NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Selegiline is an inhibitor of monamine oxidase used in the treatment of depression and as adjunctive therapy in combination with levodopa and carbidopa in the therapy of Parkinson disease. Selegiline has been associated with a low rate of serum enzyme elevations during treatment, but has not been linked to instances of clinically apparent acute liver injury.
Background
Selegiline (se le' ji leen) is a specific inhibitor of monamine oxidase (MAO) type B, which is a major enzyme in the pathway of dopamine and levodopa metabolism. As a result, selegiline results in an increase in the bioavailability of levodopa, enhancing and increasing the duration of its effects in Parkinson disease. Selegiline is also an antidepressant, its mechanism of action being inhibition of dopamine reuptake from the synaptic cleft. Selegiline was approved for use in the United States in 2006, the first MAO-B inhibitor approved for use in the therapy of Parkinson disease as an adjunct to levodopa therapy. Selegiline is available in capsules and tablets of 5 mg generically and under the brand name Eldepryl, the typical dose being 10 mg daily in two divided doses. It is also available in oral disintegrating tablets of 1.25 mg under the brand name Zelapar, which is given once or twice daily. Transdermal patches of selegiline in amounts of 6, 9 and 12 mg/24 hours are available under the brand name Emsam for treatment of depresssion, the usual dose being 6 to 12 mg daily. Common side effects include headache, nausea, dizziness, agitation, delusions, insomnia, orthostatic hypotension, dry mouth, headache and gastrointestinal upset – most of which are attributable to enhanced dopaminergic effects. In higher doses, selegiline can also inhibit MAO-A and, similar to the nonspecific MAO inhibitors, cause increased susceptibility to dietary tyramine inducing hypertensive crises (“cheese effect”).
Hepatotoxicity
Selegiline has been reported to cause serum enzyme elevations in up to 40% of patients treated long term. Although the abnormalities were usually mild and self-limiting, they were persistent with continuation of treatment in some patients, ultimately requiring drug discointuation. Selegiline has not been implicated in cases of clinically apparent acute liver injury, but such instances have been reported with other less specific MAO inhibitors.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Selegiline is extensively removed from the blood by the liver (first pass metabolism) and undergoes hepatic conjugation and elimination. The pathways of selegiline metabolism have not been well defined.
Outcome and Management
The only liver abnormalities that have attributed to selegiline have been mild and self-limiting elevations in serum enzymes. No instances of acute hepatitis, acute liver failure, chronic hepatitis or vanishing bile duct syndrome have been linked to selegiline use.
Drug Class: Antiparkinson Agents
Other Drugs in the Subclass, Selective MAO-B Inhibitors: Rasagiline, Safinamide
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Selegiline – Generic, Atapryl®, Eldepryl®
DRUG CLASS
Antiparkinson Agents
Product labeling at DailyMed, National Library of Medicine, NIH
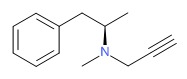
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Selegiline | 14611-51-9 | C13-H17-N |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 21 July 2017
- Zimmerman HJ. Antiparkinsonism drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 715-7.(Expert review of hepatotoxicity published in 1999; among anticholinergic agents, "only trihexyphenidyl has been incriminated in hepatic injury"; other antiparkinsonism drugs discussed include levodopa, lergotrile [no longer available], pergolide and bromocriptine, but not selegiline).
- Larrey D, Ripault MP. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier Inc, 2013, pp. 443-62.(Review of hepatotoxicity of agents acting on the central nervous system).
- Standaert DG, Roberson ED. Treatment of central nervous system degenerative disorders. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 609-28.(Textbook of pharmacology and therapeutics).
- Golbe LI. Long-term efficacy and safety of deprenyl (selegiline) in advanced Parkinson's disease. Neurology 1989; 39: 1109-11. [PubMed: 2503769](Among 22 patients with Parkinson disease treated with selegiline for up to 27 months, serum enzyme increased from baseline by 21-54% and elevations occurred in 9 [41%], peak ALT values ranging from 111-280 U/L often after 6 months of treatment).
- Hauser RA, Molho E, Shale H, Pedder S, Dorflinger EE. A pilot evaluation of the tolerability, safety, and efficacy of tolcapone alone and in combination with oral selegiline in untreated Parkinson's disease patients. Tolcapone De Novo Study Group. Mov Disord 1998; 13: 643-7. [PubMed: 9686768](Among 83 patients with Parkinson disease treated with tolcapone with or without selegiline for 8 weeks, ALT elevations occurred in 1 patient [2%] on tolcapone alone).
- Lambert D, Waters CH. Comparative tolerability of the newer generation antiparkinsonian agents. Drugs Aging 2000; 16: 55-65. [PubMed: 10733264](Review of mechanism of action, tolerability and safety of selegiline, pramipexole, ropinirole, tolcapone and entacapone in Parkinson disease).
- Shoulson I, Oakes D, Fahn S, Lang A, Langston JW, LeWitt P, Olanow CW, et al.; Parkinson Study Group. Impact of sustained deprenyl (selegiline) in levodopa-treated Parkinson's disease: a randomized placebo-controlled extension of the deprenyl and tocopherol antioxidative therapy of Parkinsonism trial. Ann Neurol 2002; 51: 604-12. [PubMed: 12112107](Assessment of long term selegiline therapy in Parkinson disease found no increase in nonmotor adverse events in comparison to placebo; no change in ALT levels or hepatotoxicity mentioned).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to agents used for Parkinson disease).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25,1425. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none of the 96 were attributed to an agent used to treat Parkinson disease).
- Drugs for Parkinson's disease. Treat Guidel Med Lett 2013; 11 (135): 101-6. [PubMed: 24165688](Concise review of recommendations for therapy of Parkinson disease with description of mechanisms of action, efficacy and adverse events).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to an agent to treat Parkinson disease).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury from the US enrolled in a prospective database between 2004 and 2012, none were attributed to an agent used to treat Parkinson disease).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Rasagiline.[LiverTox: Clinical and Researc...]Review Rasagiline.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Safinamide.[LiverTox: Clinical and Researc...]Review Safinamide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Monamine oxidase inhibitors: current and emerging agents for Parkinson disease.[Clin Neuropharmacol. 2007]Review Monamine oxidase inhibitors: current and emerging agents for Parkinson disease.Fernandez HH, Chen JJ. Clin Neuropharmacol. 2007 May-Jun; 30(3):150-68.
- Review Selegiline: initial or adjunctive therapy of Parkinson's disease?[DICP. 1991]Review Selegiline: initial or adjunctive therapy of Parkinson's disease?Fuller MA, Tolbert SR. DICP. 1991 Jan; 25(1):36-40.
- Review Tolcapone.[LiverTox: Clinical and Researc...]Review Tolcapone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Selegiline - LiverToxSelegiline - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...