NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Sarecycline is an oral, once daily, narrow spectrum tetracycline used to treat moderate-to-severe acne. Oral sarecycline use has been rarely linked to serum enzyme elevations during therapy and, although suspected of causing liver injury, it has yet to be linked to clinically apparent acute hepatic injury.
Background
Sarecycline (sar" e sye' kleen) is an oral, narrow spectrum tetracycline antibiotic with potent activity against Propionbacterium acnes, a gram-positive organism that plays an important role in the inflammatory lesions of acne vulgaris. The tetracyclines act by inhibition of protein synthesis by binding to the 30S subunit of microbial ribosomes. Human cells are less susceptible to this inhibition. Tetracyclines have been available in the United States since the 1957s and used in therapy of several bacterial infections as well as acne. More modern forms of tetracycline include doxycycline, minocycline and sarecycline which have better absorption and tissue penetration. Sarecycline also has a more limited spectrum of antibacterial activity and was developed specifically for therapy of acne and to avoid the broad spectrum complications such as Clostridium difficile colitis. Sarecycline was approved for use in moderate-to-severe acne in 2018 and is available in tablets of 60, 100 and 150 mg under the brand name Seysara. The recommended dose is based upon body weight and administered as a single tablet once daily (60 mg for 33-54 kg; 100 mg for 55-84 kg; and 150 mg for 85-136 kg). Typically, chronic long term and sometimes intermittent therapy is used for acne. Common side effects of sarecycline include gastrointestinal upset, nausea, poor appetite, diarrhea, glossitis, rash and hypersensitivity reactions. Tetracyclines can cause vertigo and tinnitus, skin photosensitivity reactions, and staining of developing teeth (in children or when taken by a pregnant mother) for which reason the tetracyclines should not be used in pregnant women or in children below the age of nine.
Hepatotoxicity
In preclinical clinical trials of sarecycline in facial acne, serum aminotransferase elevations were mild and no more frequent than with placebo or comparator arms. There were no instances of clinically apparent liver injury. Nevertheless, other tetracycline antibiotics, even when used in low doses, are well known causes of drug induced liver injury particularly when given long term. Minocycline which is also used for acne is one of the most common causes of liver injury with jaundice. The injury typically arises after months or years of therapy and frequently presents as an autoimmune-like syndrome that can mimic lupus erythematosus, rheumatoid arthritis or autoimmune hepatitis. While sarecycline has not been associated with a similar syndrome, it has been available for a short time only, and the prelicensure clinical trials were generally for 12 weeks only.
Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which sarecycline might cause liver injury is unknown. It has minimal hepatic metabolism and is not associated with significant drug-drug interactions. Minocycline associated liver injury and has been shown to have an association with carriage of the rare HLA allele B*35:02.
Outcome and Management
Patients on long term sarecycline who develop serum aminotransferase elevations should be monitored more carefully and, if ALT or AST rise above 5 times the upper limit of normal or are accompanied by jaundice or symptoms (including arthralgias and rash), sarecycline should be discontinued. Whether there is cross sensitivity to hepatic injury among the various tetracyclines is not known, but switching to another tetracycline after drug induced liver injury from another should be done with caution and careful monitoring.
Drug Class: Antiinfective Agents, Tetracyclines
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Sarecycline – Seysara®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
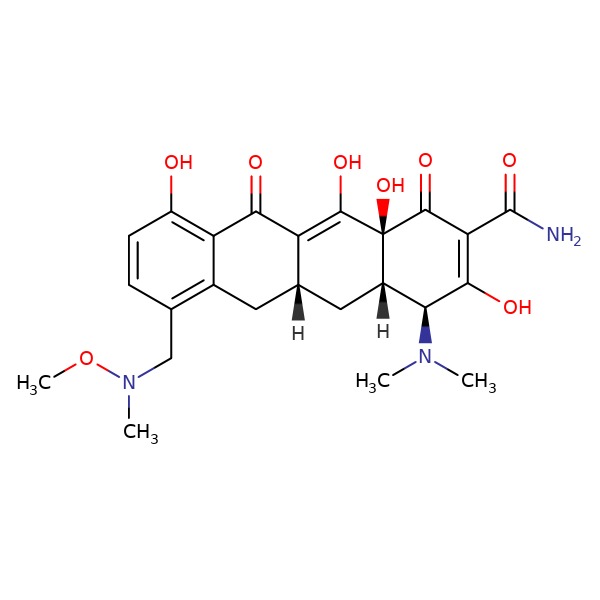
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Sarecycline | 1035654-66-0 | C24-H29-N3-O |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 10 April 2019
- Zimmerman HJ. Tetracyclines. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999. p. 599-602.(Expert review of tetracycline and liver injury published in 1999; the tetracyclines cause two forms of drug induced liver injury, microvesicular fat and liver failure occurring after 4-10 days with high doses of parenteral tetracyclines and an idiosyncratic liver injury that occurs with the oral agents, doxycycline causing a cholestatic and minocycline a hepatocellular injury which may be associated with autoimmune features).
- Moseley RH. Tetracyclines. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 468.(Expert review of tetracycline induced liver injury; mentions that the hepatotoxicity of intravenous tetracycline is of historic interest only as it is no longer given parenterally; both doxycycline and minocycline have been associated with idiosyncratic liver injury).
- MacDougall C. Protein synthesis inhibitors and miscellaneous antibacterial agents. In, Brunton LL, Hilal-Dandan R, Knollman KC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1049-65.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions that pooled safety data indicated that serious adverse events were rare [<1%] and no more common with sarecycline [n=1820] than placebo [n=1113] treatment as were discontinuations for adverse events [1.3% vs 1.3%] and “there were no adverse effects with kidney or liver dysfunction” although 5 subjects discontinued sarecycline therapy because of liver enzyme elevations [one scored as “serious”], all of which were asymptomatic and resolved with stopping). - Moore A, Green LJ, Bruce S, Sadick N, Tschen E, Werschler P, Cook-Bolden FE, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol 2018; 17: 987-96. [PubMed: 30235387](Among 968 patients, ages 9 to 45 years, with facial acne treated with sarecycline or placebo for 12 weeks in 2 randomized controlled trials, inflammatory lesions improved more with sarecycline while adverse events included nausea [5%] and vomiting [2%] and more rarely dizziness and phototoxicity [<1%]; there were “no clinically meaningful differences … in clinical laboratory” measurements in either study).
- Leyden JJ, Sniukiene V, Berk DR, Kaoukhov A. Efficacy and safety of sarecycline, a novel, once-daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris: results of a phase 2, dose-ranging study. J Drugs Dermatol 2018; 17: 333-8. [PubMed: 29537451](Among 285 patients, ages 12 to 45 years, with severe facial acne vulgaris treated with 3 doses of sarecycline or placebo once daily for 12 weeks, inflammatory lesions were improved with higher doses of sarecycline vs placebo while adverse event rates were comparable and there were no serious adverse events; no mention of ALT elevations or hepatotoxicity).
- Deeks ED. Sarecycline: first global approval. Drugs. 2019; 79: 325-9. [PMC free article: PMC6505496] [PubMed: 30659422](Review of the mechanism of action, pharmacology, clinical efficacy and safety of sarecycline from clinical trials on which its FDA approval was based; mentions that adverse events were uncommon but included nausea, vomiting, abdominal pain, sunburn, vulvovaginal candidiasis and mycotic infections; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Efficacy and Safety of Sarecycline, a Novel, Once-Daily, Narrow Spectrum Antibiotic for the Treatment of Moderate to Severe Facial Acne Vulgaris: Results of a Phase 2, Dose-Ranging Study.[J Drugs Dermatol. 2018]Efficacy and Safety of Sarecycline, a Novel, Once-Daily, Narrow Spectrum Antibiotic for the Treatment of Moderate to Severe Facial Acne Vulgaris: Results of a Phase 2, Dose-Ranging Study.Leyden JJ, Sniukiene V, Berk DR, Kaoukhov A. J Drugs Dermatol. 2018 Mar 1; 17(3):333-338.
- Once-Daily Oral Sarecycline 1.5 mg/kg/day Is Effective for Moderate to Severe Acne Vulgaris: Results from Two Identically Designed, Phase 3, Randomized, Double-Blind Clinical Trials.[J Drugs Dermatol. 2018]Once-Daily Oral Sarecycline 1.5 mg/kg/day Is Effective for Moderate to Severe Acne Vulgaris: Results from Two Identically Designed, Phase 3, Randomized, Double-Blind Clinical Trials.Moore A, Green LJ, Bruce S, Sadick N, Tschen E, Werschler P, Cook-Bolden FE, Dhawan SS, Forsha D, Gold MH, et al. J Drugs Dermatol. 2018 Sep 1; 17(9):987-996.
- Review Sarecycline: a narrow spectrum tetracycline for the treatment of moderate-to-severe acne vulgaris.[Future Microbiol. 2019]Review Sarecycline: a narrow spectrum tetracycline for the treatment of moderate-to-severe acne vulgaris.Moore AY, Charles JEM, Moore S. Future Microbiol. 2019 Sep; 14(14):1235-1242. Epub 2019 Sep 2.
- Review Sarecycline hydrochloride for the treatment of acne vulgaris.[Drugs Today (Barc). 2019]Review Sarecycline hydrochloride for the treatment of acne vulgaris.Kaul G, Saxena D, Dasgupta A, Chopra S. Drugs Today (Barc). 2019 Oct; 55(10):615-625.
- Safety and Tolerability of Sarecycline for the Treatment of Acne Vulgaris: Results from a Phase III, Multicenter, Open-Label Study and a Phase I Phototoxicity Study.[J Clin Aesthet Dermatol. 2019]Safety and Tolerability of Sarecycline for the Treatment of Acne Vulgaris: Results from a Phase III, Multicenter, Open-Label Study and a Phase I Phototoxicity Study.Pariser DM, Green LJ, Lain EL, Schmitz C, Chinigo AS, McNamee B, Berk DR. J Clin Aesthet Dermatol. 2019 Nov; 12(11):E53-E62. Epub 2019 Nov 1.
- Sarecycline - LiverToxSarecycline - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...