NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Pregabalin is an inhibitor of neuronal activity used for therapy of painful neuropathy and as an anticonvulsant. Therapy with pregabalin is not associated with serum aminotransferase elevations, and clinically apparent liver injury from pregabalin has been reported but appears to be quite rare.
Background
Pregabalin (pre gab' a lin) is a structural analogue of gamma-aminobutyric acid (GABA) but is novel in its activity, having no effects on GABA-A or GABA-B receptors. Instead, the neuronal activity of pregabalin appears to be mediated by its binding to the alpha-2-delta subunit of the presynaptic voltage-gated calcium channel which leads to a decrease in release of neuroexcitatory neurotransmitters by hyperexcited neurons. Pregabalin has been shown to be effective in reducing neuropathic pain from diabetic and postherpetic neuropathy and is an effective anticonvulsant. Pregabalin was approved for use in the United States in 2004. Current indications include diabetic and post-herpetic neuropathy and as adjunctive therapy of partial onset seizures. Pregabalin is also used for fibromyalgia and off-label for generalized anxiety disorders and migraine. Pregabalin is available in capsules in varying concentrations from 25 to 300 mg under the brand name of Lyrica. The recommended initial dose for neuropathic pain is 50 to 75 mg two to three times daily, the maximum dose being 300 mg daily. Higher doses are used in treating seizures. The dose should be increased and tapered gradually. The most common side effects of pregabalin are dose related and include peripheral edema, weight gain, dizziness, somnolence, confusion, headache, blurred vision, tremor and ataxia. Rare but potentially severe adverse events include depression, suicidal ideation and behaviors, angioedema, and hypersensitivity reactions.
Hepatotoxicity
Limited data is available on the hepatotoxicity of pregabalin. In prelicensure clinical trials in diabetic neuropathy and epilepsy, therapy with pregabalin was not associated with an increased frequency of serum aminotransferase elevations or liver toxicity. Since its approval and more wide scale use, however, pregabalin has been linked to rare instances of clinically apparent liver injury. Most cases were mild and frequently without jaundice. The latency to onset of injury was short, symptoms of liver injury arising within 3 to 14 days. Both cholestatic and hepatocellular patterns of injury have been reported. Signs of hypersensitivity (fever, rash, eosinophilia) and autoimmunity were not present. Some cases have been severe and associated with marked jaundice and prolongation of the prothrombin time, but all cases ultimately resolved after the medication was stopped without evidence of residual injury.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The low rate of significant hepatotoxicity from pregabalin may be due to its minimal hepatic metabolism and rapid urinary excretion. The injury is clearly idiosyncratic and either immunologic or metabolic causes are possible.
Outcome and Management
The case reports of hepatic injury due to pregabalin were followed by complete recovery without evidence of residual or chronic injury. There is no information about cross reactivity with other compounds having similar structure (gabapentin).
Drug Class: Anticonvulsants
CASE REPORT
Case 1. Mild cholestatic hepatitis attributed to pregabalin therapy.(1)
A 61 year old man underwent laminectomy for spinal stenosis and was started on pregabalin 2 weeks later. Within 2 days he developed dizziness, blurred vision, somnolence and fatigue, which worsened when the dose was increased from 75 to 150 mg/day one week later. Because of persistence of symptoms, blood tests were taken 11 days after starting therapy which revealed moderate enzyme elevations (Table). The patient had a history of hypertension and gout for which he took amlodipine (5 mg/day), candesartan (16 mg/day) and allopurinol (100 mg/day) chronically. Before the laminectomy, serum enzymes were tested and were normal. After surgery, he received metamizole (an NSAID not available in the US) for five days. Physical examination showed no jaundice, rash or signs of chronic liver disease. Tests for hepatitis A, B, C, D, and E, for HIV and for autoantibodies were negative. Abdominal ultrasound and MRCP were normal. Pregabalin was stopped and all symptoms except for mild fatigue, resolved rapidly. Reintroduction of pregabalin a few days later led to an immediate return of symptoms, but blood tests were not taken during the rechallenge. The abnormal enzyme values rapidly improved once pregabalin was stopped and were normal two months later.
Key Points
| Medication: | Pregabalin (75→150 mg daily) |
|---|---|
| Pattern: | Mixed (R=2.1) |
| Severity: | 1+ (enzyme elevations without jaundice) |
| Latency: | 2 days to onset of symptoms, 11 days to laboratory abnormalities |
| Recovery: | Rapid (2 to 8 weeks) |
| Other medications: | Amlodipine, candesartan, and allopurinol chronically; metamizole for 5 days 3 weeks before onset. |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | Normal | Normal | Normal | Preoperative evaluation | |
| 0 | Pregabalin started: 75 mg/day increasing to 150 mg/day one week later | ||||
| 11 days | 0 | 307 | 476 | 1.7 | GGT 1546 U/L |
| 22 days | 10 days | 37 | 174 | 1.0 | GGT 542 U/L |
| 11 weeks | 10 weeks | 32 | 125 | 0.9 | GGT 44 U/L |
| Normal Values | <40 | <130 | <1.2 | ||
Comment
Other causes of acute liver disease were excluded. The rechallenge was somewhat convincing, but the role of pregabalin in causing liver injury was not completely proven because of the lack of testing immediately before and after the two day rechallenge (on days 13-14 after starting). Based on the “R” value, the pattern of enzyme elevations was “mixed,” but the subsequent values and GGT elevations suggest that the injury was cholestatic. Most published cases of liver injury attributed to pregabalin have been marked by a short latency (3 to 14 days) and a rapidly resolving, self-limiting course.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Pregabalin – Generic, Lyrica®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Pregabalin | 148553-50-8 | C8-H17-N-O2 |
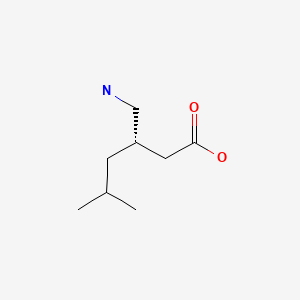
|
CITED REFERENCE
- 1.
- Crespo Pérez L, Moreira Vicente V, Manzano Fernández R, García Aguilera XA. Med Clin (Barc). 2008;130:157–8. [Cholestasis associated with pregabalin treatment] Spanish. [PubMed: 18279637]
ANNOTATED BIBLIOGRAPHY
References updated: 30 July 2020
Abbreviations used: DRESS, drug rash with eosinophilia and systemic symptoms; SJS/TEN, Stevens-Johnson syndrome and toxic epidermal necrolysis.
- Zimmerman HJ. Anticonvulsants. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 498-516.(Expert review of liver injury due to anticonvulsants published in 1999, before the clinical availability of pregabalin which is not discussed).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-41.(Review of anticonvulsant induced liver injury does not specifically discuss pregabalin).
- Smith MD, Metcalf CS, Wilcox KS. Pharmacology of the epilepsies. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 303-26.(Textbook of pharmacology and therapeutics).
- Huckle R. Pregabalin (Pfizer). Curr Opin Investig Drugs. 2004;5:82–9. [PubMed: 14983979](History of development of pregabalin and summary of information on mechanisms of action, pharmacology, pharmacokinetics, and clinical data supporting indications; no information on liver adverse events).
- Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia. 2004;45 Suppl 6:13–8. [PubMed: 15315511](Summary of pharmacology and pharmacokinetics of pregabalin; it has no hepatic metabolism and does not induce P450 enzymes).
- Kavoussi R. Pregabalin: From molecule to medicine. Eur Neuropsychopharmacol. 2006;16 Suppl 2:S128–33. [PubMed: 16765030](Summary of mechanism of action and clinical experience with pregabalin in generalized anxiety disorder; no mention of hepatic adverse events).
- Tassone DM, Boyce E, Guyer J, Nuzum D. Pregabalin: a novel gamma-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures, and anxiety disorders. Clin Ther. 2007;29:26–48. [PubMed: 17379045](Review of efficacy and safety of pregabalin; major side effects were CNS related including dizziness, somnolence, headache, and ataxia, also edema and weight gain; withdrawal symptoms included anxiety, irritability and nervousness: “…there were no significant differences in changes in blood chemistry or hematology tests…”).
- Crespo Pérez L, Moreira Vicente V, Manzano Fernández R, García Aguilera XA. Med Clin (Barc). 2008;130:157–8. [Cholestasis associated with pregabalin treatment] Spanish. [PubMed: 18279637](61 year old man with neuropathic pain after laminectomy developed symptoms of dizziness and fatigue within 2 days, and liver test abnormalities at 11 days after starting pregabalin [bilirubin 1.2 mg/dL, ALT 307 U/L, Alk P 476 U/L], symptoms recurring with rechallenge, and all signs of injury resolving within 2 months of stopping: Case 1).
- Einarsdottir S, Björnsson E. Pregabalin as a probable cause of acute liver injury. Eur J Gastroenterol Hepatol. 2008;20:1049. [PubMed: 18787478](61 year old man developed nausea followed by jaundice 8 days after starting pregabalin [bilirubin 10.7 mg/dL, ALT 35 times ULN, INR 3.8], resolving completely within 24 weeks).
- Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008;31:1448–54. [PMC free article: PMC2453685] [PubMed: 18356405](Pooled data from 7 trials of pregabalin given for 5-13 weeks vs placebo in 1510 patients; common side effects were edema, weight gain, dizziness and somnolence with “…no clinically meaningful changes in laboratory values from baseline.”).
- Stein DJ, Baldwin DS, Baldinetti F, Mandel F. Efficacy of pregabalin in depressive symptoms associated with generalized anxiety disorder: a pooled analysis of 6 studies. Eur Neuropsychopharmacol. 2008;18:422–30. [PubMed: 18359203](Pooled analysis in >1000 patients with generalized anxiety disorder showed improvement with higher doses, but provided no information on tolerance or side effects).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were linked to pregabalin).
- [Hepatic adverse effects of pregabalin (Lyrica)]. Lakartidningen 2010; 107(4): 194. Swedish. [PubMed: 20333975]
- Johannessen Landmark C, Patsalos PN. Drug interactions involving the new second- and third-generation antiepileptic drugs. Expert Rev Neurother. 2010;10:119–40. [PubMed: 20021326](Review of drug-drug interactions; pregabalin has not been implicated in clinical significant drug interactions with other major anticonvulsants).
- Doğan S, Ozberk S, Yurci A. Pregabalin-induced hepatotoxicity. Eur J Gastroenterol Hepatol. 2011;23:628. [PubMed: 21654262](28 year old woman developed jaundice 4 months after starting pregabalin [bilirubin 16.3 mg/dL, ALT 26 times ULN, INR 1.91], resolving within 2 months of stopping).
- Sendra JM, Junyent TT, Pellicer MJ. Pregabalin-induced hepatotoxicity. Ann Pharmacother. 2011;45:e32. [PubMed: 21652790](59 year old man developed serum enzyme elevations 14 days after starting pregabalin [bilirubin 1.3 mg/dL, ALT 1582 U/L, Alk P 488 U/L], resolving in 4 months; patient had preexisting abnormalities and also received levofloxacin).
- Zaccara G, Gangemi P, Perucca P, Specchio L. The adverse event profile of pregabalin: a systematic review and meta-analysis of randomized controlled trials. Epilepsia. 2011;52:826–36. [PubMed: 21320112](Analysis of 38 double-blind trials of pregabalin identified 20 adverse events that were more common with pregabalin than comparator arms and were mostly dizziness, vertigo, ataxia, diplopia, tremor, somnolence and confusion; even in non-frequently mentioned side effects, ALT elevations and hepatotoxicity were not listed).
- Toth C. Drug safety evaluation of pregabalin. Expert Opin Drug Saf. 2012;11:487–502. [PubMed: 22468635](Review of structure, mechanism of action, pharmacokinetics, clinical uses and safety of pregabalin; common side effects include dizziness, sedation, dry mouth, weight gain and edema, rash and hypersensitivity reactions are rare; no mention of hepatotoxicity or ALT changes during therapy).
- Drugs for epilepsy. Treat Guidel Med Lett. 2013;11:9–18. Erratum in Treat Guidel Med Lett 2013; 11: 112. [PubMed: 23348233](Concise review of the drugs of choice for epilepsy; pregabalin is listed as an alternate drug of choice for partial onset seizures; side effects include somnolence, dizziness, ataxia, confusion, weight gain, dry mouth, blurred vision and edema; ALT elevations and liver injury are not mentioned).
- Bamanikar A, Dhobale S, Lokwani S. Pregabalin hypersensitivity in a patient treated for postherpetic neuralgia. Indian J Pharmacol. 2013;45:522–3. [PMC free article: PMC3793527] [PubMed: 24130391](40 year old man developed fever, rash and facial swelling 2 weeks after starting pregabalin for post-herpetic neuralgia [bilirubin normal, ALT 250 U/L, Alk P normal], resolving on oral prednisolone and within a few months of stopping pregabalin).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, one of which was attributed to gabapentin but none to pregabalin ).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 7 [4%] of which were attributed to anticonvulsants including phenytoin, carbamazepine and valproate but not pregabalin).
- Devarbhavi H, Andrade RJ. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014;34:145–61. [PubMed: 24879980](Review of drug induced liver injury caused by several drug classes including antiepileptics, which account for 2-11% of all cases in various registries; a common presentation was with DRESS particularly with the older agents such as carbamazepine, phenytoin and phenobarbital and often associated with reactivation of human herpes virus 6 or 7).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were due to anticonvulsants including 3 attributed to gabapentin but only one to pregabalin; a 44 year old woman developed nausea followed by jaundice a week after starting pregabalin for peripheral neuropathy [bilirubin 16.9 mg/dL, ALT 229 U/L, Alk P 116 U/L], resolving slowly with stopping, but taking several other potential hepatotoxic agents such as mercaptopurine and simvastatin).
- Vidaurre J, Gedela S, Yarosz S. Antiepileptic drugs and liver disease. Pediatr Neurol. 2017;77:23–36. [PubMed: 29097018](Review of the use of anticonvulsants in patients with liver disease recommends use of agents that have little hepatic metabolism such as levetiracetam, lacosamide, topiramate, gabapentin and pregabalin, levetiracetam being an "ideal" first line therapy for patients with liver disease because of its safety and lack of pharmacokinetic interactions).
- Drugs for epilepsy. Med Lett Drugs Ther. 2017;59(1526):121–30. [PubMed: 28746301](Concise review of the drugs available for therapy of epilepsy lists pregabalin as an adjunctive treatment for partial seizures similar in action and efficacy to gabapentin, its most common adverse events being somnolence, dizziness, ataxia, dry mouth, blurred vision, confusion, edema, weight gain and myoclonus; no mention of ALT elevations or hepatotoxicity).
- Borrelli EP, Lee EY, Descoteaux AM, Kogut SJ, Caffrey AR. Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs: An analysis of the US Food and Drug Administration Adverse Event Reporting System. Epilepsia. 2018;59:2318–24. [PMC free article: PMC6420776] [PubMed: 30395352](Review of adverse event reports to the FDA between 2014 and 2018 identified ~2.9 million reports, 1034 for SJS/TEN, the most common class of drugs being anticonvulsants with 17 of 34 having at least one report, those most frequently linked being lamotrigine [n=106], carbamazepine [22], levetiracetam [14], phenytoin [14], valproate [9], clonazepam [8], zonisamide [7], gabapentin [4] and pregabalin [4]; no mention of accompanying liver injury or whether attribution was as a single agent or one of several).
- Cano-Paniagua A, Amariles P, Angulo N, Restrepo-Garay M. Epidemiology of drug-induced liver injury in a University Hospital from Colombia: Updated RUCAM being used for prospective causality assessment. Ann Hepatol. 2019;18:501–7. [PubMed: 31053545](Among 286 patients with liver test abnormalities seen in a single hospital in Colombia over a 1 year period, 17 were diagnosed with drug induced liver injury, the most common cause being antituberculosis therapy [n=6] followed by anticonvulsants [n=3, 1 each due to phenytoin, gabapentin and valproate]).
- Gabapentin and pregabalin: hepatic and haematological toxicity. Prescrire Int. 2014;23:267. [PubMed: 25954794](Among adverse event reports to a French pharmacovigilance registry between 1995 and 2010, there were 90 reports of liver injury attributed to gabapentin, 37 described as hepatitis and 1 with a fatal outcome; and 32 reports of liver injury attributed to pregabalin of which 2 were fatal; no details given).
- Kowar M, Friedrich C, Jacobs AH. Pregabalin als seltene Ursache einer Hepatopathie. Dtsch Med Wochenschr. 2015;140:1759–60. [Pregabalin as a rare cause of liver disease] German. [PubMed: 26583821](76 year old woman developed jaundice 15 days after starting pregabalin for back pain [bilirubin 6.0 mg/dL, ALT 2030 U/L, Alk P 650 U/L], with recovery 1-3 months after stopping).
- Düzenli T, Ata E, Kösem M, Bayram Çarli A. Pregabaline as a rare cause of hepatotoxicity. Pain Med. 2017;18:1407–8. [PubMed: 28087846](78 year old woman with painful neuropathy developed fatigue with a few days of starting pregabalin [bilirubin 0.6 mg/dL, ALT 701 U/L, Alk P 260 U/L], with rapid improvement on stopping).
- Nonopioid drugs for pain. Med Lett Drugs Ther. 2018;60(1540):25–32. [PubMed: 29422479](Concise review of nonopioid drugs that are used for pain, mentions that pregabalin is approved for use in post-herpetic and diabetic neuropathy as well as for fibromyalgia; no mention of hepatic adverse events).
- Markman J, Resnick M, Greenberg S, Katz N, Yang R, Scavone J, Whalen E, et al. Efficacy of pregabalin in post-traumatic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase 3 trial. J Neurol. 2018;265:2815–24. [PMC free article: PMC6244661] [PubMed: 30242745](Among 539 adults with post-traumatic neuropathic pain treated for 15 weeks with pregabalin or placebo, there were no differences in improvements in pain scores between the two groups while adverse events more common with pregabalin were dizziness [15% vs 4%] and somnolence [10% vs 3%]; no mention of ALT elevations or hepatotoxicity).
- Salehifar E, Janbabaei G, Hendouei N, Alipour A, Tabrizi N, Avan R. Comparison of the efficacy and safety of pregabalin and duloxetine in taxane-induced sensory neuropathy: a randomized controlled trial. Clin Drug Investig. 2020;40:249–57. [PubMed: 31925721](Among 82 patients with post-taxane chemotherapy neuropathy treated with pregabalin or duloxetine for 6 weeks, response rates were 93% for pregabalin vs 38% for duloxetine and adverse events were mild-to-moderate, dizziness and somnolence more common with pregabalin, nausea and vomiting with duloxetine; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Ameliorative Effects Of N-Acetylcysteine As Adjunct Therapy On Symptoms Of Painful Diabetic Neuropathy.[J Pain Res. 2019]Ameliorative Effects Of N-Acetylcysteine As Adjunct Therapy On Symptoms Of Painful Diabetic Neuropathy.Heidari N, Sajedi F, Mohammadi Y, Mirjalili M, Mehrpooya M. J Pain Res. 2019; 12:3147-3159. Epub 2019 Nov 19.
- Duloxetine for the treatment of painful diabetic peripheral neuropathy in Venezuela: economic evaluation.[Medwave. 2015]Duloxetine for the treatment of painful diabetic peripheral neuropathy in Venezuela: economic evaluation.Carlos F, Espejel L, Novick D, López R, Flores D. Medwave. 2015 Sep 25; 15(8):e6265. Epub 2015 Sep 25.
- Glycemic and serum lipid control in patients with painful diabetic peripheral neuropathy treated with pregabalin.[J Diabetes Complications. 2017]Glycemic and serum lipid control in patients with painful diabetic peripheral neuropathy treated with pregabalin.Parsons B, Emir B. J Diabetes Complications. 2017 Feb; 31(2):489-493. Epub 2016 Mar 17.
- Review The effect of pregabalin on pain-related sleep interference in diabetic peripheral neuropathy or postherpetic neuralgia: a review of nine clinical trials.[Curr Med Res Opin. 2010]Review The effect of pregabalin on pain-related sleep interference in diabetic peripheral neuropathy or postherpetic neuralgia: a review of nine clinical trials.Roth T, van Seventer R, Murphy TK. Curr Med Res Opin. 2010 Oct; 26(10):2411-9.
- Review Pregabalin in the Management of Painful Diabetic Neuropathy: A Narrative Review.[Diabetes Ther. 2019]Review Pregabalin in the Management of Painful Diabetic Neuropathy: A Narrative Review.Azmi S, ElHadd KT, Nelson A, Chapman A, Bowling FL, Perumbalath A, Lim J, Marshall A, Malik RA, Alam U. Diabetes Ther. 2019 Feb; 10(1):35-56. Epub 2018 Dec 18.
- Pregabalin - LiverToxPregabalin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...