NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Propranolol is a nonselective beta-adrenergic receptor blocker (beta-blocker) that is widely used for the therapy of hypertension, cardiac arrhythmias, angina pectoris and hyperthyroidism. Propranolol has yet to be convincingly associated with clinically apparent liver injury and is often used in patients with liver disease and cirrhosis.
Background
Propranolol (proe pran' oh lol) was the prototype beta-blocker developed for therapy of hypertension and is considered nonselective, acting on both the beta-1 and beta-2 adrenergic receptors. Beta-1 adrenergic blockade reduces the heart rate and myocardial contractility by slowing AV conduction and suppressing automaticity. Beta-2 blockade also affects peripheral vascular resistance and can cause bronchospasm and hypoglycemia. The beta-2 blockade is responsible for the majority of adverse effects associated with propranolol. Propranolol was approved for use in the United States in 1967 and is still commonly used, with several million prescriptions filled yearly. Propranolol is indicated for the management of hypertension, myocardial infarction, angina pectoris, idiopathic hypertrophic subaortic stenosis and prevention of migraine and vascular headaches. Propranolol is also used to treat essential tremor, to decrease the heart rate in patients with hyperthyroidism, and to prevent recurrent variceal hemorrhage. Propranolol is available in multiple generic forms and under the trade name of Inderal in tablets of 10, 20, 40, 60, 80 and 90 mg, as well as extended or sustained release capsules of 60, 80, 120 and 160 mg. Liquid formulations for oral and parenteral administration are also available. The typical dose of propranolol in adults is 60 to 240 mg daily in 2 divided doses (one with extended release formulations), with subsequent dose modification based upon efficacy and tolerance. Common side effects of propranolol include bradycardia, hypotension, fatigue, dizziness, depression, memory loss, impotence, cold limbs and less commonly severe hypotension, heart failure and bronchospasm. Sudden withdrawal can trigger rebound hypertension. Beta-blockers are contraindicated in patients with asthma, bradycardia and heart failure and should be used cautiously in the elderly and in patients with diabetes.
Hepatotoxicity
Mild-to-moderate elevations in serum aminotransferase levels occur in less than 2% of patients on propranolol and are usually transient and asymptomatic, resolving even with continuation of therapy. Despite its widespread use, propranolol has not been convincingly linked to instances of clinically apparent liver injury; the few cases reported have generally occurred in patients who were receiving other well known hepatotoxic agents or were associated with elevations in serum enzymes only without jaundice.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Propranolol undergoes rapid first-pass metabolism by the liver, but has little or no effect on P450 activity. The reason why it rarely causes liver injury is unknown; other beta-blockers with similar chemical structures have been linked to cases of clinically apparent, idiosyncratic liver injury.
References to the safety and potential hepatotoxicity of propranolol are provided in the overview on Beta-Adrenergic Receptor Antagonists, last updated in June 2019.
Drug Class: Beta-Adrenergic Receptor Antagonists
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Propranolol – Generic, Inderal®
DRUG CLASS
Beta-Adrenergic Receptor Antagonists
Product labeling at DailyMed, National Library of Medicine, NIH
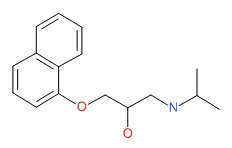
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Propranolol | 525-66-6 | C16-H21-N-O2 |
 |
- PubChem SubstanceRelated PubChem Substances
- Review Timolol.[LiverTox: Clinical and Researc...]Review Timolol.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Beta Adrenergic Blocking Agents.[LiverTox: Clinical and Researc...]Review Beta Adrenergic Blocking Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Pindolol.[LiverTox: Clinical and Researc...]Review Pindolol.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Nadolol.[LiverTox: Clinical and Researc...]Review Nadolol.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review [Propranolol--a place in the modern therapy].[Wiad Lek. 2006]Review [Propranolol--a place in the modern therapy].Olakowska E, Olakowski M. Wiad Lek. 2006; 59(5-6):388-91.
- Propranolol - LiverToxPropranolol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...