NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The thiazides are the most commonly used oral diuretics and are widely used in the therapy of hypertension and congestive heart failure, as well as the treatment of edema due to local, renal and hepatic causes. Only rare instances of clinically apparent liver injury have been linked to use of thiazide diuretics.
Background
The benzothiazide diuretics are structurally related drugs that act by inhibition of sodium (and chloride) transport in the distal convoluted tubule by binding to and inhibiting the Na+-Cl- symporter. As a result, there is increased excretion of sodium and water and an associated loss of potassium. Chronic therapy may also result in increased calcium and magnesium loss. The thiazide diuretics are grouped together based upon shared chemical, sulfonamide-like, structure. More recently non-benzothiazide drugs with a similar mechanism of action have been developed (metolazone, indapamide), which are referred to as thiazide-like diuretics. The thiazide and thiazide-like diuretics are all available generically and differ largely in their pharmacokinetic properties of oral availability, relative potency, serum and effective half-life and route of elimination. The general indications for the thiazide diuretics are treatment of hypertension, congestive heart failure and edema.
Thiazide diuretics are available in multiple forms and all are available generically. Bendroflumethiazide (ben' droe floo" me thye' a zide) is available in tablets of 2.5 mg in generic forms; recommended oral doses in adults are 2.5 to 10 mg in two divided doses. It is also available in a fixed dose (5 mg) with nadolol (40 or 80 mg) generically and under the brand name Corzide.
Chlorothiazide (klor" oh thye' a zide) is available in tablets of 250 and 500 mg generically and under the trade name of Diuril; recommended oral doses in adults are 500 to 1000 mg once or twice daily. It is also available as a lyophilized powder for injection in vials of 500 mg.
Chlorthalidone (klor thal' i done) is one of the most frequently used oral thiazide diuretics. It is available in tablets of 25 and 50 mg generically and under the brand name of Thalitone; recommended oral doses in adults are 25 to 100 mg once daily or 100 mg every other day.
Hydrochlorothiazide (hye' droe klor" oh thye' a zide) is available in tablets of 25 and 50 mg and as capsules of 12.5 mg generically and under the trade names of Hydrodiuril, Microzide and Esidrix; recommended oral doses in adults are 12.5 to 50 mg daily given in one or two divided doses.
Methyclothiazide (meth" i kloe thye' a zide) was previously available in tablets of 2.5 and 5 mg generically and under the trade name of Enduron; recommended oral doses in adults were 2.5 to 5 mg once daily.
Polythiazide (pol" ee thye' a zide) is available in tablets of 1, 2 and 4 mg generically and under the trade name of Renese; typical oral doses in adults are 2 to 4 mg in one or two divided doses daily.
Metolazone (me tol' a zone) is a thiazide-like diuretic that is available as tablets of 2.5 and 5 mg generically and under the trade name of Zaroxolyn; recommended oral doses in adults are 2.5 to 20 mg once daily.
Indapamide (in dap' a mide) is a thiazide-like diuretic that is available as tablets of 1.25 and 2.5 mg generically and under the trade name of Lozol; recommended oral doses in adults are 1.25 to 5 mg once daily.
The thiazide diuretics are some of the most commonly used medications and are usually well tolerated except in high doses. Common side effects of the thiazide and thiazide-like diuretics can include nausea, dizziness, headache, polyuria, dehydration, dry mouth, hyponatremia, hypokalemia and hypomagnesia. Chronic therapy may be associated with hyperuricemia and gout, hyperglycemia and hyperlipidemia, and possibly an increased risk of cholecystitis. Rare adverse reactions include pancreatitis and hypersensitivity reactions. Many of the thiazide diuretics are also available in fixed dose combination with other antihypertensive medications or with potassium-sparing diuretics.
Hepatotoxicity
The thiazide diuretics have not been shown to cause serum aminotransferase elevations to an appreciable extent, and are often used as a control group in assessing adverse events including serum aminotransferase elevations of newer antihypertensive medications. Despite their widespread use, the thiazide diuretics have only rarely been implicated in cases of clinically apparent acute liver injury. No clear signature or clinical pattern has been demonstrated in the rare case reports and in some instances other potentially hepatotoxic medications were being used and other possible diagnoses were present. The usual latency period to onset has been short (few days to several weeks) and the pattern of serum enzyme elevations has ranged from hepatocellular, to mixed and cholestatic (Cases 1 and 2). Immunoallergic features were uncommon as was autoantibody formation. Recovery was usually rapid upon stopping. At present, only hydrochlorothiazide has been implicated in more than 3 instances, while chlorothiazide, indapamide, metolazone and polythiazide have been implicated in one or two instances in causing drug induced liver injury. Hydrochlorothiazide is the most commonly used thiazide diuretic and it is not surprising that it has been implicated most commonly. Regardless, liver injury from thiazide diuretics is exceedingly rare. The similarity in chemical structure among the thiazide diuretics suggests that liver injury might be a class effect.
Hydrochlorothiazide likelihood score: C (probable rare cause of clinically apparent liver injury).
Chlorothiazide, indapamide, metolazone and polythiazide likelihood score: D (possible rare causes of clinically apparent liver injury).
Chlorthalidone, Bendroflumethiazide, and methyclothiazide likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
Some instances of hepatic injury attributed to the thiazide diuretics have appeared to be due to metabolic idiosyncrasy.
Outcome and Management
The few instances of hepatic injury attributed to thiazide diuretics have been self-limited and rapidly reversed upon stopping the medication. There have been no convincing instances of acute liver failure or prolonged jaundice or vanishing bile duct syndrome associated with the thiazide diuretics. There have been no reports of cross challenges among the different thiazide and thiazide-like diuretics.
Drug Class: Diuretics, Thiazide Diuretics
CASE REPORTS
Case 1. Acute anicteric liver injury due to hydrochlorothiazide.(1)
A 72 year old woman with hypertension developed anorexia, nausea and right upper quadrant abdominal pain 6 days after starting hydrochlorothiazide. She had no history of liver disease and had normal liver tests shortly before starting therapy. She took no other medications except for calcium and did not drink alcohol or have risk factors for viral hepatitis. Physical examination demonstrated tenderness over the liver, but no jaundice. Laboratory tests showed elevations in serum aminotransferase levels, alkaline phosphatase and gamma glutamyl transpeptidase (GGT) (Table). White blood cell counts were normal. Tests for hepatitis A, B and C were negative as were autoantibodies. Liver ultrasound was normal. Hydrochlorothiazide was stopped and symptoms resolved within days. Serum aminotransferase levels were normal within two weeks and alkaline phosphatase and GGT within three weeks of stopping treatment.
Key Points
| Medication: | Hydrochlorothiazide (25 mg daily) |
|---|---|
| Pattern: | Mixed (R=3.4) |
| Severity: | 1+ (enzyme elevations and symptoms) |
| Latency: | 6 days to onset of symptoms |
| Recovery: | 2-3 weeks |
| Other medications: | Calcium |
Laboratory Values
Comment
The acute liver injury was mild but associated with symptoms arising within days of starting a low dose of hydrochlorothiazide. While the rapidity of onset suggests a hypersensitivity reaction, there were no other manifestations of an immunoallergic reaction (fever, rash, eosinophilia). Hydrochlorothiazide is one of the most frequently used medications in the world, but has only rarely been implicated in acute liver injury. Most large cases series of drug induced liver injury have not included cases attributable to thiazide diuretics.
Case 2. Recurrent acute liver injury due to hydrochlorothiazide.(2)
A 47 year old man developed jaundice 20 days after starting hydrochlorothiazide (50 mg daily) for hypertension. He had no history of liver disease, jaundice, alcohol abuse or risk factors for viral hepatitis. He was taking no other medications. He complained of change in taste and abdominal upset, but no fever, rash or itching. Physical examination showed jaundice, but no evidence of chronic liver disease. Laboratory testing showed a total serum bilirubin of 9.5 mg/dL (direct 6.0 mg/dL) and marked elevations in serum aminotransferase levels (ALT 640 U/L, AST 1000 U/L), with minimal increase in alkaline phosphatase (5.4 Bodansky Units) and gamma glutamyl transpeptidase (60 U/L). The total white count was normal without eosinophilia. The prothrombin time and serum albumin values were normal. Tests for hepatitis B were negative as were autoantibodies. Hydrochlorothiazide was stopped and laboratory tests fell to normal within a month. Because of continuing hypertension, hydrochlorothiazide was restarted. Seventeen days later, he presented again with jaundice and serum aminotransferase levels were again elevated. A liver biopsy showed acute hepatocellular necrosis and inflammation compatible with an acute hepatitis due to a medication. Tests for hepatitis A and B and autoantibodies were negative. Hydrochlorothiazide was stopped again and laboratory values fell rapidly into the normal range. Several years later, he was seen at another medical center in another country and hydrochlorothiazide was restarted. Eighteen days later, he presented for the third time with jaundice. Stopping the medication led to improvements in laboratory tests that were normal two months later.
Key Points
| Medication: | Hydrochlorothiazide (50 mg daily) |
|---|---|
| Pattern: | Hepatocellular (R=13) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 17-20 days to onset of jaundice on three occasions |
| Recovery: | 4-8 weeks |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| 0 | Hydrochlorothiazide (50 mg daily) given for 20 days | ||||
| 3 weeks | 0 | 640 | 5.4 | 9.5 | Admission |
| 7 weeks | 4 weeks | Normal | Normal | Normal | |
| 0 | Hydrochlorothiazide (50 mg daily) given for 17 days | ||||
| 2.5 weeks | 0 | 900 | 5.8 | ||
| 9 weeks | 7 weeks | Normal | Normal | Normal | |
| Hydrochlorothiazide (50 mg daily) given for 18 days | |||||
| 2.5 weeks | 0 | 1450 | 8.2 | ||
| 10 weeks | 8 weeks | Normal | Normal | Normal | |
| Normal Values | <35 | <4.5 | <1.2 | ||
Comment
A remarkably convincing case history of recurrent acute hepatitis arising 2 to 3 weeks after starting hydrochlorothiazide for hypertension. With each exposure the pattern of injury was hepatocellular, and the clinical phenotype was a mild acute hepatitis with rapid recovery upon stopping the medication. With each reexposure, the liver injury appeared again, each time without signs of hypersensitivity (no rash, fever or eosinophilia) and without shortening of the latency or worsening of the clinical syndrome. These features suggest that metabolic idiosyncrasy rather than hypersensitivity was the cause of the hepatic injury. Despite their use in millions of patients worldwide over the previous 50 years, this is one of the only convincing published cases of hepatotoxicity from thiazide diuretics.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Bendroflumethiazide – Generic, Naturetin®
Chlorothiazide – Generic, Diuril®
Chlorthalidone – Generic, Hygroton®
Hydrochlorothiazide – Generic, Esidrix®
Indapamide – Generic, Lozol®
Methyclothiazide – Generic
Metolazone – Generic, Zaroxolyn®
Polythiazide – Generic, Renese®
DRUG CLASS
Diuretics
COMPLETE LABELING (Bendroflumethiazide)
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Bendroflumethiazide | 73-48-3 | C15-H14-F3-N3-O4-S2 |
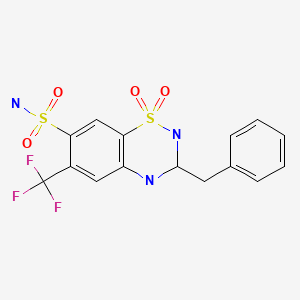
|
| Chlorothiazide | 58-94-6 | C7-H6-Cl-N3-O4-S2 |
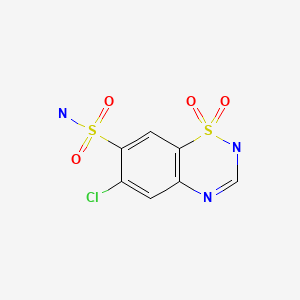
|
| Chlorthalidone | 77-36-1 | C14-H11-Cl-N2-O4-S |
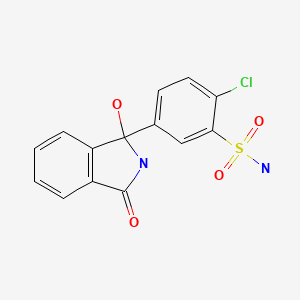
|
| Hydrochlorothiazide | 58-93-5 | C7-H8-Cl-N3-O4-S2 |
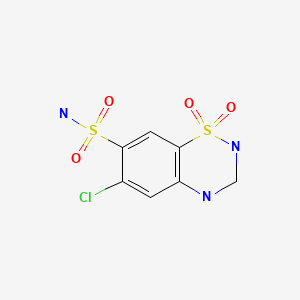
|
| Indapamide | 26807-65-8 | C16-H16-Cl-N3-O3-S |
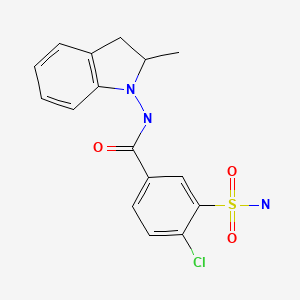
|
| Methyclothiazide | 135-07-9 | C9-H11-Cl2-N3-O4-S2 |
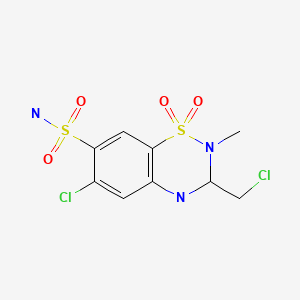
|
| Metolazone | 17560-51-9 | C16-H16-Cl-N3-O3-S |
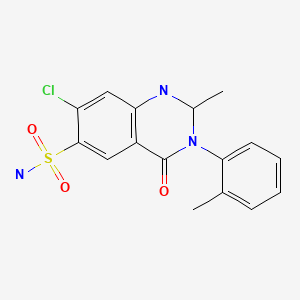
|
| Polythiazide | 346-18-9 | C11-H13-Cl-F3-N3-O4-S3 |
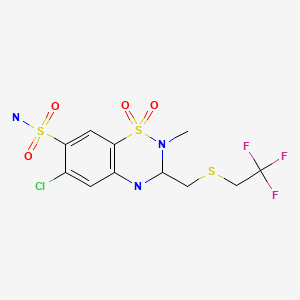
|
CITED REFERENCES
- 1.
- Arinzon Z, Alexander P, Berner Y. Hydrochlorothiazide induced hepato-cholestatic liver injury. Age Ageing. 2004;33:509–10. [PubMed: 15271638]
- 2.
- Anez MS, Dickson G, Zabala R, Zabaleta P, Pacheco A, Briceno D. Gastroenterol Hepatol. 1981;4:476–8. [Acute hepatitis due to hydrochlorothiazide: report of a case]
ANNOTATED BIBLIOGRAPHY
References updated: 13 October 2021
- Zimmerman HJ. Diuretic drugs. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 662-4.(Expert review of hepatotoxicity of diuretics published in 1999 mentions that clinically apparent liver injury due to diuretics is rare; cholestatic jaundice with features of hypersensitivity have been reported with some thiazide diuretics).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 519-40.(Review of hepatotoxicity of cardiovascular agents, mentions that thiazide diuretics can rarely cause cholestatic hepatitis).
- Jackson EK. Drugs affecting renal excretory function. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 445-70.(Textbook of pharmacology and therapeutics).
- Drerup AL, Alexander WA, Lumb GD, Cummins AJ, Clark GM. Jaundice occurring in a patient treated with chlorothiazide. N Engl J Med. 1958;259:534–6. [PubMed: 13578100](62 year old woman developed pruritus within 4 days and jaundice after 14 days of starting chlorothiazide [bilirubin 17.1 mg/dL, Alk P 14.6 BU, cholesterol 990 mg/dL], biopsy showing intrahepatic cholestasis and complete recovery 2 months after stopping chlorothiazide; had hepatomegaly and xanthelasma before treatment).
- Husebye KO. Jaundice with persisting pericholangiolitic inflammation in a patient treated with chlorothiazide. Report of a case. Am J Dig Dis. 1964;9:439–46. [PubMed: 14171621](58 year old woman developed jaundice after 3 years of therapy with chlorothiazide, but 10 weeks after restarting [bilirubin 6.1 mg/dL, AST 87 U/L, Alk P 8 times ULN], jaundice resolving within 6 weeks of stopping therapy, but persistent Alk P elevation and autopsy 10 months later showed periportal fibrosis and inflammation suggestive of primary biliary cirrhosis).
- Weisburst M, Self T, Peace R, Cooper J. Jaundice and rash associated with the use of phenobarbital and hydrochlorothiazide. South Med J. 1976;69:126–7. [PubMed: 128822](18 year old woman developed rash, fever and jaundice 6 weeks after starting hydrochlorothiazide and phenobarbital [bilirubin 7.2 mg/dL, AST 250 U/L, Alk P 350 U/L], resolving in 2 weeks on methylprednisolone; DRESS syndrome probably due to phenobarbital rather than diuretic).
- Gangitano JL, Foster SH, Contro RM. Nonfatal methazolamide-induced aplastic anemia. Am J Ophthalmol. 1978;86:138–9. [PubMed: 677225](83 year old man developed aplastic anemia 3 months after starting methazolamide for glaucoma; no mention of liver test abnormalities).
- Butaeye P, Hubschman B, Derrien G. Nouv Presse Med. 1979;8:1516. [Hepatitis during indapamide therapy] French. [PubMed: 471729](63 year old woman developed fatigue 8 months after starting indapamide and propranolol for hypertension [bilirubin 2.4 mg/dL, ALT 412 U/L, Alk P 31 U/L], with positive lymphocyte stimulation to indapamide and resolution upon stopping).
- Rosenberg L, Shapiro S, Slone D, Kaufman DW, Miettinen OS, Stolley PD. Thiazides and acute cholecystitis. N Engl J Med. 1980;303:546–8. [PubMed: 7402220](Case control study of 419 patients with acute cholecystitis and 1676 controls found increased frequency of recent use of thiazides [14% vs 8%: relative risk of 2.0]).
- Porter JB, Jick H, Dinan BJ. Acute cholecystitis and thiazides. N Engl J Med. 1981;304:954–5. [PubMed: 7207544](Two case control studies; one in Boston of 176 cases of acute cholecystitis and 704 controls found no association with recent thiazide use [5% vs 4%] and similar results from study in Seattle of 70 cases and 274 controls [14% vs 15%] for an overall relative risk of 1.0).
- Bourke JB, Langman MJ. Thiazide, diuretics, cholecystitis, and pancreatitis. N Engl J Med. 1981;304:233–4. [PubMed: 7442751](Case control study of 100 patients with acute pancreatitis and 100 controls found increase frequency of recent thiazide use [26% vs 11%], with highest rates in those with gallstone pancreatitis).
- Anez MS, Dickson G, Zabala R, Zabaleta P, Pacheco A, Briceno D. Gastroenterol Hepatol. 1981;4:476–8. [Acute hepatitis due to hydrochlorothiazide: report of a case](47 year old man developed jaundice 20 days after starting hydrochlorothiazide without rash, fever or eosinophilia [bilirubin 9.5 mg/dL, ALT 640 U/L, Alk P 1.3 times ULN], with recurrence twice 17 and 18 days after [bilirubin 5.8 and 8.2 mg/dL, ALT 900 and 1450 U/L], resolving in 6 weeks: Case 2, thiazide diuretics).
- McMaster KR 3rd, Hennigar GR. Drug-induced granulomatous hepatitis. Lab Invest. 1981;44:61–73. [PubMed: 7453131](Among 1500 liver biopsies done over 10 years, 6% had granulomas; diagnosis was sarcoidosis in 31%, tuberculosis 9%, medications 29% including methyldopa, hydralazine, metahydrin, metolazone, phenytoin, aspirin, isoniazid, cephalexin, penicillin, sulfonamides, procainamide, procarbazine, diazepam and BCPs).
- Van der Linden W, Ritter B, Edlund G. Acute cholecystitis and thiazides. Br Med J(Clin Res Ed). 1984;289:654–5. [PMC free article: PMC1443093] [PubMed: 6434025](In a population based study, 20% of 91 patients with acute cholecystitis versus 10% of 364 controls had recently received a thiazide diuretic: relative risk of 2.1 [1.1-3.9, 95% CI]).
- Kakar F, Weiss NS, Strite SA. Thiazide use and the risk of cholecystectomy in women. Am J Epidemiol. 1986;124:428–33. [PubMed: 3740043](Among female members of a group health cooperative, 25% of 153 patients undergoing gallbladder surgery were thiazide users vs 13% of 156 controls; relative risk 2.2 [1.2-4.0 95% CI]).
- Hourmand-Ollivier I, Dargere S, Cohen D, Galais MP, Mosquet B, Rousselot P, Dao T. Gastroenterol Clin Biol. 2000;24:464. [Fatal subfulminant hepatitis probably due to the combination benazepril-hydrochlorothiazide (Briazide)] French. [PubMed: 10844292](69 year old woman developed hepatitis 2 months after starting the combination of benazepril and hydrochlorothiazide [bilirubin 16.3 mg/dL, ALT 30 times ULN, Alk P 2 times ULN, prothrombin index 39%], with progressive hepatic failure and death).
- Gabrielli M, Gasbarrini A, Santoliquido A, Flore R, Gaetani E, Flex A, Pola P. Metolazone-induced cholestatic liver disease. Dig Liver Dis. 2001;33:501. [PubMed: 11572578](41 year old man with chronic heart failure developed jaundice 1-2 months after starting metolazone [bilirubin 29.6 mg/dL, ALT 86 U/L, Alk P 596 U/L], resolving within 3 weeks of stopping metolazone).
- Valhovd M, Kildahl-Andersen O. Tidsskr Nor Laegeforen. 2003;123:1202–3. [Drug-induced severe jaundice] Norwegian. [PubMed: 12789790](37 year old man developed jaundice 2 months after starting hydrochlorothiazide and amiloride for hypertension [bilirubin 5.8 rising to 28.3 mg/dL, ALT 2930 U/L, Alk P twice ULN, INR 1.4], with prolonged jaundice leading to 8 week course of prednisone, recovered but then developed pancytopenia: Case 1, amiloride).
- Arinzon Z, Alexander P, Berner Y. Hydrochlorothiazide induced hepato-cholestatic liver injury. Age ageing. 2004;33:509–10. [PubMed: 15271638](72 year old woman developed nausea and abdominal pain 6 days after starting hydrochlorothiazide [ALT 236 U/L, Alk P 146 U/L, bilirubin not mentioned], resolving within 4 weeks of stopping: Case 1, thiazide diuretics).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 137 [0.5%] were done for idiosyncratic drug induced acute liver failure, none were attributed to a diuretic).
- Leitzmann MF, Tsai CJ, Stampfer MJ, Willett WC, Giovannucci E. Thiazide diuretics and the risk of gallbladder disease requiring surgery in women. Arch Intern Med. 2005;165:567–73. [PubMed: 15767534](Analysis of thiazide diuretic use and gallbladder disease in the US Nurses’ Health Study of 81,351 women, ages 30-55 years, followed for ~20 years; found modest increase in relative risk of cholecystectomy in current users of thiazides [1.39 in multivariate analysis]).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](Survey of all cases of DILI with fatal outcome from Swedish Adverse Drug Reporting system from 1966-2002; 103 cases were identified one of which was associated with hydrochlorothiazide and one with amiloride use, but other more likely agents were being taken in both instances).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10-year period. Gastroenterology. 2005;129:512–21. [PubMed: 16083708](Reports of drug induced liver injury to a Spanish network found 570 cases; thiazide diuretics not mentioned as cause).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–8. [PubMed: 16054882](Survey of drug induced liver fatalities reported to WHO database between 1968-2003 revealed 4690 reports [89% from the US]; no diuretic found in the 20 most commonly implicated agents).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Population based survey of 126 cases of acute liver injury [24 with acute liver failure] due to drugs between 1993-1999 in Spain calculated relative risk of injury compared to the general population: hydrochlorothiazide was being taken by 7 and furosemide by 8 patients, but relative risk was not increased in comparison to a control group).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, no case was attributed to a diuretic).
- Drugs for hypertension. Treat Guidel Med Lett. 2009;7:1–10. [PubMed: 19107095](Brief overview of currently available drugs for hypertension with guidelines on their use and information on prices and toxicities: “thiazide diuretics are the first-line therapy for many patients with hypertension”).
- Björnsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatol. 2009;50:511–7. [PubMed: 19155082](Among 685 patients with drug induced liver injury, 23 [3.4%] were later hospitalized for chronic liver disease and 8 were showed to have cirrhosis, one of whom had acute icteric liver injury 3 years earlier attributed to the combination of amiloride and hydrochlorothiazide).
- Gabrielli M, Gasbarrini A, Santoliquido A, Flore R, Gaetani E, Flex A, Pola P. Metolazone-induced cholestatic liver disease. Dig Liver Dis. 2001;33:501. [PubMed: 11572578](42 year old man with cardiomyopathy developed jaundice 3 months after starting metolazone [bilirubin 29.6 mg/dL, ALT 86 U/L, Alk P 596 U/L], resolving within 20 days of stopping metolazone while continuing warfarin or enalapril).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](Among 313 cases of drug induced liver injury seen over a 12 year period at a large hospital in Bangalore, India, none were attributed to a diuretic).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Among 624,673 adverse event reports in children between 2000 and 2006 in the WHO VigiBase, no diuretic was mentioned among the 30 most common causes of liver injury).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which none were attributed to a diuretic).
- Taglietti F, Del Nonno F, Baiocchini A, Falasca L, Pieri S, Capone A, Grilli E, et al. Acute hepatocellular and cholestatic injury during therapy with hydrochlorothiazide – clinicohistopathologic findings: a case report. J Med Case Rep. 2010;4:332. [PMC free article: PMC2978230] [PubMed: 20964809](68 year old man developed fatigue and jaundice 10 days after starting hydrochlorothiazide and olmesartan [bilirubin 6.3 rising to 14.7 mg/dL, ALT 346 U/L, Alk P 1091 U/L], resolving within 3 months of stopping both drugs).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to a diuretic).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to a diuretic).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to a diuretic).
- Drugs for hypertension. Med Lett Drugs Ther. 2020;62(1598):73–80. [PubMed: 32555118](Concise summary of efficacy, safety, and costs of drug therapy of hypertension including the diuretics, focusing upon relative usefulness; no mention of hepatic adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review [Which place for thiazide and thiazide-like diuretics in patients with type 2 diabetes ?].[Rev Med Liege. 2018]Review [Which place for thiazide and thiazide-like diuretics in patients with type 2 diabetes ?].Scheen AJ, Krzesinski JM. Rev Med Liege. 2018 Apr; 73(4):176-182.
- Review The use of thiazides in chronic kidney disease.[Curr Hypertens Rev. 2014]Review The use of thiazides in chronic kidney disease.Karadsheh F, Weir MR. Curr Hypertens Rev. 2014; 10(2):81-5.
- [Thiazide and Thiazide-Like Diuretics in Therapy of Arterial Hypertension].[Kardiologiia. 2019][Thiazide and Thiazide-Like Diuretics in Therapy of Arterial Hypertension].Orlova YA, Kurlykina NV, Seredenina EM. Kardiologiia. 2019 Dec 13; 59(11):84-94. Epub 2019 Dec 13.
- Review Type 2 Diabetes and Thiazide Diuretics.[Curr Diab Rep. 2018]Review Type 2 Diabetes and Thiazide Diuretics.Scheen AJ. Curr Diab Rep. 2018 Feb 5; 18(2):6. Epub 2018 Feb 5.
- Review Diuretics in the treatment of hypertension. Part 1: thiazide and thiazide-like diuretics.[Expert Opin Pharmacother. 2014]Review Diuretics in the treatment of hypertension. Part 1: thiazide and thiazide-like diuretics.Tamargo J, Segura J, Ruilope LM. Expert Opin Pharmacother. 2014 Mar; 15(4):527-47. Epub 2014 Jan 20.
- Thiazide Diuretics - LiverToxThiazide Diuretics - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...