NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Solifenacin is an anticholinergic and antispasmodic agent used to treat urinary incontinence and the overactive bladder syndrome. Solifenacin has not been implicated in causing liver enzyme elevations or clinically apparent acute liver injury.
Background
Solifenacin (soe" li fen' a sin) is an anticholinergic agent that inhibits muscarinic actions of acetylcholine on autonomic nerve endings, decreasing secretions and inhibiting gastrointestinal and bladder motility. Solifenacin increases the bladder capacity and decreases contractions and the urgency of urination. Solifenacin was approved for use in the United States in 2004 and it remains in clinical use with more than a million prescriptions written yearly. Current indications are for treatment of overactive bladder and symptoms of urgency and urinary frequency in adults. It is available in tablets of 5 and 10 mg generically and under the brand name VESIcare. The recommended adult oral dose is 5 to 10 mg once daily. Common side effects are those of parasympathetic stimulation and include dryness of the mouth and eyes, decreased sweating, headache, visual blurring, constipation, urinary retention, restlessness, confusion and hallucinations. Rare but potentially severe adverse reactions include acute narrow angle glaucoma, acute urinary retention, gastric retention and stasis, neurologic symptoms and worsening of neurologic diseases, prolongation of the QTc interval, and severe hypersensitivity reactions.
Hepatotoxicity
Like most anticholinergic agents, solifenacin has not been linked to liver enzyme elevations during therapy or to instances of clinically apparent liver injury with jaundice. In multiple prospective clinical trials of solifenacin in patients with overactive bladder syndrome, ALT elevations were reported in less than 1% of treated subjects, rates similar to that of placebo-recipients. Despite widespread clinical use for almost two decades, there has been only a single published case report of possible liver injury due to darifenacin use. An elderly woman with end stage liver injury developed transient elevations of serum aminotransferases and alkaline phosphatase without jaundice two weeks after starting solifenacin. Thus, liver injury due to solifenacin must be rare if it occurs at all.
Likelihood score: D (possible, very rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which solifenacin might cause liver injury unknown. It is metabolized in the liver by microsomal P450 enzymes, predominantly CYP 3A4 and 2D6. Despite this, drug-drug interactions are uncommon. A major reason for its safety may relate to the low daily dose.
Drug Class: Anticholinergic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Solifenacin – Generic, VESIcare®
DRUG CLASS
Anticholinergic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
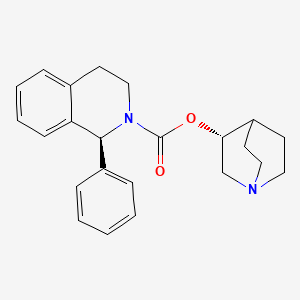
| Solifenacin | 242478-37-1 | C23-H26-N2-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 15 July 2023
Abbreviations: ER, extended release.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999 before the availability of darifenacin and other therapies of overactive bladder syndrome).
- Brown JH, Brandl K, Wess J. Therapeutic uses of muscarinic receptor antagonists: Muscarinic receptor agonists and antagonists. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 156-9.(Textbook of pharmacology and therapeutics).
- Cardozo L, Lisec M, Millard R, van Vierssen Trip O, Kuzmin I, Drogendijk TE, Huang M, Ridder AM. Randomized, double-blind placebo-controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol. 2004;172(5 Pt 1):1919-24. [PubMed: 15540755](Among 907 adults with overactive bladder syndrome treated with solifenacin [5 or 10 mg] or placebo once daily for 12 weeks, the mean number of daily micturitions decreased more with solifenacin [-2.4 and -2.8 vs -1.6] as did episodes of urgency and incontinence, while side effect rates were similar except for dry mouth [8% and 23% vs 2%], constipation [4% and 9% vs 2%] and blurred vision [4% and 6% vs 2%] and there were “no safety problems or clinically relevant changes in … laboratory values”).
- Chapple CR, Rechberger T, Al-Shukri S, Meffan P, Everaert K, Huang M, Ridder A; YM-905 Study Group. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int. 2004;93:303-10. [PubMed: 14764127](Among 1077 adults with overactive bladder syndrome treated with solifenacin [5 and 10 mg], tolterodine [4 mg], or placebo daily for 12 weeks, both agents led to a significant decrease in numbers of micturitions and episodes of urgency and incontinence, with similar rates of adverse events, and “there were no clinically relevant changes in … laboratory values”).
- Chapple CR, Martinez-Garcia R, Selvaggi L, Toozs-Hobson P, Warnack W, Drogendijk T, Wright DM, et al.; STAR study group. A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: results of the STAR trial. Eur Urol. 2005;48:464-70. [PubMed: 15990220](Among 1200 adults with overactive bladder syndrome treated with solifenacin [5 or 10 mg] or tolterodine ER [4 mg] once daily for 12 weeks, numbers of daily micturitions decreased in both groups [-2.5 vs -2.2], and adverse event rates were similar [dry mouth 18% vs 15%, constipation 3.2% vs 1.3%, blurred vision 0.7% vs 1.7%], but no mention of ALT elevations or hepatotoxicity).
- Novara G, Galfano A, Secco S, D'Elia C, Cavalleri S, Ficarra V, Artibani W. A systematic review and meta-analysis of randomized controlled trials with antimuscarinic drugs for overactive bladder. Eur Urol 2008; 54: 740-63. [PubMed: 18632201](Systematic review of efficacy and safety of drugs for overactive bladder including tolterodine, propiverine, solifenacin, darifenacin, fesoterodine and oxybutynin; common side effects included dry mouth and constipation; hepatotoxicity and ALT elevations were not mentioned).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to anticholinergics or drugs for overactive bladder).
- Chu F, Smith N, Uchida T. Efficacy and safety of solifenacin succinate 10 mg once daily: a multicenter, phase III, randomized, double-blind, placebo-controlled, parallel-group trial in patients with overactive bladder. Curr Ther Res Clin Exp. 2009;70:405-20. [PMC free article: PMC3969973] [PubMed: 24692834](Among 672 adults with overactive bladder syndrome treated with solifenacin [10 mg] vs placebo once daily for 12 weeks, decline in daily micturitions was greater with solifenacin [-3.0 vs -1.5] and adverse events were mild to moderate in intensity, including dry mouth [27% vs 4%], constipation [17% vs 3%] and blurred vision [3.5% vs 1.2%], and there were no treatment related serious adverse events or deaths).
- Herschorn S, Stothers L, Carlson K, Egerdie B, Gajewski JB, Pommerville P, Schulz J, et al. Tolerability of 5 mg solifenacin once daily versus 5 mg oxybutynin immediate release 3 times daily: results of the VECTOR trial. J Urol 2010; 183: 1892-8. [PubMed: 20303119](Among 132 patients treated with solifenacin or oxybutynin for 8 weeks, dry mouth was less common with solifenacin; hepatic side effects and ALT values were not reported).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to anticholinergics or drugs for overactive bladder syndrome).
- Shamliyan T, Wyman JF, Ramakrishnan R, Sainfort F, Kane RL. Benefits and harms of pharmacologic treatment for urinary incontinence in women: a systematic review. Ann Intern Med 2012; 156: 861-74. [PubMed: 22711079](Systematic review of the safety and efficacy of drugs used for urinary incontinence including fesoterodine, tolterodine, oxybutynin, solifenacin and trospium; most had modest effectiveness; hepatotoxicity was not mentioned).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to mirabegron or drugs for overactive bladder syndrome).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, but none were attributed to drugs used for overactive bladder syndrome).
- Maman K, Aballea S, Nazir J, Desroziers K, Neine ME, Siddiqui E, Odeyemi I, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2014;65:755-65. [PubMed: 24275310](Systematic review of literature on medical therapies for overactive bladder identified 44 controlled trials demonstrating similar efficacy among 6 anticholinergics and a single beta-3 adrenergic agonist [mirabegron] when compared to placebo, but less dry mouth with mirabegron than with anticholinergic agents; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN Prospective Study. Gastroenterology 2015; 148: 1340-1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to mirabegron or other agents for overactive bladder syndrome).
- Batista JE, Kölbl H, Herschorn S, Rechberger T, Cambronero J, Halaska M, Coppell A, et al.; BEYOND study group. The efficacy and safety of mirabegron compared with solifenacin in overactive bladder patients dissatisfied with previous antimuscarinic treatment due to lack of efficacy: results of a noninferiority, randomized, phase IIIb trial. Ther Adv Urol 2015; 7: 167-79. [PMC free article: PMC4580095] [PubMed: 26445596](Among 1887 patients with overactive bladder syndrome treated with mirabegron or solifenacin for 12 weeks, decreases in micturition rates were similar with both drugs [-3.0 and -3.1 per day] as were rates of adverse events; no mention of ALT levels or hepatotoxicity).
- Thiagamoorthy G, Cardozo L, Srikrishna S. Drug therapy for an overactive bladder. Womens Health (Lond) 2015; 11: 445-8. [PubMed: 26238677](Overactive bladder is defined as urinary urgency, usually with frequency and nocturia with or without incontinence in the absence of infection or other known cause, medical therapy being use of anticholinergics or beta-3 adrenergic receptor agonists such as mirabegron or vibegron which have fewer side effects than typical anticholinergics).
- Masood U, Sharma A, Manocha D. Liver toxicity from solifenacin. Am J Ther. 2017;24:e505-e506. [PubMed: 27574943](84 year old woman with end-stage renal disease developed abdominal pain and vomiting and was found to have abnormal liver tests 2 weeks after starting solifenacin [bilirubin not given, ALT 639 U/L, Alk P 171 U/L], with resolution within 2-3 weeks of stopping: no information on other medications, liver imaging or HCV RNA testing).
- Drugs for overactive bladder. Med Lett Drugs Ther. 2023;65:41-45. [PubMed: 36897601](Concise review of drugs approved for therapy of overactive bladder in the US including anticholinergic agents [darifenacin, fesoterodine, oxbutynin, solifenacin, tolterodine and trospium] and beta-3 adrenergic receptor agonists [mirabegron and vibegron], including clinical efficacy, safety, and costs; no mention of ALT elevations or hepatotoxicity of any of the agent discussed).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Which anticholinergic drug for overactive bladder symptoms in adults.[Cochrane Database Syst Rev. 2012]Review Which anticholinergic drug for overactive bladder symptoms in adults.Madhuvrata P, Cody JD, Ellis G, Herbison GP, Hay-Smith EJ. Cochrane Database Syst Rev. 2012 Jan 18; 1:CD005429. Epub 2012 Jan 18.
- Review Fesoterodine.[LiverTox: Clinical and Researc...]Review Fesoterodine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Darifenacin.[LiverTox: Clinical and Researc...]Review Darifenacin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Trospium.[LiverTox: Clinical and Researc...]Review Trospium.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- The Efficacy and Safety of OnabotulinumtoxinA or Solifenacin Compared with Placebo in Solifenacin Naïve Patients with Refractory Overactive Bladder: Results from a Multicenter, Randomized, Double-Blind Phase 3b Trial.[J Urol. 2017]The Efficacy and Safety of OnabotulinumtoxinA or Solifenacin Compared with Placebo in Solifenacin Naïve Patients with Refractory Overactive Bladder: Results from a Multicenter, Randomized, Double-Blind Phase 3b Trial.Herschorn S, Kohan A, Aliotta P, McCammon K, Sriram R, Abrams S, Lam W, Everaert K. J Urol. 2017 Jul; 198(1):167-175. Epub 2017 Feb 1.
- Solifenacin - LiverToxSolifenacin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...