NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Quetiapine is an atypical antipsychotic used in the treatment of schizophrenia and bipolar disorder. Use of quetiapine has been associated with serum aminotransferase elevations and in rare instances with clinically apparent acute liver injury.
Background
Quetiapine (kwe tye' a peen) is an atypical antipsychotic and dibenzodiazepine derivative which appears to act as a dopamine (D1-4) and serotonin (5-HT2) receptor antagonist. It also may have activity against histamine and alpha adrenergic receptors. Quetiapine is indicated for the treatment of schizophrenia and as either monotherapy or adjunctive therapy for acute manic episodes or as maintenance therapy in bipolar I disorder. It is also used in treatment of depressive episodes associated with bipolar I or II disorder and for major depressive disorders in combination with antidepressants. Quetiapine was approved for use in the United States in 1997 and is still widely used with more than 10 million prescriptions filled yearly in the United States. Quetiapine is available as tablets of 25, 50, 100, 200, 300 and 400 mg generically and under the brand name Seroquel. Typical doses vary from 300 to 800 mg daily given in two divided doses. Extended release forms are also available for once daily dosing. Common side effects include dizziness, sedation, somnolence, dry mouth, constipation, weakness, postural hypotension, increased appetite and weight gain. Rare, but potentially severe adverse events include neuroleptic malignant syndrome, tardive dyskinesia, severe dyslipidemia, diabetes, weight gain, hypotension, bone marrow suppression and cataracts. Quetiapine like most atypical antipsychotic agents has a boxed warning for increased risk of death in patients with dementia-related psychosis, and like most potent antidepressants it has a boxed warning for suicidal thoughts and behaviors.
Hepatotoxicity
Liver test abnormalities may occur in up to 30% of patients on long term therapy with quetiapine, but elevations are uncommonly above 3 times the upper limit of normal. The aminotransferase abnormalities are usually mild, asymptomatic and transient, reversing even with continuation of medication. Instances of clinically apparent acute liver injury have been reported due to quetiapine, but they are rare. The onset of jaundice is within 1 to 4 weeks of starting the drug, and the pattern of serum enzyme elevations is typically hepatocellular. Signs of immunoallergic manifestations (fever, rash and eosinophilia) are rare, as are autoantibodies. Most cases are mild-to-moderate in severity and self-limited in course. Instances of DRESS syndrome and acute liver failure have been reported but not vanishing bile duct syndrome or chronic liver injury.
Likelihood score: B (likely but rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which quetiapine causes serum aminotransferase elevations is not known, but is likely due to production of a toxic intermediate by its metabolism. Quetiapine is extensively metabolized by the liver via sulfoxidation and oxidation, partially via CYP 3A4. Some instances of serum aminotransferase elevations occurring on quetiapine therapy may be due to nonalcoholic fatty liver disease caused by weight gain that occurs in at least one-quarter of treated patients, generally during the first 1 to 2 years of therapy.
Outcome and Management
The serum aminotransferase elevations that occur on quetiapine therapy are usually self-limited and usually do not require dose modification or discontinuation of therapy. Cases of clinically apparent liver injury are usually mild to moderate in severity and self-limited course. Rare instances of acute liver failure due to quetiapine have been reported, but it has not been implicated in cases of chronic liver disease or vanishing bile duct syndrome. Patients with quetiapine induced liver injury may have cross sensitivity to other atypical antipsychotics (risperidone) but usually tolerate switching to others.
Drug Class: Antipsychotic Agents, Atypicals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Quetiapine – Generic, Seroquel®
DRUG CLASS
Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Quetiapine | 111974-69-7 | C21-H25-N3-O2-S |
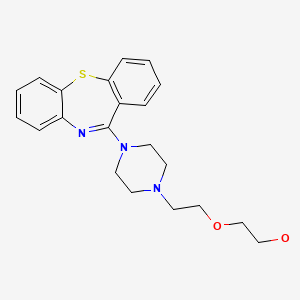
|
ANNOTATED BIBLIOGRAPHY
References updated: June 6, 2023
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- Quetiapine for schizophrenia. Med Lett Drugs Ther. 1997;39:117–8. [PubMed: 9422044](Brief review of efficacy and safety of quetiapine shortly after its approval in the US; common side effects include somnolence, dizziness, constipation, postural hypotension, dry mouth and weight gain; “increases in serum aminotransferase activity have occurred in some patients”).
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96. [PubMed: 10553730](Systematic review of 81 articles on weight change with antipsychotics, using change after 10 weeks to compare agents: clozapine +5.7, olanzapine +4.2, chlorpromazine +4.2, quetiapine +2.5, risperidone +1.7, loxapine +0.6, haloperidol +0.5, ziprasidone +0.3, molindone -0.1, and pimozide -2.7 kg).
- Balestrieri M, Vampini C, Bellantuono C. Efficacy and safety of novel antipsychotics: a critical review. Hum Psychopharmacol. 2000;15:499–512. [PubMed: 12404619](Review on efficacy and safety of quetiapine; “Mild, transient, reversible and asymptomatic elevations in the liver enzymes were seen in a small number of patients”).
- Garver DL. Review of quetiapine side effects. J Clin Psychiatry. 2000;61 Suppl 8:31–3. [PubMed: 10811241](Discussion of side effects [weight gain, sedation, extrapyramidal syndrome] of quetiapine based upon known profile of neuroreceptor blockade).
- Mullen J, Jobson MD, Sweitzer D. A comparison of the relative safety, efficacy, and tolerability of quetiapine and risperidone in outpatients with schizophrenia and other psychotic disorders: the quetiapine experience with safety and tolerability (QUEST) study. Clin Ther. 2001;23:1839–54. [PubMed: 11768836](4 month trial of quetiapine vs risperidone in 728 patients at 115 centers; no mention of ALT abnormalities or adverse events from liver injury).
- Lieberman JA, Perkins DO. Quetiapine: a 5-year update. J Clin Psychiatry. 2002;63 Suppl 13:3–4. Not in PubMed.(Editorial; quetiapine discovered in 1985 at AstraZeneca, approved in US for schizophrenia in 1997).
- Nasrallah HA, Tandon R. Efficacy, safety, and tolerability of quetiapine in patients with schizophrenia. J Clin Psychiatry. 2002;63 Suppl 13:12–20. [PubMed: 12562142](Review of 9 clinical trials of quetiapine in patients with schizophrenia; ALT elevations occurred but were mild and reversible; ALT levels were >3 times ULN in 6% of quetiapine- vs 1% of placebo-recipients, usually within the first 3 weeks of treatment and without hepatitis or jaundice).
- Choice of an antipsychotic. Med Lett Drugs Ther. 2003;45:102–4. [PubMed: 14679353](Quetiapine, a second generation antipsychotic, is generally well tolerated; no mention of hepatic effects).
- Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci. 2003;28:99–112. [PMC free article: PMC161731] [PubMed: 12670127](Review of the interactions of the atypical antipsychotics with the P450 system; clozapine metabolized by CYP1A2 and 3A4 and possibly 2C9 and 2D6; risperidone by CYP2D6 and possibly 3A4; olanzapine by CYP1A2 and possibly 2D6; quetiapine and ziprasidone by CYP3A4).
- El Hajj I, Sharara AI, Rockey DC. Subfulminant liver failure associated with quetiapine. Eur J Gastroenterol Hepatol. 2004;16:1415–8. [PubMed: 15618854](58 year old woman developed jaundice 1 month after starting quetiapine [bilirubin 17.3 mg/dL, ALT 1245 U/L, Alk P 265 U/L, ANA 1:2560], progressing to liver failure and death within 21 days; autopsy showed massive necrosis).
- Langman LJ, Kaliciak HA, Carlyle S. Fatal overdoses associated with quetiapine. J Anal Toxicol. 2004;28:520–5. [PubMed: 15516308](Three cases of overdose with quetiapine, 39-56 year olds, found dead at home soon after overdose; respiratory arrest; no information on hepatic injury).
- Rettenbacher MA, Baumgartner S, Eder-Ischia U, Edlinger M, Graziadei I, Hofer A, Huber R, et al. Association between antipsychotic-induced elevation of liver enzymes and weight gain: a prospective study. J Clin Psychopharmacol. 2006;26:500–3. [PubMed: 16974192](Prospective study of 67 patients started on atypical antipsychotics [9 on quetiapine]; ALT elevations were more frequent in 14 who gained >7% of body weight than in the 53 who did not [50% vs 19%] and mean changes in ALT, AST and GGT were greater in those who gained weight; all abnormalities were transient, asymptomatic and not associated with bilirubin elevations).
- Wright TM, Vandenberg AM. Risperidone- and quetiapine-induced cholestasis. Ann Pharmacother. 2007;41:1518–23. [PubMed: 17666578](30 year old man developed jaundice after being on risperidone and lithium for 8 years [bilirubin 4.7 mg/dL, ALT 99 U/L, Alk P 267 U/L], resolving with change of risperidone to ziprasidone, but 1 year later developed recurrent jaundice 3 weeks after starting quetiapine, having tolerated olanzapine).
- Atasoy N, Erdogan A, Yalug I, Ozturk U, Konuk N, Atik L, Ustundag Y. A review of liver function tests during treatment with atypical antipsychotic drugs: a chart review study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1255–60. [PubMed: 17600607](Retrospective analysis on 194 patients; ALT levels were >3 times ULN in 27% often in first month; among 48 receiving quetiapine, 27% had ALT elevations, 23% at 6 months, but they were modest and none had elevations greater than 3 fold).
- Torrent C, Amann B, Sanchez-Moreno J, Colom F, Feinares M, Comes M, Rosa AR, et al. Weight gain in bipolar disorder: pharmacological treatment as a contributing factor. Acta Psychiatr Scand. 2008;118:4–18. [PubMed: 18498432](Review of frequency of weight gain in patients treated for bipolar disorders, most weight gain occurred with clozapine and olanzapine, but some weight gain also with quetiapine, risperidone, lithium, valproate and gabapentin; not with aripiprazole, ziprasidone, carbamazepine or lamotrigine).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008; several antidepressants [duloxetine, sertaline, fluoxetine, amitryptilline], but none of the atypical antipsychotic agents were implicated).
- Shpaner A, Li W, Ankoma-Sey V, Botero RC. Drug-induced liver injury: hepatotoxicity of quetiapine revisited. Eur J Gastroenterol Hepatol. 2008;20:1106–9. [PubMed: 19047843](21 year old man developed jaundice 10 days after starting quetiapine [bilirubin 14.7 mg/dL, ALT 2582 U/L, Alk P 167 U/L, ANA negative, prolonged INR], resolving completely with stopping).
- Flanagan RJ. Fatal toxicity of drugs used in psychiatry. Hum Psychopharmacol. 2008;23 Suppl 1:43–51. [PubMed: 18098225](Deaths from fatal poisonings decreased in England and Wales between 1993-2004; antipsychotic overdose fatalities higher for phenothiazines than atypicals; deaths/million prescriptions being 29 for chlorpromazine, 15.5 thioridazine, 3.9 trifluoperazine, 13.3 olanzapine, 21 clozapine and 31.3 quetiapine).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including 4 due to psychotropic agents; 1 each for quetiapine, nefazodone, fluoxetine and venlafaxine).
- Naharci MI, Karadurmus N, Demir O, Bozoglu E, Ak M, Doruk H. Fatal hepatotoxicity in an elderly patient receiving low-dose quetiapine. Am J Psychiatry. 2011;168:212–3. [PubMed: 21297052](77 year old woman developed fatigue and jaundice 9 days after starting quetiapine [bilirubin 4.8 mg/dL, ALT 1565 U/L, Alk P 178 U/L, INR 4.1], with subsequent multiorgan failure and death, despite improvement in liver tests).
- Al Mutairi F, Dwivedi G, Al Ameel T. Fulminant hepatic failure in association with quetiapine: a case report. J Med Case Rep. 2012;6:418. [PMC free article: PMC3533844] [PubMed: 23234465](59 year old woman developed weakness 3 weeks and jaundice 6 weeks after starting quetiapine [bilirubin 14.3 mg/dL, ALT 711 U/L, Alk P 196 U/L, INR 2.7], with signs of hepatic failure, but ultimate slow recovery after stopping).
- Lin CH, Liu CM, Huang WL. Quetiapine-induced hepatocellular damage. Psychosomatics. 2012;53:601–2. [PubMed: 23157997](47 year old woman developed serum enzyme elevations within 3 weeks of starting quetiapine for schizophrenia [bilirubin 0.4 mg/dL, ALT 113 U/L, Alk P 547 U/L], having been normal shortly before and returning to normal within 2 weeks of decreasing the dose [from 400 to 300 mg daily] and recurring when high doses were restarted).
- Sistanizad M, Peterson GM. Drug-induced liver injury in the Australian setting. J Clin Pharm Ther. 2013;38:115–20. [PubMed: 23350857](Among 17 cases of suspected drug induced liver injury seen at a single referral hospital in Tasmania over a 12 month period, one was attributed to quetiapine occurring in a 63 year old woman with a mixed pattern of liver injury [peak ALT 467 U/L, Alk P 265 U/L] and judged as only "possibly" due to the drug).
- Greil W, Häberle A, Schuhmann T, Grohmann R, Baumann P. Age and adverse drug reactions from psychopharmacological treatment: data from the AMSP drug surveillance programme in Switzerland. Swiss Med Wkly. 2013;143:w13772. [PubMed: 23821346](Among 39,728 patients treated with psychiatric medications in Swiss hospitals between 2001 and 2010 who were monitored in a drug surveillance program, rates of severe adverse events were similar in those above and below the age of 60 [1.6% vs 1.8%], although weight gain and ALT elevations were less frequent in the elderly [details not provided]).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to quetiapine or other atypical antipsychotic agents).
- Brown ES, Davila D, Nakamura A, Carmody TJ, Rush AJ, Lo A, Holmes T, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in patients with bipolar disorder, mixed or depressed phase, and alcohol dependence. Alcohol Clin Exp Res. 2014;38:2113–8. [PMC free article: PMC4107121] [PubMed: 24976394](Among 90 patients with bipolar disorder with depression and alcohol dependence who were treated with quetiapine or placebo for 12 weeks, alcohol use was similar in the two groups; ALT levels were not mentioned, but there were no liver related serious adverse events).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to quetiapine or other atypical antipsychotic medications).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 3 of which were attributed to quetiapine).
- Vatsalya V, Pandey A, Schwandt ML, Cave MC, Barve SS, Ramchandani VA, McClain CJ. Safety assessment of liver injury with quetiapine fumarate XR management in very heavy drinking alcohol-dependent patients. Clin Drug Investig. 2016;36:935–44. [PMC free article: PMC5095696] [PubMed: 27503091](Among 218 adults with alcohol dependence treated with quetiapine or placebo for 12 weeks, there was a moderate increase in the likelihood of having ALT elevations in quetiapine treated subjects with and without preexisting elevations, although the differences [not provided] were judged to be "not clinically significant in that they required no medical management").
- Morlán-Coarasa MJ, Arias-Loste MT, Ortiz-García de la Foz V, Martínez-García O, Alonso-Martín C, Crespo J, Romero-Gómez M, et al. Incidence of non-alcoholic fatty liver disease and metabolic dysfunction in first episode schizophrenia and related psychotic disorders: a 3-year prospective randomized interventional study. Psychopharmacology (Berl). 2016;233:3947–52. [PubMed: 27620899](Among 191 schizophrenic patients treated with an atypical antipsychotic agent for at least 3 years, surrogate markers for steatosis arose in 48 [25%], most of whom had a 7% increase in body weight [n=44: 92%], increase in triglycerides [54%], total cholesterol [52%], and waist circumference [68%]; changes in regard to fatty liver did not vary by specific antipsychotic agent [46 received quetiapine]).
- Hayes JF, Marston L, Walters K, Geddes JR, King M, Osborn DP. Adverse renal, endocrine, hepatic, and metabolic events during maintenance mood stabilizer treatment for bipolar disorder. PLoS Med. 2016;13:e1002058. [PMC free article: PMC4970809] [PubMed: 27483368](Analysis of UK electronic health data on patients with bipolar disorder between 1995 and 2013, found greater than 15% weight gain to be higher with valproate [Hazard Ratio: HR=1.6], olanzapine [HR=1.8], quetiapine [HR=1.7] compared to lithium alone).
- Drugs for psychotic disorders. Med Lett Drugs Ther. 2016;58(1510):160–4. [PubMed: 27960194](Concise review of medications available in the US for therapy of psychotic disorders; mentions that olanzapine can cause aminotransferase elevations, and that olanzapine and ziprasidone can cause DRESS syndrome, but does not mention ALT elevations or hepatotoxicity for any of agents discussed, including aripiprazole, brexpiprazole, cariprazine, clozapine, quetiapine, risperidone, asenapine, iloperidone, paliperidone and lurasidone).
- Das A, Guarda LA, Allen LG. Liver injury associated with quetiapine: an illustrative case report. J Clin Psychopharmacol. 2017;37:623–5. [PubMed: 28786826](45 year old woman developed jaundice a year after starting and 3 weeks after increasing the dose of quetiapine [bilirubin 18.2 mg/dL, ALT 95 U/L, Alk P 267 U/L, INR 1.45], which improved on stopping, but documentation not provided).
- Baeza I, de la Serna E, Calvo-Escalona R, Merchán-Naranjo J, Rodríguez-Latorre P, Martínez-Cantarero MC, Andrés P, et al. One-year prospective study of liver function tests in children and adolescents on second-generation antipsychotics: is there a link with metabolic syndrome? J Child Adolesc Psychopharmacol. 2018;28:463–73. [PubMed: 29975563](Among 216 children and adolescents starting atypical antipsychotics, mean weight gain at 6 months was 6.5 kg and mean ALT levels increased by 8.6 U/L, while among 19 taking quetiapine for 6 months mean weight gain was 10.3 kg and ALT increase 42.5 U/L; increases in ALT associated most closely with development of the metabolic syndrome, mean ALT increasing by 27.8 U/L at 6 months).
- Druschky K, Toto S, Bleich S, Baumgärtner J, Engel RR, Grohmann R, Maier HB, et al. Severe drug-induced liver injury in patients under treatment with antipsychotic drugs: data from the AMSP study. World J Biol Psychiatry. 2021;22:373–386. [PubMed: 32892689](Among 246 cases of severe liver injury due to antipsychotic medications identified in a prospective registry of German psychiatric hospitals between 1993 and 2016, 46 arose in 38,349 patients [0.12%] who received clozapine [34 as a single antipsychotic agent]; other commonly implicated agents being olanzapine [n=90 of 54,822: 0.16%], quetiapine [34 of 66,209: 0.05%] and risperidone [27 of 51,683: 0.05%]; two fatal cases occurred in olanzapine-treated patients).
- Zeiss R, Hafner S, Schönfeldt-Lecuona C, Connemann BJ, Gahr M. Drug-associated liver injury related to antipsychotics: exploratory analysis of pharmacovigilance data. J Clin Psychopharmacol. 2022;42:440–444. [PubMed: 35730552](Review of the VigiBase data base of individual case safety reports on antipsychotics and liver injury found positive hepatic safety signals for olanzapine and clozapine, but none for risperidone, quetiapine, ziprasidone, asenapine, aripiprazole, brexpiprazole, and cariprazine).
- Gunther M, Dopheide JA. Antipsychotic safety in liver disease: a narrative review and practical guide for the clinician. J Acad Consult Liaison Psychiatry. 2023;64:73–82. [PubMed: 36180017](Review of the literature on hepatotoxicity of antipsychotic medications and guidance on their use in patients with liver disease characterizes chlorpromazine, clozapine, and olanzapine as having the greatest risk for causing liver injury, quetiapine and risperidone as having moderate risk, haloperidol as having low risk and paliperidone, aripiprazole, lurasidone, and loxapine as having low risk).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Quetiapine : A Review of its Use in Schizophrenia.[CNS Drugs. 1998]Quetiapine : A Review of its Use in Schizophrenia.Gunasekara NS, Spencer CM. CNS Drugs. 1998 Apr; 9(4):325-40.
- Review Summary of the comparative effectiveness review on off-label use of atypical antipsychotics.[J Manag Care Pharm. 2012]Review Summary of the comparative effectiveness review on off-label use of atypical antipsychotics.Maher AR, Theodore G. J Manag Care Pharm. 2012 Jun; 18(5 Suppl B):S1-20.
- Review Olanzapine.[LiverTox: Clinical and Researc...]Review Olanzapine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Cariprazine.[LiverTox: Clinical and Researc...]Review Cariprazine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Asenapine.[LiverTox: Clinical and Researc...]Review Asenapine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Quetiapine - LiverToxQuetiapine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...