NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Sulfadiazine is a sulfonamide antibacterial agent used in the therapy of mild-to-moderate infections due to sensitive organisms. Sulfadiazine, like other sulfonamides, is a well known cause of clinically apparent, idiosyncratic liver injury.
Background
Sulfadiazine (sul" fa dye' a zeen) is an orally administered sulfonamide antibiotic that acts by inhibition of folic acid synthesis, which is required for bacterial replication and growth. Different forms of sulfonamides have been used in clinical medicine since the 1930s. Sulfadiazine was approved for use in the United States in 1973. Current indications are many and include urinary tract infections and otitis media due to sensitive organisms. Sulfadiazine is also used as prophylaxis against rheumatic fever and meningococcal meningitis. It is used in combination with pyrimethamine for prevention and treatment of toxoplasmosis and is also effective in nocardiasis, chancroid, and trachoma. It is used as adjunctive therapy for chloroquine-resistant malaria and several forms of bacterial meningitis. Sulfadiazine is available in multiple generic forms in tablets of 500 mg. For urinary tract infections, the usual dose is 2 to 4 grams daily in 3 to 6 divided doses. Common side effects are diarrhea, nausea, gastrointestinal upset, rash and fever.
Hepatotoxicity
Sulfadiazine, like other sulfonamides, causes a characteristic idiosyncratic liver injury which has features of drug-allergy or hypersensitivity. The typical onset is sudden development of fever and rash followed by jaundice within a few days or weeks of starting the medication. The pattern of injury is typically mixed, although fatal cases are often hepatocellular and prolonged cholestatic cases have also been described. Eosinophilia or atypical lymphocytosis are also common and the clinical pattern can be considered a part of DRESS syndrome (drug rash with eosinophilia and systemic symptoms). Cases of Stevens Johnson syndrome due to sulfadiazine have also been described. Sulfonamides such as sulfadiazine have been linked to many cases of acute liver failure and as a class, the sulfonamides still rank in the top 5 to 10 causes of drug induced, idiosyncratic fulminant hepatic failure. However, most cases of sulfonamide induced liver injury resolve rapidly, usually within 2 to 4 weeks unless cholestasis is severe. Onset of injury is more rapid with rechallenge and can appear within a day or reexposure. Patients with hepatic injury due to sulfadiazine may have a history of previous exposure to the drug without injury. Sulfonamides such as sulfadiazine can also cause mild and transient ALT elevations that do not progress to jaundice or more severe liver injury either alone or as a part of a generalized hypersensitivity reaction. Sulfonamides have also been linked to hepatic granulomas.
Mechanism of Injury
The clinical pattern of injury with sulfadiazine suggests a drug-allergy or hypersensitivity mechanism, perhaps through its metabolism to a toxic, reactive or antigenic metabolite.
Outcome and Management
Sulfadiazine induced liver injury ranges in severity from anicteric and asymptomatic liver enzyme elevations, to symptomatic hepatitis with jaundice to severe acute hepatic failure. Sulfadiazine hepatotoxicity can result in acute liver failure, but most cases resolve rapidly with discontinuation of drug and full recovery is expected within 2 to 8 weeks. Severe cholestatic injury may be prolonged, and rare cases of chronic liver injury with vanishing bile duct syndrome due to sulfadiazine have been reported. The hepatic injury due to sulfonamides is usually a part of systemic hypersensitivity reaction. Rechallenge should not be done, and patients should be told that they are allergic to sulfonamides (“sulfa-drugs”) and not receive other drugs in this class. Prednisone has been used with variable success, but may be particularly helpful in patients with prominent allergic features with systemic features and fever, rash, lymphadenopathy and eosinophilia or atypical lymphocytosis.
References to the safety and potential hepatotoxicity of sulfadiazine are given in the Overview on Sulfonamides.
Drug Class: Antiinfective Agents, Sulfonamides
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Sulfadiazine – Generic, Microsulfon®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
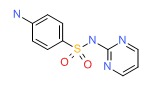
| Sulfadiazine | 68-35-9 | C10-H10-N4-O2-S |
 |
- PubChem SubstanceRelated PubChem Substances
- Review Sulfonamides.[LiverTox: Clinical and Researc...]Review Sulfonamides.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Metal sulfonamides as antibacterial agents in topical therapy.[Scand J Plast Reconstr Surg. 1...]Metal sulfonamides as antibacterial agents in topical therapy.Fox CL Jr, Modak S, Stanford JW, Fox PL. Scand J Plast Reconstr Surg. 1979; 13(1):89-94.
- Toxoplasma gondii: characterization of a mutant resistant to sulfonamides.[Exp Parasitol. 1992]Toxoplasma gondii: characterization of a mutant resistant to sulfonamides.Pfefferkorn ER, Borotz SE, Nothnagel RF. Exp Parasitol. 1992 May; 74(3):261-70.
- Mechanisms of in vitro sensitivity to sulfadiazine silver.[Arch Surg. 1983]Mechanisms of in vitro sensitivity to sulfadiazine silver.McManus AT, Denton CL, Mason AD Jr. Arch Surg. 1983 Feb; 118(2):161-6.
- Review Idiosyncratic toxicity associated with potentiated sulfonamides in the dog.[J Vet Pharmacol Ther. 2004]Review Idiosyncratic toxicity associated with potentiated sulfonamides in the dog.Trepanier LA. J Vet Pharmacol Ther. 2004 Jun; 27(3):129-38.
- Sulfadiazine - LiverToxSulfadiazine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...