NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tolmetin is a nonsteroidal antiinflammatory drug (NSAID) that is available by prescription only and used for therapy of chronic arthritis. Tolmetin is associated with low rates of serum aminotransferase elevations during therapy and has been linked to rare instances of clinically apparent drug induced liver injury.
Background
Tolmetin (tol' met in) belongs to the acetic acid derivative class of NSAIDs similar to diclofenac, sulindac and indomethacin. Like other NSAIDs, tolmetin is a cyclo-oxygenase (Cox-1 and Cox-2) inhibitor which blocks the formation of prostaglandins that are important in pain and inflammatory pathways. Tolmetin has analgesic as well as antipyretic and antiinflammatory activities. Tolmetin is one of the oldest NSAIDs in clinical use having been approved in the United States in 1976. It is currently not commonly used, having been replaced by NSAIDs with longer half-lives and better tolerance. The current indications for tolmetin include osteoarthritis, rheumatoid arthritis, ankylosing spondylitis and juvenile rheumatoid arthritis. Tolmetin is available only by prescription and in several generic forms of 200, 400 and 600 mg tablets or capsules and formerly under the brand name Tolectin. The usual dose in adults is 1200 to 1800 mg per day in divided doses. As with other NSAIDs, tolmetin is generally well tolerated, but side effects can include headache, dizziness, somnolence, dyspepsia, abdominal discomfort, diarrhea, peripheral edema and hypersensitivity reactions. Rare but serious adverse events from NSAIDs include gastrointestinal ulceration and bleeding, increased risk for cardiovascular disease, renal dysfunction and hypersensitivity reactions including anaphylaxis, exfoliative dermatitis and Stevens Johnson syndrome.
Hepatotoxicity
Up to 5% of patients taking tolmetin chronically experience at least transient serum aminotransferase elevations. These may resolve even with drug continuation. Marked aminotransferase elevations (>3 fold elevated) occur in <1% of patients. There have been no convincing cases of tolmetin induced liver injury published in the literature and tolmetin is not mentioned as an etiologic agent in large case series on drug induced liver injury or acute liver failure. Reports of liver injury including hepatitis and acute liver failure attributed to tolmetin have been received by the Food and Drug Administration. Thus, liver injury from tolmetin may occur, but is rare. The latency, clinical features and outcome of tolmetin induced liver injury have not been described.
Likelihood score: D (possible, rare cause of clinically apparent liver injury).
Mechanism of Injury
Tolmetin has a short half-life and is extensively metabolized by the liver. The mechanism of tolmetin hepatotoxicity is not known.
Outcome and Management
The asymptomatic elevations in serum aminotransferase levels are usually self-limited and resolve even with continuing tolmetin. Convincing instances of fatal and chronic cases have not been described in the literature, but tolmetin like most drugs in the acetic acid class of NSAIDs (diclofenac, indomethacin) should be considered capable of causing clinically apparent acute liver injury.
Drug Class: Nonsteroidal Antiinflammatory Drugs
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tolmetin – Generic, Tolectin®
DRUG CLASS
Nonsteroidal Antiinflammatory Drugs
Product labeling at DailyMed, National Library of Medicine, NIH
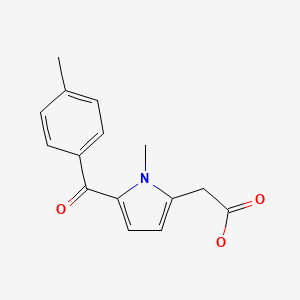
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Tolmetin | 26171-23-3 | C15-H15-N-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 March 2020
Abbreviations: NSAIDs, nonsteroidal antiinflammatory drugs.
- Zimmerman HJ. Drugs used to treat rheumatic and musculospastic disease. The NSAIDS. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 517-41.(Review of hepatotoxicity of NSAIDs published in 1999; although there are no case reports in the literature, several instances of heptocellular injury attributed to tolmetin have been reported to the FDA).
- Lewis JH, Stine JG. Nonsteroidal anti-inflammatory drugs and leukotriene receptor antagonists: pathology and clinical presentation of hepatotoxicity. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd. Amsterdam: Elsevier, 2013, pp. 369-401.(Review of hepatotoxicity of NSAIDs mentions that about 5% of recipients develop minor elevations in aminotransferase levels, but that clinically apparent liver injury from tolmetin is very rare).
- Grossner T, Smyth EM, Fitzgerald GA. Pharmacotherapy of inflammation, fever, pain, and gout. In, Brunton LL, Hilal-Dandan R, Knollman BC. Goodman & Gilman’s The pharmacological basis of therapeutics, 13th ed. New York: McGraw-Hill, 2018. pp. 685-709.(Textbook of pharmacology and therapeutics).
- O'Brien WM. Long-term efficacy and safety of tolmetin sodium in treatment of geriatric patients with rheumatoid arthritis and osteoarthritis: a retrospective study. J Clin Pharmacol. 1983;23:309–23. [PubMed: 6350377](Retrospective review of 847 geriatric patients with rheumatoid arthritis or osteoarthritis treated with tolmetin, AST levels usually decreased on treatment, but minor elevations found in 19 patients [2%]; no mention of hepatitis or jaundice).
- Jick H, Jick SS, Hunter JR, Walker AM. Follow-up study of tolmetin users. Pharmacotherapy. 1989;9:91–4. [PubMed: 2726592](Follow up of 7 years of 8370 tolmetin users for reports of hospitalization possibly caused by drug: 692 hospitalized within 90 days of prescription, but only 17 were possibly related, 11 for peptic ulcer, 1 for hepatitis, but arose >30 days after stopping and was considered unlikely to be related).
- Zimmerman HJ. Update of hepatotoxicity due to classes of drugs in common clinical use: non-steroid drugs, anti-inflammatory drugs, antibiotics, antihypertensives, and cardiac and psychotropic agents. Semin Liver Dis. 1990;10:322–8. [PubMed: 2281340](Extensive and excellent review article on liver injury due to NSAIDs; tolmetin mentioned as having led to little or no liver injury).
- Shaw GR, Anderson WR. Multisystem failure and hepatic microvesicular fatty metamorphosis associated with tolmetin ingestion. Arch Pathol Lab Med. 1991;115:818–21. [PubMed: 1863194](15 year old girl with juvenile rheumatoid arthritis developed fatigue within 10 days of starting tolmetin and was admitted with seizures, acidosis and progressive renal and pulmonary failure; ALT 440 U/L, AST 4378 U/L, LDH 7073 U/L, Alk P 212 U/L and bilirubin 1.2 mg/dL dying after 3 days; microvesicular fat on autopsy. High tolmetin levels detected suggestive of an overdose leading to shock and lactic acidosis).
- Rostom A, Goldkind L, Laine L. Nonsteroidal anti-inflammatory drugs and hepatic toxicity: a systematic review of randomized controlled trials in arthritis patients. Clin Gastroenterol Hepatol. 2005;3:489–98. [PubMed: 15880319](Review of randomized clinical trials of NSAIDS for frequency of adverse events; ALT >3 fold ULN in 0.43% of ibuprofen, 0.43% naproxen, 0.42% celecoxib, 1.8% rofecoxib, 3.55% diclofenac and 0.29% of placebo recipients, rare liver related severe adverse events or deaths with any; no mention on tolmetin).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](Survey of all cases of drug induced liver injury with fatal outcome from Swedish Adverse Drug Reporting system from 1966-2002; among 103 cases, 3 attributed to naproxen, but none to tolmetin).
- Lapeyre-Mestre M, de Castro AM, Bareille MP, Del Pozo JG, Requejo AA, Arias LM, et al. Non-steroidal anti-inflammatory drug-related hepatic damage in France and Spain: analysis from national spontaneous reporting systems. Fundam Clin Pharmacol. 2006;20:391–5. [PubMed: 16867024](Analysis of reports of liver injury from NSAIDs from France and Spain from 1982-2001; tolmetin was not listed among more than 29,000 NSAID related liver adverse event reports).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, NSAIDs were implicated as a sole agent in 8 cases [4 diclofenac, 2 celecoxib, 1 meloxicam and 1 oxaprozin] and as one of several agents in 3 cases [1 diclofenac, 1 celecoxib, 1 ibuprofen]).
- Bessone F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J Gastroenterol. 2010;16:5651–61. [PMC free article: PMC2997980] [PubMed: 21128314](Review of estimated frequency of drug induced liver injury due to NSAIDs from large published epidemiological studies; no mention or discussion of tolmetin).
- Lapeyre-Mestre M, Grolleau S, Montastruc JL., Adsociation Française des Centres Régionaux de Pharmacovigilance (CRPV). Adverse drug reactions associated with the use of NSAIDs: a case/noncase analysis of spontaneous reports from the French pharmacovigilance database 2002-2006. Fundam Clin Pharmacol. 2013;27:223–30. [PubMed: 21929527](Analysis of 42,389 spontaneous serious adverse event reports to the French Pharmacovigilance database on 8 NSAIDs between 2002 and 2006; liver adverse events were most frequent with nimesulide [0.15 per million daily doses] compared to diclofenac [0.09], ketoprofen [0.09] piroxicam [0.06], naproxen [0.04], meloxicam [0.03], and tenoxicam [0.03]; tolmetin not discussed).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 6 attributed to diclofenac [ranking 2nd], but none due to tolmetin).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, the most common class of implicated agents being NSAIDs [n=62, 32%], and specific agents were nimesulide [n=53], piroxicam [5], diclofenac [2], gold salts [1], and naproxen [1]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 28 were attributed to NSAIDs [Schmeltzer 2016]).
- Schmeltzer PA, Kosinski AS, Kleiner DE, Hoofnagle JH, Stolz A, Fontana RJ, Russo MW., Drug-Induced Liver Injury Network (DILIN). Liver injury from nonsteroidal anti-inflammatory drugs in the United States. Liver Int. 2016;36:603–9. [PMC free article: PMC5035108] [PubMed: 26601797](Among 1221 cases of drug induced liver injury enrolled in a prospective, US database between 2004 and 2014, 30 cases [2.5%] were attributed to NSAIDs, most commonly diclofenac [n=16], but also celecoxib [3], meloxicam [3], etodolac [2], ibuprofen [2], oxaprozin [2], valdecoxib [1] and sulindac [1], but none due to tolmetin).
- Donati M, Conforti A, Lenti MC, Capuano A, Bortolami O, Motola D, Moretti U, et al. DILI-IT Study Group. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case-control study in Italy. Br J Clin Pharmacol. 2016;82:238–48. [PMC free article: PMC4917796] [PubMed: 26991794](Among 179 cases of acute liver injury and 1770 controls admitted to 9 Italian hospitals between 2010 and 2014, NSAIDs used more frequently in cases compared to controls included nimesulide [17% vs 10%: odds ratio 1.88] and ibuprofen [14% vs 10%: odds ratio 1.59] and risk was higher in those taking higher doses).
- Meunier L, Larrey D. Recent advances in hepatotoxicity of non-steroidal anti-inflammatory drugs. Ann Hepatol. 2018;17:187–91. [PubMed: 29469052](Review of the hepatotoxicity of NSAIDS mentions the most commonly implicated are diclofenac, nimesulide, sulindac, ibuprofen, piroxicam, naproxen and aspirin).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Piroxicam.[LiverTox: Clinical and Researc...]Review Piroxicam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Diclofenac.[LiverTox: Clinical and Researc...]Review Diclofenac.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Ketoprofen.[LiverTox: Clinical and Researc...]Review Ketoprofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Fenoprofen.[LiverTox: Clinical and Researc...]Review Fenoprofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Flurbiprofen.[LiverTox: Clinical and Researc...]Review Flurbiprofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Tolmetin - LiverToxTolmetin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...