NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Trientine is an oral copper chelating agent used to treat Wilson disease. Trientine has not been associated with worsening of serum enzyme elevations during therapy or with cases of clinically apparent liver injury with jaundice.
Background
Trientine (trye’ en teen) is an orally available copper chelating agent that is used to treat Wilson disease, an inherited abnormality of copper metabolism that leads to excess copper accumulation and injury to liver and brain. The metabolic defect in Wilson disease is caused by mutations in ATPase7B, a hepatic enzyme responsible for transmembrane transport and excretion of copper into the bile. The metabolic detect leads to accumulation of free copper in liver and blood and secondarily in other organs, particularly brain and kidney. The disease usually presents in childhood or adolescence with neurologic syndromes, signs of advanced liver disease and hemolytic anemia. Trientine is one of several copper chelating agents that lower blood and tissue copper levels and, when given chronically, prevent copper accumulation and injury in Wilson disease. Other copper chelating agents include d-penicillamine and dimercaprol (British anti-Lewisite [BAL]). Trientine has a polyamine-like structure and chelates copper by creating a stable complex with the four nitrogens in a plantar ring. Trientine complexed to copper is excreted in the urine. Trientine was approved for use in the United States in 1969, and current formal indications are for treatment of patients with Wilson disease who are intolerant of penicillamine. Trientine is available in capsules of 250 mg generically and under the brand name Syprine. The recommended dose is 750 to 1250 mg for adults and 500 to 750 mg for children given in 2 to 4 divided doses daily initially, which can be raised to a maximum of 2000 mg daily for adults and 1500 mg daily for children. Side effects are generally mild and may include headache, arthralgias, myalgias, nausea, anorexia, diarrhea, rash and renal dysfunction. Uncommon, but potentially severe adverse events include hypersensitivity reactions, lupus-like syndromes and pancytopenia.
Hepatotoxicity
In clinical trials conducted in children with Wilson disease, serum aminotransferase levels generally improved or were stable during treatment with trientine. There have been no clinical reports of acute liver injury with jaundice attributed to trientine, even with overdoses. Patients with Wilson disease typically have mild-to-moderate serum aminotransferase elevations and may have signs and symptoms of cirrhosis. Improvement in liver injury in Wilson disease typically requires months to years of treatment.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Drug Class: Chelating Agents, Wilson Disease Agents
Other Drugs in the Subclass, Wilson Disease: Dimercaprol, Penicillamine, Zinc
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Trientine – Generic, Syprine®
DRUG CLASS
Chelating Agents
Product labeling at DailyMed, National Library of Medicine, NIH
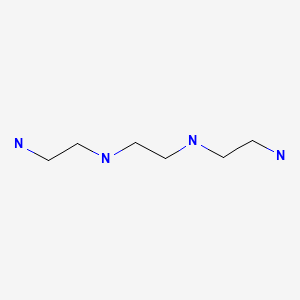
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Trientine | 112-24-3 | C6-H18-N4 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 25 July 2020
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999; trientine is not discussed).
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Textbook on hepatotoxicity; chelating agents are not discussed).
- Byrns MC, Penning TM. Treatment of metal exposure. Environmental toxicology: carcinogens and heavy metals. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1311-5.(Textbook of pharmacology and therapeutics).
- Walshe JM. Penicillamine, a new oral therapy for Wilson's disease. Am J Med. 1956;21:487–95. [PubMed: 13362281](Initial studies on efficacy of oral penicillamine [ β,β-dimethyl cysteine, a monothiol] in inducing cupruresis in Wilson disease and lack of effect of cysteine and methionine; no toxic reactions were observed).
- Walshe JM. Triethylene tetramine. Lancet. 1970;2(7664):154. [PubMed: 4194539](Early studies of trientine used chemical preparations that had to be modified for human use and induced a prompt cupruresis, but were often contaminated with impurities that could be toxic).
- Dubois RS, Rodgerson DO, Slovis TL, Hambidge KM, Bianchi TA. Triethylene tetramine dihydrochloride in Wilson’s disease. Lancet. 1970;2:775. [PubMed: 4195996](13 year old boy who developed leukopenia during penicillamine therapy responded to trientine [1 g daily] with prompt cupruresis, after which he was able to tolerate penicillamine).
- Walshe JM. Copper chelation in patients with Wilson’s disease. A comparison of penicillamine and triethylene tetramine dihydrochloride. Q J Med. 1973;42:441–52. [PubMed: 4728043](Trientine induced a cupruresis in 18 patients with Wilson disease which was less than with penicillamine, but was substantial particularly in patients who had never been treated, suggesting that it might be an alternative for patients intolerant to penicillamine therapy).
- Epstein O, Sherlock S. Triethylene tetramine dihydrochloride toxicity in primary biliary cirrhosis. Gastroenterology. 1980;78:1442–5. [PubMed: 6445305](Four patients with primary biliary cirrhosis and elevated hepatic copper levels developed intolerable side effects within two weeks of starting trientine, marked by anorexia, fatigue, epigastric pain, muscle pains, skin rash and anemia).
- Walshe JM. Treatment of Wilson’s disease with trientine(triethylene tetramine) dihydrochloride. Lancet. 1982;1:643–7. [PubMed: 6121964](Among 20 patients with Wilson disease who were intolerant of penicillamine therapy, all responded to trientine therapy although complications of penicillamine [lupus syndrome, elastosis perforans] did not always improve; no evidence of toxicity including hepatotoxicity).
- Hill GM, Brewer GJ, Juni JE, Prasad AS, Dick RD. Treatment of Wilson's disease with zinc. II. Validation of oral 64copper with copper balance. Am J Med Sci. 1986;292:344–9. [PubMed: 3799705](Assessment of copper update using a radioactive copper uptake study demonstrated that patients with Wilson disease on zinc have a low [<1%], while those on no therapy or on penicillamine or trientine had normal update levels [~6%]).
- Scheinberg IH, Jaffe ME, Sternlieb I. The use of trientine in preventing the effects of interrupting penicillamine therapy in Wilson's disease. N Engl J Med. 1987;317:209–13. [PubMed: 3600712](Eleven patients with Wilson disease who were unable to tolerate penicillamine therapy were treated successfully with trientine for 2-15 years; no mention of effect on copper levels or side effects).
- Dubois RS, Rodgerson DO, Hambidge KM. Treatment of Wilson's disease with triethylene tetramine hydrochloride (Trientine). J Pediatr Gastroenterol Nutr. 1990;10:77–81. [PubMed: 2324883](Seven patients, ages 13 to 33 years, with Wilson disease intolerant of penicillamine were treated with trientine for up to 16 years with stable disease and "no serious untoward side effects").
- Saito H, Watanabe K, Sahara M, Mochizuki R, Edo K, Ohyama Y. Triethylene-tetramine (trien) therapy for Wilson’s disease. Tohoku J Exp Med. 1991;164:29–35. [PubMed: 1926144](Among 4 Japanese patients with Wilson disease intolerant of penicillamine treated with trientine, all had increased cupruresis and clinical improvement without adverse effects).
- Morita J, Yoshino M, Watari H, Yoshida I, Motohiro T, Yamashita F, Okano Y, Hashimoto T. Wilson's disease treatment by triethylene tetramine dihydrochloride (trientine, 2HCl): long-term observations. Dev Pharmacol Ther. 1992;19:6–9. [PubMed: 1307347](19 year old girl and 14 year old boy with Wilson disease and intolerant of penicillamine therapy were treated with trientine [2-3 g daily] for 8 years, with excellent cupruresis and clinical responses and no adverse events except for low serum iron levels without anemia).
- Condamine L, Hermine O, Alvin P, et al. Acquired sideroblastic anaemia during treatment of Wilson’s disease with triethylene tetramine dihydrochloride. Br J Haematol. 1993;83:166–8. [PubMed: 8435326](15 year old girl with severe Wilson disease had marked clinical improvement, but developed sideroblastic anemia 4 months after switching from penicillamine to high doses of trientine [2250 mg daily], which resolved on lowering the dose).
- Moreno Pérez-Crespo JL, García de la Rocha ML, Martín Araguz A, Olmedilla N, Rodríguez Arias CA, Porta J, Moreno Martínez JM. Rev Neurol. 1995;23:145–7. [Wilson disease: a new case treated with trientine] Spanish. [PubMed: 8548611](20 year old woman with Wilson disease and cirrhosis with thrombocytopenia was treated with trientine rather than penicillamine and had a prompt cupruresis).
- Dahlman T, Hartvig P, Löfholm M, Nordlinder H, Lööf L, Westermark K. Long-term treatment of Wilson's disease with triethylene tetramine dihydrochloride (trientine). QJM. 1995;88:609–16. [PubMed: 7583074](Among 19 Swedish patients with Wilson disease treated with trientine for 4 months to 17 years, 16 had clinical improvement whereas one deteriorated and was switched to penicillamine; serious long term outcomes included liver failure requiring transplantation in 2, and colitis responding to withdrawal of the drug in 2 patients).
- Perry AR, Pagliuca A, Fitzsimons EJ, Mufti GJ, Williams R. Acquired sideroblastic anaemia induced by a copper-chelating agent. Int J Hematol. 1996 Jul;64:69–72. [PubMed: 8757970](19 year old girl with Wilson disease developed myasthenia gravis after 2 years of penicillamine therapy, was switched to trientine [2.4 g daily] and soon developed a sideroblastic anemia, which improved on reduction of the trientine dose [0.9 g daily]).
- Harders H, Cohnen E. Preparation of and clinical experiences with trien for the treatment of Wilson’s disease in absolute intolerance of D-penicillamine. Proc R Soc Med. 1977;70 Suppl 3:10–2. [PMC free article: PMC1543601] [PubMed: 122652](28 year old woman who was intolerant to penicillamine was successfully treated with trientine prepared by an improved method, with prompt cupruresis and improvement in neurologic symptoms).
- Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson's disease. Lancet. 2007;369(9559):397–408. [PubMed: 17276780](Review of the clinical features, pathogenesis, genetics, diagnosis and treatment including role of trientine).
- Roberts EA, Schilsky ML. AASLD. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089–111. [PubMed: 18506894](Thorough review of the cause, natural history, diagnosis and treatment of Wilson disease, with specific recommendations for use of penicillamine, trientine and zinc).
- Walshe JM. The conquest of Wilson's disease. Brain. 2009;132(Pt 8):2289–95. [PubMed: 19596747](History of the initial description of Wilson disease, its link to copper accumulation, and therapies several of which were developed by the author).
- Lu J, Poppitt SD, Othman AA, Sunderland T, Ruggiero K, Willett MS, Diamond LE, et al. Pharmacokinetics, pharmacodynamics, and metabolism of triethylenetetramine in healthy human participants: an open-label trial. J Clin Pharmacol. 2010;50:647–58. [PubMed: 20145262](Studies in 24 healthy volunteers demonstrated that trientine increases urine copper excretion and its effects were not influenced by N-acetyltransferase [NTA] 2 phenotypes).
- Weiss KH, Stremmel W. Evolving perspectives in Wilson disease diagnosis: treatment and monitoring. Curr Gastroenterol Rep. 2012;14:1–7. [PubMed: 22083169](Review of the diagnosis and management of Wilson disease, including the role of genetic testing and the choice of medical therapies).
- Weiss KH, Thurik F, Gotthardt DN, Schäfer M, Teufel U, Wiegand F, Merle U, et al. EUROWILSON Consortium. Efficacy and safety of oral chelators in treatment of patients with Wilson disease. Clin Gastroenterol Hepatol. 2013;11:1028–35.e1. [PubMed: 23542331](Retrospective analysis of 380 patients with Wilson disease from referral centers in Germany and Austria, including 141 who were treated with trientine and 326 with penicillamine, found higher rate of improvement with penicillamine, but also higher rate of adverse events leading to discontinuation [29% vs 7%], although there were no therapy related deaths; reasons for discontinuation in the trientine group included arthralgias, gastrointestinal upset, myalgias, leukopenia, rash, lupus erythematosus and increase in ANA titers; no mention of ALT elevations or hepatotoxicity).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which was attributed to trientine or other agents used to treat Wilson disease).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was attributed to penicillamine but none were attributed to trientine or other drugs for Wilson disease).
- Hashim A, Parnell N. A case of trientine overdose. Toxicol Int. 2015;22:158–9. [PMC free article: PMC4721165] [PubMed: 26862278](40 year old man with Wilson disease and cirrhosis took an overdose of trientine [60 mg] and developed dizziness, nausea and vomiting that resolved within 48 hrs; no mention of ALT elevations or hepatotoxicity).
- Ala A, Aliu E, Schilsky ML. Prospective pilot study of a single daily dosage of trientine for the treatment of Wilson disease. Dig Dis Sci. 2015;60:1433–9. [PMC free article: PMC4427615] [PubMed: 25605552](Among 8 patients with Wilson disease on long term copper chelation therapy who were switched to once-daily weight based trientine, all tolerated therapy well with stable copper balance and minor fluctuations in serum aminotransferase levels).
- Pfeiffenberger J, Beinhardt S, Gotthardt DN, Haag N, Freissmuth C, Reuner U, Gauss A, et al. Pregnancy in Wilson's disease: management and outcome. Hepatology. 2018;67:1261–9. [PubMed: 28859232](Among 282 pregnancies in 136 patients with Wilson disease analyzed in a retrospective, multicenter study, spontaneous abortion was less common among patients being treated with penicillamine [17%] or zinc [10%] than untreated patients [41%], which was proportionally greater than with trientine [28%] and similar to that in patients who discontinued therapy during pregnancy [36%]; worsening of liver disease arose in some patients on treatment but was self-limiting and resolved after delivery; birth defects were found in 7 of 209 [3%] newborns overall and in 4 of 98 women taking penicillamine).
- Roberts EA. Update on the diagnosis and management of Wilson disease. Curr Gastroenterol Rep. 2018;20:56. [PubMed: 30397835](Review of recent advances in the understanding of the pathogenesis, clinical features, diagnosis, and treatment of Wilson disease).
- Appenzeller-Herzog C, Mathes T, Heeres MLS, Weiss KH, Houwen RHJ, Ewald H. Comparative effectiveness of common therapies for Wilson disease: A systematic review and meta-analysis of controlled studies. Liver Int. 2019;39:2136–52. [PubMed: 31206982](Systematic review of literature on therapies of Wilson disease found no differences in outcomes with penicillamine vs zinc, but higher rates of adverse events and discontinuations with penicillamine; no mention of hepatotoxicity or ALT elevations).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Trientine tetrahydrochloride versus penicillamine for maintenance therapy in Wilson disease (CHELATE): a randomised, open-label, non-inferiority, phase 3 trial.[Lancet Gastroenterol Hepatol. ...]Trientine tetrahydrochloride versus penicillamine for maintenance therapy in Wilson disease (CHELATE): a randomised, open-label, non-inferiority, phase 3 trial.Schilsky ML, Czlonkowska A, Zuin M, Cassiman D, Twardowschy C, Poujois A, Gondim FAA, Denk G, Cury RG, Ott P, et al. Lancet Gastroenterol Hepatol. 2022 Dec; 7(12):1092-1102. Epub 2022 Sep 30.
- Efficacy and safety of oral chelators in treatment of patients with Wilson disease.[Clin Gastroenterol Hepatol. 2013]Efficacy and safety of oral chelators in treatment of patients with Wilson disease.Weiss KH, Thurik F, Gotthardt DN, Schäfer M, Teufel U, Wiegand F, Merle U, Ferenci-Foerster D, Maieron A, Stauber R, et al. Clin Gastroenterol Hepatol. 2013 Aug; 11(8):1028-35.e1-2. Epub 2013 Mar 28.
- Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease.[Arch Neurol. 2006]Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease.Brewer GJ, Askari F, Lorincz MT, Carlson M, Schilsky M, Kluin KJ, Hedera P, Moretti P, Fink JK, Tankanow R, et al. Arch Neurol. 2006 Apr; 63(4):521-7.
- Review Wilson Disease Agents.[LiverTox: Clinical and Researc...]Review Wilson Disease Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Trientine induced colitis during therapy for Wilson disease: a case report and review of the literature.[BMC Pharmacol Toxicol. 2015]Review Trientine induced colitis during therapy for Wilson disease: a case report and review of the literature.Boga S, Jain D, Schilsky ML. BMC Pharmacol Toxicol. 2015 Nov 20; 16:30. Epub 2015 Nov 20.
- Trientine - LiverToxTrientine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...