NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tasimelteon is a melatonin receptor agonist that is used for the treatment of non-24 hour sleep-wake disorder in blind individuals. Tasimelteon therapy is associated with a low rate of serum enzyme elevations, but has not been implicated in cases of clinically apparent liver injury.
Background
Tasimelteon (tas" i mel' tee on) is a synthetic melatonin receptor agonist with affinity for both the melatonin type 1 and type 2 receptors (MT1 and MT2). These receptors are believed to be involved in the maintenance of the circadian rhythm that regulates the normal sleep-wake cycle. Melatonin itself has been proposed as therapy of sleep disturbances including insomnia and jet lag, but systematic reviews and metaanalyses of controlled trials of various melatonin formulations have failed to demonstrate consistent efficacy. In contrast, melatonin receptor agonists such as ramelteon and tasimelteon were found to have effects on the circadian rhythm and sleep patterns. Tasimelteon was approved for use in “non-24 hour sleep-wake disorder” in the United States in 2014, the second melatonin receptor antagonist approved for use in sleep disorders. Whereas ramelteon has been approved for insomnia, tasimelteon is used more for shifting of sleep-wake patterns and may take weeks to have its full effect. In experimental simulations of jet lag, tasimelteon has been shown to increase sleep time. Tasimelteon is available in 20 mg capsules under the brand name Hetlioz. The recommended dose is 20 mg taken orally before bedtime at the same time every night. Side effects are few, but may include daytime somnolence, fatigue, dizziness, headache and vivid or abnormal dreams.
Hepatotoxicity
In several clinical trials, tasimelteon was found to be well tolerated. Serum enzyme elevations occurred in up to 10% of tasimelteon treated patients compared to 5% of placebo controls, but instances of clinically apparent liver injury were not reported. In a combined analysis of 6 trials of tasimelteon given for an average of 1 year, ALT elevations above 3 times the ULN arose in 6.5% of tasimelteon treated subjects, but no elevations were above 10 times ULN, and none were associated with symptoms or jaundice. Most elevations were single values and resolved spontaneously without dose reduction or discontinuation. Tasimelteon has been available for a limited period of time, but has not been linked to instances of clinically apparent liver injury. Tasimelteon is metabolized by the cytochrome P450 system (predominantly CYP 1A2 and CYP3A4), which can result in significant drug-drug interactions, strong inhibitors of the enzymes increasing serum concentrations of tasimelteon and strong inducers decreasing them.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Drug Class: Sedatives and Hypnotics
Other Drugs in the Subclass, Melatonin and its Analogues: Melatonin, Ramelteon
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tasimelteon – Hetlioz®
DRUG CLASS
Sedatives and Hypnotics
Product labeling at DailyMed, National Library of Medicine, NIH
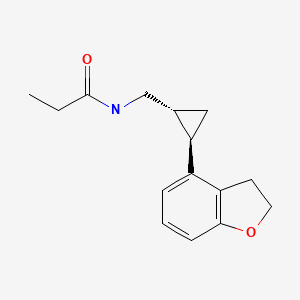
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Tasimelteon | 609799-22-6 | C15-H19-N-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 August 2020
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 731-4.(Expert review of hepatotoxicity published in 1999; melatonin, ramelteon and tasimelteon are not discussed).
- Larrey D, Ripault MP. Anxiolytic agents. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 455-6.(Review of hepatotoxicity of hypnotics and sedatives discusses benzodiazepines, buspirone and valerian, all of which have been linked to rare cases of liver injury; melatonin, ramelteon and tasimelteon are not discussed).
- Mihic SJ, Mayfield J, Harris RA. Hypnotics and sedatives. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 339-53.(Textbook of pharmacology and therapeutics).
- Penev PD, Zee PC. Melatonin: a clinical perspective. Ann Neurol. 1997;42:545–53. [PubMed: 9382465](Discussion of physiological functions of melatonin and potential clinical uses).
- Seabra ML, Bignotto M, Pinto LR Jr, Tufik S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J Pineal Res. 2000;29:193–200. [PubMed: 11068941](Controlled trial of melatonin [10 mg daily] vs placebo for 28 days in 40 volunteers found no changes or abnormalities of serum bilirubin, ALT, AST or Alk P during the 4 weeks of treatment).
- Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Vohra S, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ. 2006;332:385–93. [PMC free article: PMC1370968] [PubMed: 16473858](Systematic review of literature on efficacy and safety of melatonin for sleep disorders; in 9 randomized controlled trials there was no overall evidence of benefit; in 10 studies evaluating safety there was no evidence of adverse effects occurring more often than with placebo, with use limited to 3 months).
- Mayer G, Wang-Weigand S, Roth-Schechter B, Lehmann R, Staner C, Partinen M. Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. Sleep. 2009;32:351–60. [PMC free article: PMC2647789] [PubMed: 19294955](Controlled trial of 6 month course of ramelteon vs. placebo in 451 adults with primary chronic insomnia; adverse effects were similar between the two groups; no mention of ALT levels).
- Rajaratnam SM, Polymeropoulos MH, Fisher DM, Roth T, Scott C, Birznieks G, Klerman EB. Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Lancet. 2009;373(9662):482–91. [PubMed: 19054552](Controlled trial of 3 doses of tasimelteon vs placebo in 411 patients with transient insomnia found improvements in time to sleep latency and total sleep time, while the “frequency and severity of adverse events were similar across treatment groups”).
- Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012;349:91–104. [PMC free article: PMC3242827] [PubMed: 21939733](Review of the biologic basis of circadian rhythm including the role of melatonin).
- Johnsa JD, Neville MW. Tasimelteon: a melatonin receptor agonist for non-24-hour sleep-wake disorder. Ann Pharmacother. 2014;48:1636–41. [PubMed: 25204464](Systematic review of published literature on use of tasimelteon mentions that the most common adverse event was headache [<10%] and that “fewer [1% to 10%] patients experienced: increased alanine transaminase…”).
- Tasimelteon (Hetlioz) for non-24-hour sleep-wake disorder. Med Lett Drugs Ther. 2014;56(1441):34–5. [PubMed: 24759294](Concise review of the efficacy, safety and cost of tasimelteon shortly after its approval for use in the US mentions that the most common adverse events are headache [17%], elevated aminotransferase levels [10%], and nightmares or unusual dreams [10%]).
- Leger D, Quera-Salva MA, Vecchierini MF, Ogrizek P, Perry CA, Dressman MA. Safety profile of tasimelteon, a melatonin MT1 and MT2 receptor agonist: pooled safety analyses from six clinical studies. Expert Opin Drug Saf. 2015;14:1673–85. [PubMed: 26393492](In a combined analysis of 2 open label and 4 controlled trials of tasimelteon with a total of 259 patient years of exposure, common adverse events included headache [15%], ALT elevations [6.5%] and abnormal dreams [5.5%], but none led to premature discontinuation; 15 ALT elevations above 3 times ULN [2 were above 5 but none were above 10 times ULN], none were associated with jaundice or symptoms, and all except one were a single elevated value, the exception being a patient with pre-existing mild elevations).
- Lockley SW, Dressman MA, Licamele L, Xiao C, Fisher DM, Flynn-Evans EE, Hull JT, et al. Tasimelteon for non-24-hour sleep-wake disorder in totally blind people (SET and RESET): two multicentre, randomised, double-masked, placebo-controlled phase 3 trials. Lancet. 2015;386(10005):1754–64. [PubMed: 26466871](Among 84 totally blind individuals treated with tasimelteon or placebo 1 hour before bedtime for 26 weeks, clinical responses occurred in 24% vs none and adverse events included headache [17% vs 7%], elevated liver enzymes [10% vs 5%] and abnormal dreams [10% vs none], but there were no deaths and no serious adverse events that were considered related to tasimelteon).
- Advice for travelers. Med Lett Drugs Ther. 2019;61(1582):153–60. [PubMed: 31599872](Advice for travelers mentions drugs for jet lag including zolpidem, melatonin, ramelteon and tasimelteon, but does not discuss adverse side effects).
- Polymeropoulos CM, Mohrman MA, Keefe MS, Brzezynski JL, Wang J, Prokosch LS, Polymeropoulos VM, et al. Efficacy of tasimelteon (HETLIOZ®) in the treatment of jet lag disorder evaluated in an 8-h phase advance model; a multicenter, randomized, double-blind, placebo-controlled trial. Front Neurol. 2020;11:611. [PMC free article: PMC7381312] [PubMed: 32754110](Among 320 healthy travelers treated with tasimelteon or placebo in a single dose before an 8 hour phase activated bedtime, sleep time increased by an average of 60 minutes and decreased next-day sleepness; the only adverse event reported being headache [5% vs 2.5%]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Tasimelteon for the treatment of non-24-hour sleep-wake disorder.[Drugs Today (Barc). 2015]Review Tasimelteon for the treatment of non-24-hour sleep-wake disorder.Neubauer DN. Drugs Today (Barc). 2015 Jan; 51(1):29-35.
- Tasimelteon for treating non-24-h sleep-wake rhythm disorder.[Expert Opin Pharmacother. 2019]Tasimelteon for treating non-24-h sleep-wake rhythm disorder.Nishimon S, Nishimon M, Nishino S. Expert Opin Pharmacother. 2019 Jun; 20(9):1065-1073. Epub 2019 Apr 16.
- Review Tasimelteon (Hetlioz™): A New Melatonin Receptor Agonist for the Treatment of Non-24-Hour Sleep-Wake Disorder.[J Pharm Pract. 2015]Review Tasimelteon (Hetlioz™): A New Melatonin Receptor Agonist for the Treatment of Non-24-Hour Sleep-Wake Disorder.Bonacci JM, Venci JV, Gandhi MA. J Pharm Pract. 2015 Oct; 28(5):473-8. Epub 2014 Aug 3.
- Review Tasimelteon: A Review in Non-24-Hour Sleep-Wake Disorder in Totally Blind Individuals.[CNS Drugs. 2016]Review Tasimelteon: A Review in Non-24-Hour Sleep-Wake Disorder in Totally Blind Individuals.Keating GM. CNS Drugs. 2016 May; 30(5):461-8.
- Safety profile of tasimelteon, a melatonin MT1 and MT2 receptor agonist: pooled safety analyses from six clinical studies.[Expert Opin Drug Saf. 2015]Safety profile of tasimelteon, a melatonin MT1 and MT2 receptor agonist: pooled safety analyses from six clinical studies.Leger D, Quera-Salva MA, Vecchierini MF, Ogrizek P, Perry CA, Dressman MA. Expert Opin Drug Saf. 2015; 14(11):1673-85. Epub 2015 Sep 22.
- Tasimelteon - LiverToxTasimelteon - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...