NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Temsirolimus is an inhibitor of cell proliferation and anticancer agent that is used as treatment of advanced renal cell cancer. Temsirolimus therapy is frequently associated with mild serum enzyme elevations, but has yet to be linked to instances of clinically apparent liver injury with jaundice.
Background
Temsirolimus (tem" sir oh' li mus) is an ester of sirolimus, both of which bind to the same intracellular receptor as tacrolimus and cyclosporine, but which block the "mammalian target of rapamycin" (mTOR) rather than calcineurin. mTOR is a serine/threonine kinase which plays an important role in signaling pathways of several cytokines and growth factors, which are involved in carcinogenesis and cancer progression. Inhibition of mTOR causes a decrease in protein synthesis and cell cycle arrest. Temsirolimus therapy has been shown to inhibit progression and prolong survival in patients with advanced and metastatic renal cell cancer. Temsirolimus was approved for use in the United States in 2007 and current indications are limited to therapy of advanced renal cell cancer. Temsirolimus is being actively investigated as therapy of other solid tumors. Temsirolimus is available as liquid solution for injection in vials of 25 mg/mL under the brand name of Torisel. The recommended dose is 25 mg intravenously once weekly until disease progression or unacceptable toxicity. Temsirolimus has many, largely dose dependent, side effects including hypersensitivity reactions, oral ulcers, diarrhea, nausea, poor appetite, fatigue, peripheral edema, rash, and anemia. Uncommon but potentially severe adverse events include hyperglycemia, thrombocytopenia, hypophosphatemia, hyperlipidemia, interstitial pneumonitis and severe hypersensitivity reactions including Stevens Johnson syndrome and angioedema.
Hepatotoxicity
Serum aminotransferase elevations occur in 30% to 40% and alkaline phosphatase in 60% to 70% of patients receiving temsirolimus, but the abnormalities are usually mild, asymptomatic and self-limiting, rarely requiring dose modification or discontinuation. Elevations of liver enzymes above 5 times the upper limit of normal occur in only 1% to 3% of patients. Since approval and wide spread clinical use, there have been no case reports of clinically apparent liver injury attributed to temsirolimus use. Temsirolimus, like sirolimus, is immunosuppressive, and reactivation of hepatitis B is considered a possible complication of therapy. Yet despite more than 10 years of clinical use, there have been no reports of reactivation of hepatitis B attributed to temsirolimus therapy. Thus, acute liver injury with jaundice due to temsirolimus is probably quite rare, if it occurs at all. Hypersensitivity reactions to temsirolimus infusions are not uncommon (for which reason premedication with an antihistamine is recommended) and instances of Stevens Johnson syndrome have been reported.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
Temsirolimus undergoes extensive hepatic metabolism, largely via the cytochrome P450 system (CYP 3A4). Liver injury might be due to a direct effect of temsirolimus or to a toxic intermediate of its metabolism. Temsirolimus is prone to drug-drug interactions if used with inhibitors or inducers of the cytochrome P450 drug metabolizing enzyme CYP 3A4.
Outcome and Management
Acute, symptomatic liver injury associated with temsirolimus therapy has not been described, and the serum enzyme elevations associated with its use are usually mild and transient, resolving spontaneously or with dose modification. Because temsirolimus can lead to reactivation of chronic hepatitis B, routine screening of patients for HBsAg before starting therapy is advisable. Patients who develop reactivation should be treated with an oral nucleoside analogue with potent activity against hepatitis B (entecavir or tenofovir). Temsirolimus is an ester of and partially metabolized to sirolimus and cross sensitivity to adverse effects between these two agents is likely. Whether such cross sensitivity extends to other inhibitors of mTOR (such as everolimus) or calcineurin (cyclosporine, tacrolimus) is not known.
Drug Class: Antineoplastic Agents, Miscellaneous; Transplant Drugs
Other Drugs with Similar Intracellular Actions: Cyclosporine, Everolimus, Mycophenolate, Sirolimus, Tacrolimus
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Temsirolimus – Generic, Torisel®
DRUG CLASS
Antineoplastic Agents; Transplant Drugs
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Temsirolimus | 162635-04-3 | C56-H87-N-O16 |
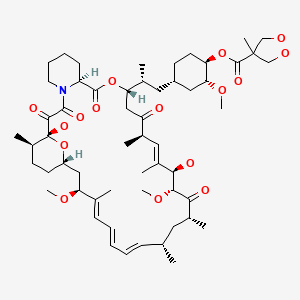
|
ANNOTATED BIBLIOGRAPHY
References updated: 04 November 2020
- Zimmerman HJ. Cyclosporine. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp.697-8.(Expert review of hepatotoxicity published in 1999 before the availability of temsirolimus).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Pathway targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL, Hillal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1203-36.(Textbook of pharmacology and therapeutics).
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, et al. Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. [PubMed: 17538086](Controlled trial of temsirolimus vs interferon alfa vs the combination in 626 patients with advanced renal cell carcinoma found prolongation of survival with temsirolimus compared to interferon [10.9 vs 7.3 months]; AST elevations occurred in 8% [>5 times ULN in 1%] of temsirolimus vs 14% of interferon [>5 times ULN in 4%] treated subjects).
- Bellmunt J, Szczylik C, Feingold J, Strahs A, Berkenblit A. Temsirolimus safety profile and management of toxic effects in patients with advanced renal cell carcinoma and poor prognostic features. Ann Oncol. 2008;19:1387–92. [PubMed: 18385198](Analysis of adverse events reported in trials of temsirolimus therapy of renal carcinoma; no discussion of hepatotoxicity or serum enzyme elevations).
- Simpson D, Curran MP. Temsirolimus: in advanced renal cell carcinoma. Drugs. 2008;68:631–8. [PubMed: 18370442](Concise review of the mechanism of action, pharmacology, efficacy and safety of temsirolimus in advanced renal cell carcinoma; no mention of serum enzyme elevations or hepatotoxicity).
- Bhatia S, Thompson JA. Temsirolimus in patients with advanced renal cell carcinoma: an overview. Adv Ther. 2009;26:55–67. [PubMed: 19172239](Review of the mechanism of action, efficacy and safety of temsirolimus mentions that therapy is associated with elevations in ALT and Alk P, but does not mention clinically apparent liver injury).
- Hudes GR, Berkenblit A, Feingold J, Atkins MB, Rini BI, Dutcher J. Clinical trial experience with temsirolimus in patients with advanced renal cell carcinoma. Semin Oncol. 2009;36 Suppl 3:S26–36. [PubMed: 19963097](Review of results of clinical trials of temsirolimus in advanced renal cell carcinoma; no mention of hepatotoxicity or serum enzyme elevations).
- Knox JJ, Qin R, Strosberg JR, Tan B, Kaubisch A, El-Khoueiry AB, Bekaii-Saab TS, et al. A phase II trial of bevacizumab plus temsirolimus in patients with advanced hepatocellular carcinoma. Invest New Drugs. 2015;33:241–6. [PubMed: 25318437](Among 28 patients with advanced hepatocellular carcinoma treated with temsirolimus, only 5 patients had a partial response and adverse events were common including fatigue, cytopenias, mucositis, diarrhea and bleeding episodes; no mention of ALT elevations but liver function was maintained).
- Yeo W, Chan SL, Mo FK, Chu CM, Hui JW, Tong JH, Chan AW, et al. Phase I/II study of temsirolimus for patients with unresectable hepatocellular carcinoma (HCC)- a correlative study to explore potential biomarkers for response. BMC Cancer 20152; 15: 395. [PMC free article: PMC4434865] [PubMed: 25962426](Among 36 patients with advanced hepatocellular carcinoma treated with temsirolimus in an open label phase II study, only one patient had a partial response and adverse events included mucositis, rash, fatigue, cough, fever, anorexia, insomnia, diarrhea, thrombocytopenia, abdominal and head pain, hyperglycemia, and thrombocytopenia; no mention of ALT elevation or change in hepatic status).
- Minguet J, Smith KH, Bramlage CP, Bramlage P. Targeted therapies for treatment of renal cell carcinoma: recent advances and future perspectives. Cancer Chemother Pharmacol. 2015;76:219–33. [PubMed: 25963382](Review of current and future targeted therapies for renal cancer discusses tyrosine kinase inhibitors and mTor inhibitors including everolimus and temsirolimus; no mention of ALT elevations or hepatotoxicity).
- Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, Offner F, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–8. [PubMed: 26673811](Among 280 patients with refractory mantle cell lymphoma treated with temsirolimus or ibrutinib, progression free survival was longer with ibrutinib [14.6 vs 6.2 months], which was also better tolerated; no mention of ALT elevations or hepatotoxicity of either drug).
- Pulido M, Roubaud G, Cazeau AL, Mahammedi H, Vedrine L, Joly F, Mourey L, et al. Safety and efficacy of temsirolimus as second line treatment for patients with recurrent bladder cancer. BMC Cancer. 2018;18:194. [PMC free article: PMC5816357] [PubMed: 29454321](Among 54 patients with bladder cancer treated with temsirolimus, partial responses occurred in 3 and the authors concluded that temsirolimus “had potential benefit for a subset of bladder cancer patients”; no mention of ALT elevations or hepatotoxicity).
- Sugiyama S, Sato K, Shibasaki Y, Endo Y, Uryu T, Toyoshima Y, Oya M, et al. Real-world use of temsirolimus in Japanese patients with unresectable or metastatic renal cell carcinoma: recent consideration based on the results of a post-marketing, all-case surveillance study. Jpn J Clin Oncol. 2020;50:940–7. [PMC free article: PMC7401718] [PubMed: 32458996](Among 1001 Japanese patients with renal cancer treated with temsirolimus followed in a postmarketing study, 78% had adverse events, most commonly stomatitis [27%], interstitial lung disease [17%], thrombocytopenia [17%], hyperglycemia [10%], and rash [7%], but no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Everolimus.[LiverTox: Clinical and Researc...]Review Everolimus.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Mitomycin.[LiverTox: Clinical and Researc...]Review Mitomycin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Temsirolimus, an mTOR inhibitor for treatment of patients with advanced renal cell carcinoma.[Clin J Oncol Nurs. 2008]Review Temsirolimus, an mTOR inhibitor for treatment of patients with advanced renal cell carcinoma.Malizzia LJ, Hsu A. Clin J Oncol Nurs. 2008 Aug; 12(4):639-46.
- Vorinostat enhances the activity of temsirolimus in renal cell carcinoma through suppression of survivin levels.[Clin Cancer Res. 2010]Vorinostat enhances the activity of temsirolimus in renal cell carcinoma through suppression of survivin levels.Mahalingam D, Medina EC, Esquivel JA 2nd, Espitia CM, Smith S, Oberheu K, Swords R, Kelly KR, Mita MM, Mita AC, et al. Clin Cancer Res. 2010 Jan 1; 16(1):141-53. Epub 2009 Dec 22.
- Review Axitinib.[LiverTox: Clinical and Researc...]Review Axitinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Temsirolimus - LiverToxTemsirolimus - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...