valproate metabolites]; hypothesized that injury was due to carnitine deficiency related inhibition of mitochondrial beta oxidation).
- Sugimoto T, Nishida N, Yasuhara A, Ono A, Sakane Y, Matsumura T. Reye-like syndrome associated with valproic acid. Brain Dev. 1983;5:334–7. [PubMed: 6412580](13 year old girl on chronic valproate therapy had seizures and was admitted in shock with high serum ammonia 400 µM, ALT 1454 U/L, bilirubin 1.2 mg/dL, LDH 29,184 U/L, died 3 days later; questioned whether it was due to Reyes syndrome vs ischemic hepatitis).
- de Wolff FA, Peters AC, van Kempen GM. The effects of valproic acid on liver function. Arch Toxicol Suppl. 1983;6:369–73. [PubMed: 6138013](Serum levels of GGT were not elevated in 32 children with epilepsy being treated with valproate).
- Perucca E, Hedges A, Makki KA, Ruprah M, Wilson JF, Richens A. A comparative study on the relative enzyme inducing properties of anticonvulsant drugs in epileptic patients. Br J Clin Pharmacol. 1984;18:401–10. [PMC free article: PMC1463658] [PubMed: 6435654](Cross sectional study of antipyrine clearance in 122 patients with epilepsy, found dose dependent increase in clearance in those on phenytoin, phenobarbital and carbamazepine, but not on valproate).
- Keene DL, Humphreys P, Carpenter B, Fletcher JP. Valproic acid producing a Reye-like syndrome. J Canad Sci Neurol 1982; 435-7. [PubMed: 6817907](3 year old boy with severe seizure disorder and status epilepticus developed coma and acute liver failure on valproate [2 years], phenytoin and ethosuximide with AST >4500 U/L, normal bilirubin and valproate levels; subsequently had worsening neurological status, liver biopsy showing macro and microvesicular fat, ballooning, cholestasis and inflammation, similar but not completely typical of Reyes syndrome).
- Coulter DL. Carnitine deficiency: a possible mechanism for valproate hepatotoxicity. Lancet. 1984;1:689. [PubMed: 6142383](Letter in which author proposes that valproate lowers carnitine levels, which impairs metabolism of fatty acids and mitochondrial function causing hyperammonemia and liver injury, recommends supplementation).
- Green SH. Sodium valproate and routine liver function tests. Arch Dis Child. 1984;59:813–4. [PMC free article: PMC1628698] [PubMed: 6435543](Editorial suggesting that monitoring liver tests is unlikely to be helpful in predicting idiosyncratic reactions to valproate).
- Powell-Jackson PR, Tredger JM, Williams R. Hepatotoxicity to sodium valproate: a review. Gut. 1984;25:673–81. [PMC free article: PMC1432377] [PubMed: 6428980](Review of literature and own cases of valproate liver injury; describe three forms, hepatitis-like syndrome arising 4-16 weeks after starting therapy with high fatality rate; a Reyes-like syndrome and hyperammonemia with minimal liver injury).
- Murphy JV, Marquardt KM, Shug AL. Valproic acid associated abnormalities of carnitine metabolism. Lancet. 1985;1:820–1. [PubMed: 2858698](6 month old infant with Reyes-like syndrome [ALT 9690 U/L] had low carnitine levels that increased with recovery; testing 13 asymptomatic children on valproate found low levels ~40 μM compared to 50 μM in controls).
- Blaw ME, Belknap WM. Valproate hepatotoxicity. Pediatr Neurol. 1985;1:320. [PubMed: 3939748](Report a fatal case of valproate hepatotoxicity [no specific information given], recommending prompt investigation if symptoms develop on valproate, particularly during first month of therapy).
- Dickinson RG, Bassett ML, Searle J, Tyrer JH, Eadie MJ. Valproate hepatotoxicity: a review and report of two instances in adults. Clin Exp Neurol. 1985;21:79–91. [PubMed: 3939614](Review of literature and 2 case reports; 19 year old male with drowsiness starting 2 months after increasing valproate dose [bilirubin ~5.0 mg/dL, AST 3x ULN, Alk P ~4x ULN], resolving rapidly upon stopping; 25 year old man with liver injury arising 1 month after starting valproate with biopsy showing centrolobular necrosis).
- Møller P, Henriksen O. Tidsskr Nor Laegeforen. 1986;106:940–1. [Severe liver involvement during use of sodium valproate] Norwegian. [PubMed: 3088761](27 year old man developed agitation within days of starting valproate [ALT 313 U/L, bilirubin and Alk P normal], which rapidly improved on stopping, but the patient died suddenly 6 weeks later).
- Ratnaike RN, Schapel GJ, Purdie G, Rischbieth RHC, Hoffmann S. Hyperammonaemia and hepatotoxicity during chronic valproate therapy: enhancement by combination with other antiepileptic drugs. Br J Clin Pharmacol. 1986;22:100–3. [PMC free article: PMC1401093] [PubMed: 3091053](Cross sectional analysis of ammonia levels and liver tests in 81 patients with epilepsy; higher average levels of ammonia in patients on valproate [37.1 vs 28.7 μmol], slightly higher rate of elevations in those receiving other agents [phenytoin, carbamazepine] as well).
- Colletti RB, Trainer TD, Krawisz BR. Reversible valproate fulminant hepatic failure. J Pediatr Gastroenterol Nutr. 1986;5:990–4. [PubMed: 3098946](12 year old boy developed fever and rash followed by confusion 2-3 months after starting valproate [bilirubin 4.6 mg/dL, ALT 2425 U/L, Alk P 289 U/L, ammonia 66 μmol/L, prothrombin time 15 sec], improvement beginning a week after stopping, values ultimately normal; liver biopsy showing microvesicular steatosis and focal necrosis).
- Laub MC, Paetzke-Brunner I, Jaeger G. Serum carnitine during valproic acid therapy. Epilepsia. 1986;27:559–62. [PubMed: 3093213](Cross sectional study of 21 patients on valproate, 21 on other anticonvulsants, and 21 controls; found lower carnitine levels in patients on valproate, but small differences and no response in one patient with fatal hyperammonemia).
- Dreifuss FE. Fatal liver failure in children on valproate. Lancet. 1987;1:47–8. [PubMed: 2879132](Letter pointing out safety of valproate if used in children above the age of 2, without familial history of liver disease, in low doses without concurrent aspirin or during febrile illness and rapid investigation of symptoms).
- Scheffner D, König S, Rauterberg-Ruland I, Kochen W, Hofmann WJ, Unkelbach S. Fatal liver failure in 16 children with valproate therapy. Epilepsia. 1988;29:530–42. [PubMed: 3137017](Analysis of 16 patients with fatal valproate hepatotoxicity in Germany between 1977 and 1987; ages 1-17, 6 on monotherapy, onset occurred mostly in first 6 months; typical presentation with nausea, abdominal pain and apathy followed by jaundice, biopsies show micro- and macro-vesicular fat and centrilobular necrosis, often with fibrosis; estimated fatality rate 1:5,000, recommended monitoring for first 6 months).
- Dreifuss FE, Santilli N, Langer DH, Sweeney KP, Moline KA, Menander KB. Valproic acid hepatic fatalities: a retrospective review. Neurology. 1987;37:379–85. [PubMed: 3102998](Review of all the reports of fatal liver injury from valproate reported to drug company and FDA between 1978 and 1984 found 37 cases; highest number in children <2 years, with coincidental neurologic problems and polytherapy with other anticonvulsants; onset usually within first 90 days; variable presentations and laboratory abnormalities).
- Iinuma K, Hayasaka K, Narisawa K, Tada K, Hori K. Hyperamino-acidaemia and hyperammonaemia in epileptic children treated with valproic acid. Eur J Pediatr. 1988;148:267–9. [PubMed: 3145882](Among 75 children with epilepsy, serum ammonia levels were elevated in 20% [8/41] of those on valproate, but in none of 34 children taking other anticonvulsants).
- Dreifuss FE, Langer DH, Moline KA, Maxwell JE. Valproic acid hepatic fatalities. II. US experience since 1984. Neurology. 1989;39:201–7. [PubMed: 2492646](During further follow up of national data, there was an increase use of valproate, but a decrease in incidence of valproate hepatotoxicity; thus in 1985-86, only four cases were identified, 3 with polytherapy, 3 in children with other medical conditions, clinical onset occurred after 60-105 days of therapy).
- Kuhara T, Inoue Y, Matsumoto M, Shinka T, Matsumoto I, Kawahara N, Sakura N. Markedly increased omega-oxidation of valproate in fulminant hepatic failure. Epilepsia. 1990;31:214–7. [PubMed: 2108017](Abnormal metabolites of valproate found in urine of child with fatal Reyes-like syndrome on long term valproate [ALT 5330, LDH 18405, ammonia 212 mg/L and rising bilirubin], abnormalities suggested increase in P-450 metabolism of valproate).
- Appleton RE, Farrell K, Applegarth DA, Dimick JE, Wong LT, Davidson AG. The high incidence of valproate hepatotoxicity in infants may relate to familial metabolic defects. Can J Neurol Sci. 1990;17:145–8. [PubMed: 2113424](Two unrelated infants developed fatal, acute liver failure after 2-3 days of valproate, both had siblings with developmental delay and liver abnormalities and steatosis not on valproate, suggesting that valproate may not have been responsible for liver injury in the index patients).
- Wyllie E, Wyllie R. Routine laboratory monitoring for serious adverse effects of antiepileptic medications: the controversy. Epilepsia. 1991;32 Suppl 5:S74–9. [PubMed: 1743173](The effectiveness of monitoring serum enzyme levels in patients starting anticonvulsant therapy in preventing acute liver failure is controversial and unproven; this approach is most appropriate for valproate, but probably only during the first 6 months and in high risk subjects).
- Binek J, Hany A, Egloff B, Heer M. Schweiz Med Wochenschr. 1991;121:228–33. [Acute fatal liver insufficiency due to valproic acid therapy] German. [PubMed: 2008603](21 year old man developed jaundice 12 weeks after starting valproate [bilirubin 10.2 mg/dL, ALT 500 U/L, Alk P 348 U/L, prothrombin index 19%], followed by progressive multiorgan failure and death; autopsy showing massive necrosis and steatosis).
- Bell EA, Shaefer MS, Markin RS, Wood RP, Langnas AN, Stratta RJ, Shaw BW Jr. Treatment of valproic acid-associated hepatic failure with orthotopic liver transplantation. Ann Pharmacother. 1992;26:18–21. [PubMed: 1606338](23 year old woman developed lethargy and confusion followed by coma 6 weeks after starting valproate [bilirubin 3.1 mg/dL, ALT 10,640 U/L, Alk P 197 U/L, prothrombin time 54 sec, ammonia 127 umol/L], with severe metabolic acidosis and progressive liver failure, undergoing liver transplant within 48 hours of presentation).
- Murphy JV, Groover RV, Hodge C. Hepatotoxic effects in a child receiving valproate and carnitine. J Pediatr. 1993;123:318–20. [PubMed: 8345435](17 month old child with developmental delay developed severe liver injury arising 9 weeks after starting valproate [bilirubin 2.3 rising to 26.5 mg/dL, ALT 552 U/L, Alk P 360 U/L, prothrombin time 24.7 sec], with progressive hepatic failure and death 6 weeks later, autopsy showing massive necrosis and fibrosis).
- Siemes H, Nau H, Schultze K, Wittfoht W, Drews E, Penzien J, Seidel U. Valproate (VPA) metabolites in various clinical conditions of probable VPA-associated hepatotoxicity. Epilepsia. 1993;34:332–46. [PubMed: 8453944](Analysis of plasma valproate levels and its metabolites in 470 patients with epilepsy found no consistent level or pattern of metabolites in patients with liver injury compared to those without).
- König SA, Simes H, Blaker F, Boenigk E, Gross-Selbeck G, Hanefeld F, Haas N, et al. Severe hepatotoxicity during valproate therapy: an update and report of eight new fatalities. Epilepsia. 1994;35:1005–15. [PubMed: 7925143](Review and update of fatal cases of valproate hepatotoxicity from Germany-Switzerland, risk factors were found to be age <2 years and cases were rare in patients above 18 years; onset can occur >6 months after onset, often presenting with worsening seizures, nausea and apathy after a febrile illness).
- Plantin P, Cartier H, Le Bihan G, Clouard P, Lellouche F, Leroy JP. Presse Med. 1995;24:1624. [Drug hypersensitivity syndrome during treatment with valproic acid] French. [PubMed: 8545377](41 year old woman developed fever followed by rash 6 weeks after starting valproate [bilirubin 6.0 mg/dL, ALT 559 U/L, eosinophils 20%], resolving within 4 weeks of stopping).
- Krähenbühl S, Mang G, Kupferschmidt H, Meier PJ, Krause M. Plasma and hepatic carnitine and coenzyme A pools in a patient with fatal, valproate induced hepatotoxicity. Gut. 1995;37:140–3. [PMC free article: PMC1382786] [PubMed: 7672665](39 year old woman with bipolar disorder developed stupor 4 months after starting valproate [bilirubin 8.5 mg/dL, ALT 35 U/L, prothrombin index 39%, ammonia 75 µmol/L] and deteriorated after a “cardiovascular collapse”, dying of multiorgan failure).
- Böhles H, Sewell AC, Wenzel D. The effect of carnitine supplementation in valproate-induced hyperammonaemia. Acta Paediatr. 1996;85:446–9. [PubMed: 8740302](In an uncontrolled study, carnitine supplementation was followed by decreases in ammonia levels and increases in free carnitine levels in 14 patients with hyperammonia on valproate).
- Bryant AE, Dreifuss FE. Valproic acid hepatic fatalities, III: US experience since 1986. Neurology. 1996;46:465–9. [PubMed: 8614514](Third retrospective study of US experience with fatal hepatotoxicity from valproate summaries 29 cases; risk factors were age <2 years and polytherapy [~1:600 treated subjects]; risk decreased with age so that in adults risk is ~1:30,000, but risk in adults also increased with polytherapy).
- Wallace SJ. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf. 1996;15:378–93. [PubMed: 8968693](Systematic review; ALT elevations occur in 4% of children on phenytoin, 6% on valproate, 1% on carbamazepine and not reported on tiagabine or gabapentin).
- Schwabe MJ, Dobyns WB, Burke B, Armstrong DL. Valproate-induced liver failure in one of two siblings with Alpers disease. Pediatr Neurol. 1997;16:337–43. [PubMed: 9258971](Siblings with Alpers syndrome developed progressive developmental delay and seizures, one dying of acute liver failure 1 month after starting valproate).
- Pessayre D, Mansouri A, Haouzi D, Fromenty B. Hepatotoxicity due to mitochondrial dysfunction. Cell Biol Toxicol. 1999;15:367–73. [PubMed: 10811531](Review of the role of mitochondrial dysfunction in drug induced liver injury focusing upon valproate).
- Hamer HM, Morris HH. Hypersensitivity syndrome to antiepileptic drugs: a review including new anticonvulsants. Cleve Clin J Med. 1999;66:239–45. [PubMed: 10199060](Hypersensitivity occurs in 1-5/10,000 users of aromatic anticonvulsants, higher risk in African Americans and affected siblings; liver involvement common, but most cases anicteric; other manifestations include facial edema, lymphadenopathy, bone marrow aplasia, pseudolymphoma, thyroiditis, interstitial nephritis).
- McLaughlin DB, Eadie MJ, Parker-Scott SL, Addison RS, Henderson RD, Hooper WD, Dickinson RG. Valproate metabolism during valproate-associated hepatotoxicity in a surviving adult patient. Epilepsy Res. 2000;41:259–68. [PubMed: 10962217](Valproate is a simple branched chain fatty acid, but has complex metabolism undergoing β-oxidation; 33 year old man developed jaundice and marked ALT elevations [~100x ULN] 7 months after starting valproate, initially had high oxo-VPA relative to valproate suggesting impaired β-oxidation and mitochondrial dysfunction; cause or effect unclear).
- Picart N, Périole B, Mazereeuw J, Bonafé JL. Presse Med. 2000;29:648–50. [Drug hypersensitivity syndrome to valproic acid] [PubMed: 10780197](28 year old woman developed fever and maculo-papular generalized rash 3 weeks after starting valproate with adenopathy, eosinophilia and ALT 176 U/L, but normal bilirubin and Alk P, rapid resolution and positive skin reaction to valproate 4 months later).
- Delarue A, Paut O, Guys JM, Montfort MF, Lethel V, Roquelaure B, Pellissier JF, et al. Inappropriate liver transplantation in a child with Alpers-Huttenlocher syndrome misdiagnosed as valproate-induced acute liver failure. Pediatr Transplant. 2000;4:67–71. [PubMed: 10731063](3 year old boy developed acute liver failure within a few months of starting valproate for seizures [bilirubin not given; ALT 196 U/L, prothrombin index 24%], with progressive hepatic failure and liver transplantation within 3 weeks of onset, child dying 4 months later of progressive neurological deterioration).
- Kayihan N, Nennesmo I, Ericzon BG, Németh A. Fatal deterioration of neurological disease after orthotopic liver transplantation for valproic acid-induced liver damage. Pediatr Transplant. 2000;4:211–4. [PubMed: 10933322](13 year old girl with progressive neurologic disease since age 3 to 5 years developed acute liver failure 5 months after starting valproate leading to liver transplantation, with subsequent rapid neurological deterioration and death 6 months later).
- Thomson MA, Lynch S, Strong R, Shepherd RW, Marsh W. Orthotopic liver transplantation with poor neurologic outcome in valproate-associated liver failure: a need for critical risk-benefit appraisal in the use of valproate. Transplant Proc. 2000;32:200–3. [PubMed: 10701024](5 children, ages 1 to 4 years, with acute liver failure from valproate [onset after 2-4 months] had poor neurological outcomes after liver transplantation, explant showing microvesicular steatosis and massive necrosis).
- Spahr L, Negro F, Rubbia-Brandt L, Marinescu O, Goodman K, Jordan M, Frossard JL, Hadenque A. Acute valproate-associated microvesicular steatosis: could the [13C]methionine breath test be useful to assess liver mitochondrial function? Dig Dis Sci. 2001;46:2758–61. [PubMed: 11768270](24 year old woman took an overdose of valproate [~7 grams] and presented with drowsiness and marked rise in ALT and fall in prothrombin over next 2 days, but without jaundice; liver biopsy showed microvesicular steatosis and swollen mitochondria, abnormal C-methionine breath test, rapidly improved).
- Chen WT, Yen DJ, Yu HY, Liao KK. Valproate-induced encephalopathy. Zhonghua Yi Xue Za Zhi (Taipei). 2001;64:474–8. [PubMed: 11720147]
- Lott RS, Helmboldt KM, Madaras-Kelly KJ. Retrospective evaluation of the effect of valproate therapy on transaminase elevations in patients with hepatitis C. Pharmacotherapy. 2001;21:1345–51. [PubMed: 11714207](Serial ALT levels in 214 HCV positive patients, 28 on valproate, found no correlation with valproate use).
- Barrueto F Jr, Hack JB. Hyperammonemia and coma without hepatic dysfunction induced by valproate therapy. Acad Emerg Med. 2001;8:999–1001. [PubMed: 11581089](41 year old man developed altered mental status while on chronic valproate therapy [3 years], with ammonia of 377 µM but normal liver tests and normal valproate levels).
- Bohan TP, Helton E, McDonald I, König SA, Gazitt S, Sugimoto T, Scheffner D, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56:1405–9. [PubMed: 11376200](Analysis of 123 patients with valproate hepatotoxicity in international registry demonstrating marked improvement in survival with use of carnitine; there was 100% survival if iv carnitine was started within 5 days of symptomatic onset, compared to >80% fatality rate before introduction of carnitine).
- DeVivo DC. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2002;58:507–8. [PubMed: 11839873](Letter in response to Bohan et al. suggesting routine oral carnitine supplementation in patients on valproate).
- Clarkson A, Choonara I. Surveillance for fatal suspected adverse drug reactions in the UK. Arch Dis Child. 2002;87:462–6. [PMC free article: PMC1755830] [PubMed: 12456539](Between 1964 and 2002 in the UK, there were 331 reported deaths in children due to medications, including 50 due to liver failure, 21 of which were attributed to valproate).
- Longin E, Teich M, Koelfen W, König S. Topiramate enhances the risk of valproate-associated side effects in three children. Epilepsia. 2002;43:451–4. [PubMed: 11952778](3 children, ages 1-9 years on valproate for few months to 5 years developed apathy and hypothermia 4-8 weeks after adding topiramate with ammonia elevations [one with ALT elevation as well], rapidly reversed by stopping either agent, positive rechallenge in one; topiramate appears to enhance risk of hyperammonemia due to valproate).
- Vossler DG, Wilensky AJ, Cawthon DF, Abson Kraemer DL, Ojemann LM, Caylor LM, Morgan JD. Serum and CSF glutamine levels in valproate-related hyperammonemic encephalopathy. Epilepsia. 2002;43:154–9. [PubMed: 11903461](In assessing 7 patients with hyperammonemia on valproate, elevations in glutamine found in CSF; serum valproate levels were usually normal).
- Huang Y-L, Hong H-S, Wang Z-W, Kuo T-T. Fatal sodium valproate-induced hypersensitivity syndrome with lichenoid dermatitis and fulminant hepatitis. J Am Acad Dermatol. 2003;49:316–9. [PubMed: 12894087](2 year old girl treated with valproate for myoclonic jerks developed fever and rash after 17 days followed by jaundice [bilirubin 37.8 mg/dL, ALT 3037 U/L, protime 50.2 sec], progressing to acute liver failure and death; biopsy showing microvesicular steatosis and necrosis).
- Romero-Falcón A, de la Santa-Belda E, García-Contreras R, Varela JM. A case of valproate-associated hepatotoxicity treated with L-carnitine. Eur J Intern Med. 2003;14:338–40. [PubMed: 13678762](16 year old male developed lethargy and jaundice 6 weeks after starting valproate for epilepsy [bilirubin 21 mg/dL, AST 3887 U/L, Alk P 406 U/L, normal valproate levels], given carnitine and recovered in ~3 weeks).
- Ee LC, Shepherd RW, Cleghorn GJ, Lewindon PJ, Fawcett J, Strong RW, Lynch SV. Acute liver failure in children: A regional experience. J Paediatr Child Health. 2003;39:107–10. [PubMed: 12603798](Among 26 children with acute liver failure referred to the Queensland Transplant Service between 1984 and 2000; 2 were transplanted for valproate liver injury, both of whom subsequently died of neurological disease despite good graft function).
- Arévalo-Lorido JC, Carretero-Gómez J, Bureo-Dacal JC, Montero-Leal C, Bureo-Dacal P. Antiepileptic drug hypersensitivity syndrome in a patient treated with valproate. Br J Clin Pharmacol. 2003;55:415–6. [PMC free article: PMC1884237] [PubMed: 12680893](36 year old man developed fever, rash, eosinophilia and lymphadenopathy 1 month after starting carbamazepine, improving with corticosteroid therapy, but recurring when valproate was started 2 weeks later).
- Bumb A, Diederich N, Beyenburg S. Adding topiramate to valproate therapy may cause reversible hepatic failure. Epileptic Disord. 2003;5:157–9. [PubMed: 14684351](51 year old woman with resistant epilepsy had topiramate added to valproate and developed confusion and liver injury [bilirubin not given, ALT 464 U/L, GGT 569 U/L, ammonia 88 μg/dL], improved on withdrawal of valproate; hypothesized that topiramate changed pharmacokinetics of valproate).
- Nakazato Y, Ando S, Yamamoto T, Tamura N, Shimazu K. Rinsho Shinkeigaku. 2004;44:682–5. [Valproate-induced hyperammonemic encephalopathy in a patient with Sjögren's syndrome] Japanese. [PubMed: 15568484]
- McCall M, Bourgeois JA. Valproic acid-induced hyperammonemia: a case report. J Clin Psychopharmacol. 2004;24:521–6. [PubMed: 15349008](62 year old woman on long term valproate developed dizziness, fatigue and confusion with normal valproate levels but 3 fold elevation in ammonia, minimal AST elevation, improvement with stopping; unclear what triggered the event).
- Yehya N, Saldarini CT, Koski ME, Davanzo P. Valproate-induced hyperammonemic encephalopathy. J Am Acad Child Adolesc Psychiatry. 2004;43:926–7. [PubMed: 15266186](9 year old boy with severe behavioral problems developed recurrent episodes of confusion and aggression on valproate with elevated ammonia levels improving with stopping valproate, liver tests normal).
- Luef GJ, Waldmann M, Sturm W, Naser A, Trinka E, Unterberger I, Bauer G, et al. Valproate therapy and nonalcoholic fatty liver disease. Ann Neurol. 2004;55:729–32. [PubMed: 15122714](Among 45 patients with epilepsy, ultrasound showed fatty liver in 63% of patients on valproate but only 23% on carbamazepine; ALT and AST similar, insulin resistance and overweight were more common with valproate treatment).
- Lokrantz CM, Eriksson B, Rosén I, Asztely F. Hyperammonemic encephalopathy induced by a combination of valproate and pivmecillinam. Acta Neurol Scand. 2004;109:297–301. [PubMed: 15016014](72 year old woman on valproate for 8 months developed stupor and coma 2 weeks after starting pivmecillinam for urinary tract infection [liver tests normal, ammonia 113 µM], pivmecillinam known to decrease carnitine levels).
- Weng TI, Shih FF, Chen WJ. Unusual causes of hyperammonemia in the ED. Am J Emerg Med. 2004;22:105–7. [PubMed: 15011224](Four case reports demonstrating various causes of hyperammonemia without liver disease; partial ornithine carbamoyltransferase deficiency, anticancer chemotherapy and valproate).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants done in the United States between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, of which 10 were attributed to phenytoin, 10 to valproate and 1 to carbamazepine, but none to any other anticonvulsants).
- Roepke S, Treudler R, Anghelescu I, Orfanos CE, Tebbe B. Valproic Acid and hypersensitivity syndrome. Am J Psychiatry. 2004;161:579. [PubMed: 14992991](48 year old man developed fever, rash and lymphadenopathy 3 weeks after starting valproate with eosinophilia [24%] and slight elevations in “levels of transaminases”, resolving with corticosteroid therapy).
- Rahman M, Haider N. Anticonvulsant hypersensitivity syndrome from addition of lamotrigine to divalproex. Am J Psychiatry. 2005;162:1021. [PubMed: 15863816](50 year old woman with bipolar disorder on valproate developed fever, rash and eosinophilia [5%] 2-3 weeks after starting lamotrigine [bilirubin and Alk P normal, ALT 186 U/L], resolving soon after stopping both valproate and lamotrigine).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](36 years of reporting to Swedish registry identified 103 cases of acute liver failure due to drugs; valproate listed as causing only one case).
- Lheureux PE, Penaloza A, Zahir S, Gris M. Science review: carnitine in the treatment of valproic acid-induced toxicity–what is the evidence? Crit Care. 2005;9:431–40. [PMC free article: PMC1297603] [PubMed: 16277730](Review of literature on efficacy of carnitine in reversing toxicity of valproate overdose, acute liver injury and hyperammonemia; efficacy largely based on use of historical controls).
- Ortiz-Sáenz de Santa María R, Santiago-Fernández C, Cano-Del Pozo M, et al. Rev Neurol. 2005;41:766–7. [Treatment with valproate and hepatic steatosis] [PubMed: 16355364]
- Cuturic M, Abramson RK. Acute hyperammonemic coma with chronic valproic acid therapy. Ann Pharmacother. 2005;39:2119–23. [PubMed: 16288075](56 year old woman on long term valproate for epilepsy developed episodic confusion after minor increase in valproate dose and stopping other agents; valproate dose increased and patient worsened and found to have high ammonia with normal liver tests).
- Sato K, Ueda Y, Ueno K, Okamoto K, Iizuka H, Katsuda S. Hepatocellular carcinoma and nonalcoholic steatohepatitis developing during long-term administration of valproic acid. Virchows Arch. 2005;447:996–9. [PubMed: 16133366](64 year old man treated with valproate for 17 years developed hepatocellular carcinoma with steatosis, Mallory bodies and bridging fibrosis, possibly related to nonalcoholic steatohepatitis).
- Rath A, Naryanan TJ, Chowdhary GV, Murthy JM. Valproate-induced hyperammonemic encephalopathy with normal liver function. Neurol India. 2005;53:226–8. [PubMed: 16010067](Review of 5 cases of hyperammonemia on valproate; 3 monotherapy, onset after 4-90 days with decreased sensorium, episodic vomiting, all had normal ALT, resolved with stopping without use of carnitine).
- Bauer MS. Fatal hepatic failure and valproate. Am J Psychiatry. 2005;162:192. [PubMed: 15625224](51 year old man with bipolar disorder developed jaundice 3 months after starting valproate with previously normal liver tests [bilirubin 9.1 mg/dL, ALT 3208 U/L, Alk P 172 U/L, protime 18.8 sec], died awaiting liver transplantation).
- Kimmel RJ, Irwin SA, Meyer JM. Valproic acid-associated hyperammonemic encephalopathy: a case report from the psychiatric setting. Int Clin Psychopharmacol. 2005;20:57–8. [PubMed: 15602119](50 year old woman on long term valproate for psychiatric reasons developed confusion and coma with high ammonia levels 4 months after adding lamotrigine, responding to stopping therapy).
- Koenig SA, Buesing D, Longin E, Oehring R, Haussermann P, Kluger G, Lindmayer F, et al. Valproic acid-induced hepatopathy: nine new fatalities in Germany from 1994 to 2003. Epilepsia. 2006;47:2027–31. [PubMed: 17201699](Survey of all German members of International League Against Epilepsy identified 31 cases of valproate liver injury between 1994 and 2003; 9 fatal and 22 recovered cases; latency of 1-6 months, ALT 113-2160 U/L).
- Kakinuma H, Fujiki T, Nakamura T, Takahashi H. Valproate hepatotoxicity in a 5-year-old boy with cerebral palsy due to neonatal asphyxia. Pediatr Int. 2006;48:631–3. [PubMed: 17168987](5 year old boy with cerebral palsy had fever and status epilepticus and was treated with valproate, developing acute liver failure within a few days [bilirubin initially 1.0 mg/dL, ALT 8290 U/L, NH3 112 μg/mL], slow recovery and neurological residual; no metabolic abnormality found).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–8. [PubMed: 16054882](In WHO database of fatal adverse drug reactions from 1968-2003 there were 4690 reports of drug induced liver fatality: valproate was the third most common cause [~130 cases]).
- Tzoulis C, Engelsen BA, Telstad W, Aasly J, Zeviani M, Winterthun S, Ferrari G, et al. The spectrum of clinical disease caused by the A467T and W748S POLG mutations: a study of 26 cases. Brain. 2006;129:1685–92. [PubMed: 16638794](Clinical features of patients with p.A467T and/or p.W748S variants in DNA polymerase gamma; onset of epilepsy, headache, ataxia or speech delay usually in the teen years with relentless course of myoclonus and ataxia, 2 died of liver failure, but 7 developed acute liver failure after valproate therapy of seizures).
- Horvath R, Hudson G, Ferrari G, Futterer N, Ahola S, Lamantea E, Prokisch H, et al. Phenotypic spectrum associated with mutations of the mitochondrial polymerase gamma gene. Brain. 2006;129:1674–84. [PubMed: 16621917](Analysis of “large number” of patients with suspected mitochondrial disease [Alpers, familial progressive external ophthalmoplegia, late onset ataxia] for the mitochondrial DNA polymerase gamma sequence; identified frequent but heterogenous variants, p.A467T being common with encephalopathy and liver disease [often triggered by valproate], but found in other syndromes).
- Alqahtani S, Federico P, Myers RP. A case of valproate-induced hyperammonemic encephalopathy: look beyond the liver. CMAJ. 2007;177:568–9. [PMC free article: PMC1963357] [PubMed: 17846437](18 year old woman developed stupor and asterixis 2 weeks after starting valproate, with high ammonia levels and normal liver tests, and recovery within a week after stopping).
- Wadzinski J, Franks R, Roane D, Bayard M. Valproate-associated hyperammonemic encephalopathy. J Am Board Fam Med. 2007;20:499–502. [PubMed: 17823470](2 women, ages 29 and 51, developed hyperammonemia with confusion after 7 days and 5 months of high doses of valproate [normal bilirubin and ALT], with rapid improvement on stopping).
- Velioğlu SK, Gazioğlu S. Non-convulsive status epilepticus secondary to valproic acid-induced hyperammonemic encephalopathy. Acta Neurol Scand. 2007;116:128–32. [PubMed: 17661800](19 year old male developed stupor, coma and seizures 4 days after starting valproate, found to have high ammonia levels with normal ALT, bilirubin and valproate levels, resolving with stopping).
- Carr RB, Shrewsbury K. Hyperammonemia due to valproic acid in the psychiatric setting. Am J Psychiatry. 2007;164:1020–7. [PubMed: 17606652](45 year old woman treated with valproate for psychiatric disorder developed confusion after 6 days, later found with high ammonia levels despite normal ALT levels, improvement with stopping).
- Attilakos A, Voudris KA, Katsarou E, Prassouli A, Mastroyianni S, Garoufi A. Transient decrease in serum albumin concentrations in epileptic children treated with sodium valproate monotherapy. Clin Neuropharmacol. 2007;30:145–9. [PubMed: 17545749](26 children given valproate were monitored with liver tests at 0, 6, 12 and 24 months: only 1 had abnormal ALT [44 U/L], GGT normal in all, minimal overall increase in ALT and AST and decrease in albumin levels).
- Tang W. Drug metabolite profiling and elucidation of drug-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2007;3:407–20. [PubMed: 17539747](Review of role of drug metabolic studies in elucidating cause of hepatotoxicity).
- Chicharro AV, de Marinis AJ, Kanner AM. The measurement of ammonia blood levels in patients taking valproic acid: looking for problems where they do not exist? Epilepsy Behav. 2007;11:361–6. [PubMed: 17845866](Review of literature on ammonia levels in patients on valproate, consistent finding of levels increasing 2 fold, but usually without symptoms and not requiring dose modification).
- Russell S. Carnitine as an antidote for acute valproate toxicity in children. Curr Opinion Pediatr. 2007;19:206–10. [PubMed: 17496767](Short review of valproate toxicity and clinical use of carnitine).
- Gerstner T, Bauer MO, Longin E, Bell N, Koenig SA. Reversible hepatotoxicity, pancreatitis, coagulation disorder and simultaneous bone marrow suppression with valproate in a 2-year-old girl. Seizure. 2007;16:554–6. [PubMed: 17493839](2 year old girl on valproate for over a year had severe liver injury [bilirubin not given, ALT 2510 U/L, lipase 725 U/L, normal valproate and ammonia levels], resolving upon stopping).
- Chou HF, Yang RC, Chen CY, Jong YJ. Valproate-induced hyperammonemic encephalopathy. Pediatr neonatol. 2008;49:201–4. [PubMed: 19133574](Adolescent developed dizziness within days of starting valproate, with normal ALT but ammonia five-fold elevated, rapid recovery with stopping).
- Tsai M-F, Chen C-Y. Valproate-induced hyperammonemic encephalopathy treated by hemodialysis. Ren Fail. 2008;30:822–4. [PubMed: 18791959](14 year old girl developed dizziness within days of restarting higher doses of valproate with high ammonia and valproate levels, but normal ALT and bilirubin; the syndrome reversed within 12 hours with hemodialysis).
- Nicolai J, Gunning B, Leroy PL, Ceulemans B, Vles JS. Acute hepatic injury in four children with Dravet syndrome: valproic acid, topiramate or acetaminophen? Seizure. 2008;17:92–7. [PubMed: 17697789](4 children with severe infantile myoclonic epilepsy on chronic therapy with topiramate and valproate had sudden and transient onset of marked elevations in ALT [2229, 5898, 280 and 480 U/L] and hyperammonemia or coagulopathy without jaundice shortly after febrile illness and/or use of acetaminophen; possibly Reye syndrome due to valproate and topiramate, rapid recovery despite continuing anticonvulsants).
- Dewan P, Aggarwal A, Faridi MM. Effect of phenytoin and valproic acid therapy on serum lipid levels and liver function tests. Indian Pediatr. 2008;45:855–8. [PubMed: 18948659](Cross sectional analysis of lipid and liver test results in 52 children on phenytoin or valproate showing minimal differences between groups).
- Subhash HS, Heddle RJ, Schultz DW, Ring J, Thompson CH. Hepatic encephalopathy precipitated by sodium valproate therapy. Med J Aust. 2008;188:549. [PubMed: 18459937](71 year old woman developed confusion 2 years after starting valproate with no change in liver tests; chronic Alk P elevations subsequently shown to be due to primary biliary cirrhosis).
- McFarland R, Hudson G, Taylor RW, Green SH, Hodges S, McKiernan PJ, Chinnery PF, Ramesh V. Reversible valproate hepatotoxicity due to mutations in mitochondrial DNA polymerase gamma (POLG1). Arch Dis Child. 2008;93:151–3. [PubMed: 18208989](2 year old boy developed stupor 2 months after starting valproate [bilirubin 0.6 rising to 6.4 mg/dL, ALT 249 U/L], who subsequently developed liver failure but recovered; gene sequencing showed several mutations in DNA polymerase gamma).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand. 2008;118:281–90. [PubMed: 18341684](Review of hepatotoxicity of all anticonvulsants; elevations in ALT or Alk P in up to 44% of users of valproate but serious toxicity occurs in only ~1:15,000 patients, higher in young children; 4 types of injury; asymptomatic enzyme elevations, acute hepatotoxicity, Reye syndrome and hyperammonemia).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, et al. Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, valproate accounted for 6 cases, lamotrigine 5, phenytoin 5, gabapentin and topiramate 1 each).
- Neyns B, Hoorens A, Stupp R. Valproic acid related idiosyncratic drug induced hepatotoxicity in a glioblastoma patient treated with temozolomide. Acta Neurol Belg. 2008;108:131–4. [PubMed: 19239041](48 year old patient developed cholestatic hepatitis during therapy with temozolomide, cilengitide and valproate, which improved when all drugs were stopped and did not recur with restarting temozolomide).
- Mata Zubillaga D, Prieto Espuñes S, Ferrero de la Mano L, Herrero Mendoza B. An Pediatr (Barc). 2008;69:492–3. [Valproic acid induced-idiosyncratic hepatotoxicity] Spanish. [PubMed: 19128756](7 year old girl developed liver injury on valproate [peak bilirubin 9.5 mg/dL, ALT 2050 U/L, Alk P normal], resolving within a few weeks of stopping).
- Verrotti A, Di Marco G, la Torre R, Pelliccia P, Chiarelli F. Nonalcoholic fatty liver disease during valproate therapy. Eur J Pediatr. 2009;168:1391–4. [PubMed: 19184102](11 year old girl developed nonalcoholic fatty liver disease after 19 kg weight gain attributed to valproate, ALT levels rising from 30 to 80 U/L and returning to normal with weight loss after stopping valproate).
- Franciotta D, Kwan P, Perucca E. Genetic basis for idiosyncratic reactions to antiepileptic drugs. Curr Opin Neurol. 2009;22:144–9. [PubMed: 19262378](Review of the genetic associations with hypersensitivity reactions to anticonvulsant medications; closest association has been with HLA-B*15:02 and Stevens-Johnson syndrome after aromatic anticonvulsants).
- Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila). 2009;47:101–11. [PubMed: 19280426](Review of the mechanism of action of carnitine in valproate hepatotoxicity and the clinical evidence of its efficacy, concludes that intravenous carnitine "does not appear to be harmful and could be beneficial").
- Waring WS, Nixon AC. Acute liver impairment after sodium valproate overdose. BMJ Case Rep 2009; 2009. pii: bcr06.2008.0057. [PMC free article: PMC3027997] [PubMed: 21686945](36 year old woman took an overdose of valproate [32 g] and developed nausea 36 hours later [bilirubin 1.5 mg/dL, ALT 761 U/L], symptoms resolving in 4 days and liver enzymes by 6 weeks).
- Stewart JD, Horvath R, Baruffini E, Ferrero I, Bulst S, Watkins PB, Fontana RJ, et al. Polymerase γ gene POLG determines the risk of sodium valproate-induced liver toxicity. Hepatology. 2010;52:1791–6. [PMC free article: PMC3841971] [PubMed: 21038416](Among 17 US patients identified with valproate hepatotoxicity, 8 had genetic variations in the mitochondrial DNA polymerase gamma gene, a rate more than 20-fold higher than population controls).
- Nishri D, Blumkin L, Lev D, Leshinsky-Silver E, Abu-Rashid M, Birch R, Zuberi SM, Lerman-Sagie T. Hepatic coma culminating in severe brain damage in a child with a SCN1A mutation. Eur J Paediatr Neurol. 2010;14:456–9. [PubMed: 20392657](11 month old boy developed status epilepticus and "fulminant liver failure" 2 months after starting valproate for myoclonic epilepsy [bilirubin not given, ALT peak 20,657 U/L, ammonia 380 μmol/L], with brain edema and residual neurologic defects, the mitochondrial DNA polymerase gamma gene was normal but variation found in the alpha-1 subunit gene of the voltage-gated neuronal sodium channel [SCN1A]).
- Saneto RP, Lee IC, Koenig MK, Bao X, Weng SW, Naviaux RK, Wong LJ. POLG DNA testing as an emerging standard of care before instituting valproic acid therapy for pediatric seizure disorders. Seizure. 2010;19:140–6. [PMC free article: PMC3099441] [PubMed: 20138553](Among 4 Japanese children with acute liver failure attributed to valproate [3 fatal] seen at a single referral center, two were homozygous for POLG mutations [p.R597W or p.A467T] and two were compound heterozygotes [p.A467T/p.Q68X and p.L83P/p.C888S] indicative of Alpers-Huttenlocher syndrome).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 [11%] were attributed to drug induced liver injury of which 11 were due to antiepileptic drugs, 2 due to valproate).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; 3 [1.0%] were attributed to valproate, none of which were fatal).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, valproate accounting for 208 cases [2.3%: ranking third] for an adjusted risk odds ratio of 4.0).
- Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N., Drug-Induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN Prospective Study. J Pediatr Gastroenterol Nutr. 2011;53:182–9. [PMC free article: PMC3634369] [PubMed: 21788760](Among 30 children with drug induced liver injury enrolled in a prospective US database between 2004 and 2008, 8 were due to anticonvulsants [lamotrigine in 3, valproate in 3, phenytoin in 1, and carbamazepine in 1], none of which were fatal or led to chronic injury).
- Rupasinghe J, Jasinarachchi M. Progressive encephalopathy with cerebral oedema and infarctions associated with valproate and diazepam overdose. J Clin Neurosci. 2011;18:710–1. [PubMed: 21349718](29 year old man developed stupor and coma within hours of taking an overdose of valproate [4 g] and diazepam [75 mg], with normal bilirubin and liver enzymes but high ammonia [348 μmol/L], and cerebral edema resulting in residual neurological defects).
- Verrotti A, Agostinelli S, Parisi P, Chiarelli F, Coppola G. Nonalcoholic fatty liver disease in adolescents receiving valproic acid. Epilepsy Behav. 2011;20:382–5. [PubMed: 21256090](Among 86 adolescents on valproate, fatty liver was present in 36% by ultrasound and in a similar proportion [35%] of 43 weight-matched controls).
- Chaudrey KH, Naser TB, Steinberg A, Avashia KD, Nouri-Kolouri M, Asadi S, Irshad Khan SI, Ihsan M. Thinking beyond the obvious: hepatotoxicity secondary to idiosyncratic depakote toxicity. Am J Ther. 2012;19:403–6. [PubMed: 21248613](25 year old woman developed confusion while taking valproate and was found to have metabolic acidosis [bilirubin and Alk P not given, ALT 90 U/L, ammonia 143 µmol/L, valproate levels normal], resolving rapidly upon stopping).
- Mindikoglu AL, King D, Magder LS, Ozolek JA, Mazariegos GV, Shneider BL. Valproic acid-associated acute liver failure in children: case report and analysis of liver transplantation outcomes in the United States. J Pediatr. 2011;158:802–7. [PMC free article: PMC3075355] [PubMed: 21167499](Among 98 children who underwent liver transplantation for drug induced liver injury in the US between 1987 and 2009, 17 [17%] had valproate induced injury, 15 of whom were less than 8 years old and had poor posttransplant survival compared to controls transplanted for acute liver failure from other agents [20% vs 69% survival at 1 year]).
- Tarafdar S, Slee M, Ameer F, Doogue M. A case of valproate induced hyperammonemic encephalopathy. Case Rep Med. 2011;2011:969505. [PMC free article: PMC3099231] [PubMed: 21629819](36 year old man on valproate developed confusion after an alcoholic binge [ammonia 248 µmol/L, valproate levels normal], resolving within 3 days of stopping).
- Ghozzi H, Hakim A, Sahnoun Z, Ben Mahmoud L, Atheymen R, Hammami S, Zeghal K. Rev Neurol (Paris). 2011;167:600–6. [Relationship between plasma concentrations of valproic acid and hepatotoxicity in patients receiving high doses] [PubMed: 21492891](In testing 425 patients, no association was found with side effects and plasma levels of valproate).
- Pronicka E, Weglewska-Jurkiewicz A, Pronicki M, Sykut-Cegielska J, Kowalski P, Pajdowska M, Jankowska I, et al. Drug-resistant epilepsia and fulminant valproate liver toxicity. Alpers-Huttenlocher syndrome in two children confirmed post mortem by identification of p.W748S mutation in POLG gene. Med Sci Monit. 2011;17:CR203–9. [PMC free article: PMC3539522] [PubMed: 21455106](Retrospective analysis of 28 children with mitochondrial encephalopathy found p.W748S mutation in the polymerase gamma gene [typical of Alpers-Huttenlocher Syndrome] in two, both of whom died of liver failure a few months after starting valproate for seizures; liver tissue showed mitochondrial depletion).
- Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, Roujeau JC. The DRESS syndrome: a literature review. Am J Med. 2011;124:588–97. [PubMed: 21592453](Systematic review of literature on DRESS identified 172 cases due to 44 drugs, most frequently carbamazepine [27%], allopurinol [11%] and lamotrigine [6%]; no cases were linked to valproate).
- Sedky K, Nazir R, Joshi A, Kaur G, Lippmann S. Which psychotropic medications induce hepatotoxicity? Gen Hosp Psychiatry. 2012;34:53–61. Review. [PubMed: 22133982](Review of hepatotoxicity of psychotropic medications with summary of literature).
- Hunter MF, Peters H, Salemi R, Thorburn D, Mackay MT. Alpers syndrome with mutations in POLG: clinical and investigative features. Pediatr Neurol. 2011;45:311–8. [PubMed: 22000311](Clinical description of 12 children with Alpers syndrome who were homozygotes or compound heterozygotes for mutations in the polymerase gamma gene; all had developmental delay and seizures starting before 2 years of age and died by age 11, often with cirrhosis or acute liver failure particularly if treated with valproate).
- Prins MC, van Meijel JJ. A case of hyperammonaemic encephalopathy due to valproic acid. Neth J Med. 2011;69:389–91. [PubMed: 21978982](57 year old man developed progressive stupor while on chronic valproate therapy [liver tests normal, ammonia 132 µmol/L, valproate levels high], resolving rapidly upon stopping).
- Bota RG, Ligasan AP, Najdowski TG, Novac A. Acute hypersensitivity syndrome caused by valproic acid: a review of the literature and a case report. Perm J. 2011;15:80–4. [PMC free article: PMC3140754] [PubMed: 21841930](25 year old woman with bipolar disorder developed fever within one week of starting valproate, followed by jaundice and rash [bilirubin 10.2 mg/dL, ALT 767 U/L, Alk P 681 U/L], treated with corticosteroids and resolving within a month of stopping).
- Holroyd S, Overdyke JT. Hyperammonemia associated with valproic acid use in elderly psychiatric patients. J Neuropsychiatry Clin Neurosci. 2012;24:372–4. [PubMed: 23037652](Retrospective analysis of 12 elderly subjects treated with valproate for 1 to 60 months; liver tests were normal, but 10 [83%] had elevated ammonia levels and 3 had history of confusion or delirium).
- Schmid MM, Freudenmann RW, Keller F, Connemann BJ, Hiemke C, Gahr M, Kratzer W, et al. Non-fatal and fatal liver failure associated with valproic acid. Pharmacopsychiatry. 2013;46:63–8. [PubMed: 22915484](Between 1993 and 2009, 132 cases of severe valproate hepatotoxicity were reported to the German Federal regulatory agency, of which 34 [25%] were fatal).
- Chatzistefanidis D, Georgiou I, Kyritsis AP, Markoula S. Functional impact and prevalence of polymorphisms involved in the hepatic glucuronidation of valproic acid. Pharmacogenomics. 2012;13:1055–71. [PubMed: 22838952](Review of the metabolism of valproate which is mainly via glucuronidation mediated by UDP glucuronsyltransferases [UGTs] in the liver).
- van Zoelen MA, de Graaf M, van Dijk MR, Bogte A, van Erpecum KJ, Rockmann H, Maarschalk-Ellerbroek LJ. Valproic acid-induced DRESS syndrome with acute liver failure. Neth J Med. 2012;70:155. [PubMed: 22516584](26 year old man developed fever, rash, lymphadenopathy, facial edema and jaundice 6 weeks after starting valproate [bilirubin 10.9 mg/dL, ALT 2800 U/L, Alk P 456 U/L, protime 21.6 sec], eventually resolving after stopping and with corticosteroid therapy: Case 3).
- Lin YH, Chien YL. Valproic acid-related anticonvulsant hypersensitivity syndrome and subsequent olanzapine-related neutropenia and thrombocytopenia: a case report. J Clin Psychopharmacol. 2012;32:132–3. [PubMed: 22217947](27 year old woman developed fever and rash 12 days after starting valproate [bilirubin and Alk P not given; ALT 107 U/L], resolving rapidly upon stopping and with prednisolone therapy, later developing neutropenia on olanzapine).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, 2 of which were attributed to phenytoin, but none were linked to valproate use).
- Drugs for epilepsy. Treat Guidel Med Lett. 2013;11:9–18. Erratum in Treat Guidel Med Lett. 2013; 11: 112. [PubMed: 23348233](Concise review of drugs of choice for epilepsy; valproate is effective in several forms of seizures and is also used to treat migraine headaches and bipolar disorder; serious adverse effects are uncommon, but fatal liver failure has been reported particularly in children but also in adults).
- Rej S, Chen T, Edwards M, Zicherman V. Fulminant hepatic failure in the context of reinstituting valproate use. Psychosomatics. 2014;55:303–4. [PubMed: 24360531](61 year old woman with bipolar disorder restarted valproate [1000 mg daily] and developed fatigue, abdominal pain and delirium one month later [bilirubin 0.3 mg/dL, ALT >5000 U/L, Alk P not given, INR 1.9, glucose 79 mg/dL] with immediate improvement on stopping and normal liver tests within 1 week).
- Star K, Edwards IR, Choonara I. Valproic acid and fatalities in children: a review of individual case safety reports in VigiBase. PLoS One. 2014;9:e108970. [PMC free article: PMC4193865] [PubMed: 25302991](Analysis of the WHO VigiBase registry of fatal drug adverse events in children reported between 1977 and 2012 identified 268 instances attributed to valproate, including 156 for hepatotoxicity mostly in children ages 6 and below but occurring at any age).
- Chait Mermelstein A, Mermelstein J, Adam T, Brody BD, Dubin MJ. Oxcarbazepine-induced liver injury after sensitization by valproic acid: a case report. Bipolar Disord. 2016;18:307–9. [PubMed: 27041538](46 year old woman with bipolar disorder treated with valproate for 3 years was found to have abnormal liver enzymes elevations [ALT 163 U/L, Alk P 180 UL, bilirubin normal] which improved on stopping valproate, but ALT rose again when she was started on oxcarbazepine which resolved on stopping and recurred on restarting).
- Mnif L, Sellami R, Masmoudi J. Valproic Acid and Hepatic Steatosis: A Possible Link? About a Case Report. Psychopharmacol Bull. 2016;46:59–62. [PMC free article: PMC5044469] [PubMed: 27738381](47 year old man with bipolar disorder on valproate developed abnormal liver tests [bilirubin normal, ALT 123 U/L] which rose further on continuation but resolved with switching to lithium; body mass index of 32 k/m2 and no data on weight change).
- Gayam V, Khalid M, Shrestha B, Hossain MR, Dahal S, Garlapati P, Gill A, Mandal AK, Sangha R. Drug-induced liver injury: an institutional case series and review of literature. J Investig Med High Impact Case Rep. 2018;6:2324709618761754. [PMC free article: PMC5858623] [PubMed: 29568780](Three cases of liver injury, case 3 being a 27 year old African-American man who developed liver injury 1 week after starting valproate [bilirubin 1.5 m/dL, ALT 7000 U/L, Alk P 96 U/L, INR 2.4, ammonia 184 µmol/L], resolving rapidly after stopping).
- Gayam V, Mandal AK, Khalid M, Shrestha B, Garlapati P, Khalid M. Valproic acid induced acute liver injury resulting in hepatic encephalopathy- a case report and literature review. J Community Hosp Intern Med Perspect. 2018;8:311–4. [PMC free article: PMC6197012] [PubMed: 30356994](Same case as described above [Gayam 2018]).
- Bassett JT, Rodriguez B, Mulligan L, Fontana RJ. Acute liver failure in a military recruit treated with valproic acid and harboring a previously unrecognized POLG-1 mutation. Epilepsy Behav Rep. 2019;12:100342. [PMC free article: PMC6883298] [PubMed: 31799506](20 year old man with new onset seizure disorder developed fatigue followed by jaundice and change in mental status after starting valproate [bilirubin 4.5 mg/dL, ALT 283 U/L, INR 3.1], biopsy showing microvesicular fat and necrosis and with progressive course ultimately requiring liver transplantation, sequencing of POLG-1 showed p.A467T).
- Walker CP, Deb S. Rhabdomyolysis and hepatotoxicity from valproic acid: case reports. J Pharm Pract. 2019:897190019882880. [PubMed: 31658868](Two patients with schizophrenia on long term valproate [5 and 7 years] and multiple other medications presented with acute severe rhabdomyolysis [bilirubin 1.1 and normal, ALT 22 and 54 U/L, CPK 15,368 and 21,980 U/L], with subsequent acute renal and hepatic failure, one fatal and one resolving after extensive intensive care management with continuation of valproate).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 7 [4%] of which were attributed to anticonvulsants including 3 due to valproate, one of which was fatal).
- Devarbhavi H, Andrade RJ. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014;34:145–61. [PubMed: 24879980](Review of drug induced liver injury caused by several drug classes including antiepileptics, which account for 2-11% of all cases in various registries; a common presentation is with DRESS, particularly with the older agents such as carbamazepine, phenytoin and phenobarbital and often associated with reactivation of human herpes virus 6 or 7).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were due to anticonvulsants including 12 due to phenytoin, 9 lamotrigine, 7 valproate, 4 carbamazepine, 3 gabapentin, 2 topiramate and 1 each for ethosuximide, fosphenytoin, and pregabalin).
- Chalasani N, Reddy KRK, Fontana RJ, Barnhart H, Gu J, Hayashi PH, Ahmad J, Stolz A, Navarro V, Hoofnagle JH. Idiosyncratic drug induced liver injury in African-Americans is associated with greater morbidity and mortality compared to Caucasians. Am J Gastroenterol. 2017;112:1382–8. [PMC free article: PMC5667647] [PubMed: 28762375](Among subjects enrolled in a US prospective database of drug induced liver injury, causes more frequent in African Americans than Caucasian were trimethoprim/sulfamethoxazole, methyldopa, phenytoin and allopurinol, and African Americans were more likely to have severe skin adverse events [2.1% vs 0.4%], fatal or transplantation outcomes [10% vs 6%] as well as chronic injury [24% vs 16%]).
- Vidaurre J, Gedela S, Yarosz S. Antiepileptic drugs and liver disease. Pediatr Neurol. 2017;77:23–36. [PubMed: 29097018](Review of the use of anticonvulsants in patients with liver disease recommends use of agents that have little hepatic metabolism such as levetiracetam, lacosamide, topiramate, gabapentin and pregabalin, levetiracetam being an "ideal" first line therapy for patients with liver disease because of its safety and lack of pharmacokinetic interactions).
- Drugs for epilepsy. Med Lett Drugs Ther. 2017;59(1526):121–30. [PubMed: 28746301](Concise review of the drugs available for therapy of epilepsy lists valproate as approved as monotherapy or adjunctive therapy for several forms of seizures and as generally well tolerated, common adverse effects being drowsiness, cognitive changes, nausea, weight gain, hyperinsulinemia, lipid abnormalities, and menstrual disturbances, and uncommon serious adverse events include fatal liver failure, particularly in children below the age of 2 years, hyperammonemia, pancreatitis, interstitial nephritis, parkinsonism and edema).
- Borrelli EP, Lee EY, Descoteaux AM, Kogut SJ, Caffrey AR. Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs: An analysis of the US Food and Drug Administration Adverse Event Reporting System. Epilepsia. 2018;59:2318–24. [PMC free article: PMC6420776] [PubMed: 30395352](Review of adverse event reports to the FDA between 2014 and 2018 identified ~2.9 million reports, 1034 for SJS/TEN, the most common class of drugs implicated being anticonvulsants with 17 of 34 approved antiepileptics with at least one report, most frequent being lamotrigine [n=106], carbamazepine [22], levetiracetam [14], phenytoin [14], valproate [9], clonazepam [8] and zonisamide [7]; no mention of accompanying liver injury or whether attribution was as a single agent or one of several).
- Haznedar P, Doğan Ö, Albayrak P, Öz Tunçer G, Teber S, Deda G, Eminoglu FT. Effects of levetiracetam and valproic acid treatment on liver function tests, plasma free carnitine and lipid peroxidation in childhood epilepsies. Epilepsy Res. 2019;153:7–13. [PubMed: 30925397](In a cross sectional analysis of 51 children with epilepsy treated with valproate or levetiracetam, serum ALT, AST and GGT levels were in the normal range in all and did not differ from a group of healthy controls).
- Cano-Paniagua A, Amariles P, Angulo N, Restrepo-Garay M. Epidemiology of drug-induced liver injury in a University Hospital from Colombia: Updated RUCAM being used for prospective causality assessment. Ann Hepatol. 2019;18:501–7. [PubMed: 31053545](Among 286 patients with liver test abnormalities seen in a single hospital in Colombia over a 1 year period, 17 were diagnosed with drug induced liver injury, the most common cause being antituberculosis therapy [n=6] followed by anticonvulsants [n=3, 1 each due to phenytoin, gabapentin and valproate]).
- Kapur J, Elm J, Chamberlain JM, Barsan W, Cloyd J, Lowenstein D, Shinnar S, et al. NETT and PECARN Investigators. Randomized trial of three anticonvulsant medications for status epilepticus. N Engl J Med. 2019;381:2103–13. [PMC free article: PMC7098487] [PubMed: 31774955](Among 384 adults and children with status epilepticus not responding to benzodiazepines who were treated with intravenous levetiracetam, fosphenytoin or valproate, seizure control at 60 minutes was similar in all 3 groups [47%, 45%, and 46%], and adverse event rates were similar while hepatic failure occurred in one patient on fosphenytoin and abnormal liver tests were reported in one patient on valproate).
- Chamberlain JM, Kapur J, Shinnar S, Elm J, Holsti M, Babcock L, Rogers A, et al. Neurological Emergencies Treatment Trials; Pediatric Emergency Care Applied Research Network investigators. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. 2020;395:1217–24. [PMC free article: PMC7241415] [PubMed: 32203691](An extension of the trial reported by Kapur [2019] allowed for analysis of response rates by age group, control of seizures was similar with the three agents across all age groups and adverse event rates were also similar).
- Sridharan K, Daylami AA, Ajjawi R, Ajooz HAMA. Drug-induced liver injury in critically ill children taking antiepileptic drugs: a retrospective study. Curr Ther Res Clin Exp. 2020;92:100580. [PMC free article: PMC7138958] [PubMed: 32280391](Among 41 children admitted to a pediatric intensive care unit who received anticonvulsants for seizure control, 5 of 9 receiving phenobarbital alone, 9 of 12 receiving phenytoin alone but none of six receiving valproate developed liver test abnormalities [all hepatocellular], the timing, height and outcome of which were not given).
- Vignesh V, Rameshkumar R, Mahadevan S. Comparison of phenytoin, valproate and levetiracetam in pediatric convulsive status epilepticus: a randomized double-blind controlled clinical trial. Indian Pediatr. 2020;57:222–7. [PubMed: 32198861](Among 110 children with status epilepticus not responding to intravenous benzodiazepines who were treated with intravenous infusions of phenytoin, valproate or levetiracetam, the rate of seizure control was similar in the 3 groups and adverse events were rare; no mention of liver injury or ALT elevations).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults.[Cochrane Database Syst Rev. 2013]Review Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults.Linde M, Mulleners WM, Chronicle EP, McCrory DC. Cochrane Database Syst Rev. 2013 Jun 24; 2013(6):CD010611. Epub 2013 Jun 24.
- Review [Brief history of the development of valproate in bipolar disorders].[Encephale. 2001]Review [Brief history of the development of valproate in bipolar disorders].Lempérière T. Encephale. 2001 Jul-Aug; 27(4):365-72.
- Review Lessons learned from the discovery of sodium valproate and what has this meant to future drug discovery efforts?[Expert Opin Drug Discov. 2020]Review Lessons learned from the discovery of sodium valproate and what has this meant to future drug discovery efforts?Janković SM, Janković SV. Expert Opin Drug Discov. 2020 Nov; 15(11):1355-1364. Epub 2020 Jul 20.
- [Prescribing valproate to girls and women of childbearing age in Germany : Analysis of trends based on claims data].[Bundesgesundheitsblatt Gesundh...][Prescribing valproate to girls and women of childbearing age in Germany : Analysis of trends based on claims data].Wentzell N, Haug U, Schink T, Engel S, Liebentraut J, Linder R, Onken M, Schaefer C, Dathe K. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018 Aug; 61(8):1022-1029.
- Use of melatonin versus valproic acid in prophylaxis of migraine patients: A double-blind randomized clinical trial.[Restor Neurol Neurosci. 2017]Use of melatonin versus valproic acid in prophylaxis of migraine patients: A double-blind randomized clinical trial.Ebrahimi-Monfared M, Sharafkhah M, Abdolrazaghnejad A, Mohammadbeigi A, Faraji F. Restor Neurol Neurosci. 2017; 35(4):385-393.
- Valproate - LiverToxValproate - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...