NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Pravastatin is a commonly used cholesterol lowering agent (statin) that is associated with mild, asymptomatic and self-limited serum aminotransferase elevations during therapy, and rarely with clinically apparent acute liver injury.
Background
Pravastatin (pra" va stat' in) is an orally available inhibitor of hepatic 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the major rate-limiting enzyme in cholesterol synthesis. Like other members of its class (the “statins”), pravastatin lowers total serum cholesterol and low densitylipoprotein (LDL) concentrations, thereby reducing the risk of atherosclerosis and its complications – myocardial infarction and stroke. Pravastatin was approved for use in the United States in 1991 and continues to be widely used with more than 9 million prescriptions filled yearly. Current indications are for treatment of hypercholesterolemia in persons at high risk for coronary, cerebrovascular and peripheral artery disease. Pravastatin is available in tablets of 10, 20, 40 and 80 mg in several generic forms and under the brand name of Pravachol. The recommended dose in adults is 40 to 80 mg once daily. Common side effects include muscle cramps, joint aches, abdominal pain, nausea, headache and weakness, symptoms that occur with all of the currently available statins. Rare but potentially severe adverse events include liver injury, myopathy, rhabdomyolysis, and immune-mediated necrotizing myopathy.
Hepatotoxicity
Pravastatin therapy is associated with mild, asymptomatic and usually transient serum aminotransferase elevations. In summary analyses of large scale studies with prospective monitoring, ALT elevations above normal occurred in 3% to 7% of patients; but levels above 3 times the upper limit of normal (ULN) occurred in less than 1.2% of both pravastatin- as well as in placebo-treated subjects. Most of these elevations were self-limited and did not require dose modification. Pravastatin has been only rarely associated with clinically apparent hepatic injury with symptoms or jaundice at a rate estimated to be 1 per 100,000 users or less. In the case reports, latency varied from 2 to 9 months and the pattern of serum enzyme elevations from cholestatic to hepatocellular. Recovery was complete within a few months. Rash, fever and eosinophilia were uncommon as were autoantibodies, but few cases have been reported and the full clinical syndrome not well defined. Pravastatin appears to be less likely to cause clinically apparent liver injury than atorvastatin, simvastatin and rosuvastatin.
Likelihood score: B (likely cause of clinically apparent liver injury).
Mechanism of Injury
The cause of hepatic injury from pravastatin is unknown. Pravastatin has only minimal hepatic metabolism and most is excreted unchanged in the urine. The mild, self-limited ALT elevations may be due to production of a minor toxic intermediate of metabolism and the reversal of these elevations due to adaptation. The idiosyncratic, clinically apparent liver injury associated with pravastatin may be due to immune mediated responses.
Outcome and Management
The product labels for most statins recommend screening for liver test abnormalities before starting therapy and repeating tests as clinically indicated. The mild ALT elevations associated with pravastatin therapy are usually self-limited and do not require dose modification, although pravastatin should be stopped if ALT levels rise above 10-fold the ULN, or persist in being above 5-fold elevated or are associated with symptoms. In the clinically apparent liver injury attributed to pravastatin, recovery was usually complete within 1 to 2 months. In view of the wide scale use of pravastatin, clinically apparent and severe liver injury is extraordinarily rare. Recurrence of injury with rechallenge has been reported and should be avoided. Switching therapy to another statin after pravastatin induced injury can lead to recurrence and should be done with careful monitoring.
Drug Class: Antilipemic Agents
Other Drugs in the Subclass, Statins: Atorvastatin, Ezetimibe [used in combination], Fluvastatin, Lovastatin, Pitavastatin, Rosuvastatin, Simvastatin
CASE REPORT
Case 1. Acute cholestatic hepatitis attributed to pravastatin therapy.(1)
A 57 year old man developed abdominal pain and nausea followed by fever and jaundice 6 weeks after starting pravastatin (20 mg daily) for long standing hypercholesterolemia. He had a history of coronary artery disease and had been treated with beta blockers and various cholesterol lowering drugs, including fenofibrate and simvastatin in the past. At the time of presentation, he was taking only pravastatin and metoprolol, both of which were discontinued promptly. He denied alcohol use and had no risk factors for viral hepatitis. Physical examination showed jaundice and hepatic tenderness but no rash, fever, or signs of chronic liver disease. Laboratory results showed a cholestatic pattern of serum enzyme elevations and hyperbilirubinemia (Table). Tests for hepatitis A, B and C were negative as were autoantibodies. Ultrasound and CT of the abdomen showed no evidence of biliary obstruction and ERCP was normal. A liver biopsy showed intrahepatic cholestasis compatible with drug induced liver injury. He was treated with ursodiol (750 mg daily). Once pravastatin was stopped, symptoms and liver test abnormalities improved rapidly and were completely normal 7 weeks later.
Key Points
| Medication: | Pravastatin (20 mg daily) |
|---|---|
| Pattern: | Mixed (R=2.8) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 6 weeks |
| Recovery: | ~7 weeks |
| Other medications: | Metoprolol |
Laboratory Values
| Time After Starting | Time After Stopping | ALT* (U/L) | Alk P* (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 6 weeks | 0 | 421 | 482 | 13.6 | Admission |
| 2 days | 260 | 11.8 | |||
| 8 weeks | 2 weeks | 151 | 3.7 | ||
| 9 weeks | 3 weeks | 255 | 3.0 | Liver biopsy | |
| 10 weeks | 4 weeks | 210 | 1.9 | Discharge | |
| 3 months | 7 weeks | 40 | Normal | 0.5 | Outpatient follow up |
| Normal Values | <40 | <130 | <1.2 | ||
- *
Some values estimated from Figure 1.
Comment
The onset of injury within 2 months of starting pravastatin and resolution within 2 months of stopping is supportive evidence that this represented drug induced liver disease due to pravastatin. All other causes of acute liver injury were satisfactorily excluded. Metoprolol had been used for a longer period and, like other beta-blockers, is a rare cause of drug induced liver injury. The pattern of serum enzyme elevations was considered “mixed” but the clinical presentation, symptoms and liver histology were more cholestatic. This patient had previously tolerated simvastatin without obvious liver injury. Cross susceptibility to cholestatic hepatitis from the statins is frequent but not invariable.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Pravastatin – Generic, Pravachol®
DRUG CLASS
Antilipemic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Pravastatin | 81093-37-0 | C23-H36-O7 |
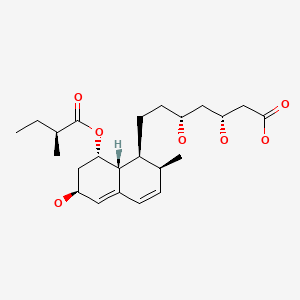
|
CITED REFERENCE
- 1.
- Hartleb M, Rymarczyk G, Januszewski K. Acute cholestatic hepatitis associated with pravastatin. Am J Gastroenterol. 1999;94:1388–90. [PubMed: 10235223]
ANNOTATED BIBLIOGRAPHY
References updated: 01 December 2021
Abbreviations used: ANA, antinuclear antibody; HDL, high density lipoprotein; LDL, low density lipoprotein; OD, odds ratio.
- Zimmerman HJ. Drugs used in the treatment of hypercholesterolemia and hyperlipidemia. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 660-2.(Expert review of hepatotoxicity published in 1999; the statins have dose related hepatic effects in guinea pigs and rabbits and transient elevations in aminotransferases occur in 1-5% of humans treated; several cases of clinically apparent liver injury from lovastatin and simvastatin have been published).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic medications. Lipid lowering agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 519-40.(Review of hepatotoxicity of lipid lowering agents; asymptomatic elevations in aminotransferases are common in patients receiving statins, but clinically significant hepatotoxicity is rare).
- Gurgle H, Blumenthal DK. Drug therapy for dyslipidemias. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 605-618.(Textbook of pharmacology and therapeutics; “Serious hepatotoxicity is rare and unpredictable, with a rate of about 1 case per million person-years of use.” Multiple academic societies and the FDA recommend testing all patients for routine liver tests before starting statins but monitoring or retesting only if symptoms arise).
- Ballarè M, Campanini M, Airoldi G, Zaccala G, Bertoncelli MC, Cornaglia G, Porzio M, et al. Hepatotoxicity of hydroxy-methyl-glutaryl-coenzyme A reductase inhibitors. Minerva Gastroenterol Dietol. 1992;38:41–4. [PubMed: 1520752](Prospective monitoring identified ALT elevations in 5% of 100 patients on simvastatin and 4.5% of 90 on pravastatin).
- The Lovastatin Pravastatin Study Group. A multicenter comparative trial of lovastatin and pravastatin in the treatment of hypercholesterolemia. Am J Cardiol. 1993;71:810–5. [PubMed: 8456759](Controlled trial of lovastatin [20 to 80 mg] vs pravastatin [10 to 40 mg] daily for 18 weeks in 672 hypercholesterolemic patients; ALT elevations >3 times ULN occurred in 1 lovastatin and 2 pravastatin treated patients; no clinically apparent liver injury mentioned).
- Morris R, Robinson G, Tilyard M, Gurr E. Pravastatin and risk factor modification in patients with moderate primary hypercholesterolaemia. NZ Med J. 1996;109:319–22. [PubMed: 8816723](In a prospective controlled trial in 78 patients, transient liver test abnormalities occurred in 3 patients on pravastatin [18%] and 7 [18%] on placebo; none led to discontinuation and none had symptoms or jaundice).
- Hartleb M, Rymarczyk G, Januszewski K. Acute cholestatic hepatitis associated with pravastatin. Am J Gastroenterol. 1999;94:1388–90. [PubMed: 10235223](57 year old man developed jaundice, 2 months after starting pravastatin [bilirubin 13.6 mg/dL, ALT 421 U/L, Alk P 482 U/L], resolving within 8 weeks of stopping: Case 1).
- Heuer T, Gerards H, Pauw M, Gabbert HE, Reis HE. Med Klin (Munich). 2000;95:642–4. [Toxic liver damage caused by HMG-CoA reductase inhibitor] German. [PubMed: 11143546](4 patients with liver injury due to statins: 3 simvastatin and 1 pravastatin with jaundice arising 8-24 months after starting [bilirubin 1.9 to 7.4 mg/dL, ALT 39 to 841 U/L, Alk P 266 to 353], resolving within 3 months of stopping).
- Punthakee Z, Scully LJ, Guindi MM, Ooi TC. Liver fibrosis attributed to lipid lowering medications: two cases. J Intern Med. 2001;250:249–54. [PubMed: 11555130](39 year old man developed fever and weakness 9 months after starting pravastatin [ALT ~500 U/L, but no jaundice], resolving rapidly with stopping and then recurring after 22 months of simvastatin therapy [ALT ~2800 U/L, ANA negative], biopsy showing chronic hepatitis whereas his enzymes remained normal over the next 6 years on no-statin therapy).
- Hartleb M, Biernat L, Kochel A. Drug-induced liver damage--a three-year study of patients from one gastroenterological department. Med Sci Monit. 2002;8:CR292–6. [PubMed: 11951073](14 patients with drug induced liver injury seen in one hospital [Silesian Medical University] over 3 year period; due to amoxicillin/clavulanate in 3, antituberculosis agents 2, pravastatin 2, fluvastatin 1, and 6 other agents in 1 each; 2 pravastatin cases in 62 and 57 year olds with onset after 4 and 7 weeks [bilirubin 1.0 and 13.6 mg/dL, ALT 3.4 and 10.5 times ULN, Alk P 1.0 and 4.4 times ULN], one case with rapid recovery upon stopping and the other [with jaundice] protracted).
- Batey RG, Harvey M. Cholestasis associated with the use of pravastatin sodium. Med J Aust. 2002;176:561. [PubMed: 12064992](64 year old woman developed abnormal liver tests 4 months after starting pravastatin [bilirubin 0.8 mg/dL, ALT 85 U/L, Alk P 362 U/L], improving on stopping and rising again with restarting, decreasing upon stopping but not to normal; never jaundiced or symptomatic).
- Pfeffer MA, Keech A, Sacks FM, Cobbe SM, Tonkin A, Byington RP, Davis BR, et al. Safety and tolerability of pravastatin in long-term clinical trials: prospective Pravastatin Pooling (PPP) Project. Circulation. 2002;105:2341–6. [PubMed: 12021218](Controlled trial of pravastatin vs placebo for 5 years in ~18,000 patients with hypercholesterolemia; no differences in rates of adverse events, gallstones in 1.9% vs 2.1% [pravastatin vs placebo], any abnormal ALT in 8.8% vs 8.2% and ALT >3 times ULN in 1.4% vs 1.3%).
- Rosenson RS, Bays HE. Results of two clinical trials on the safety and efficacy of pravastatin 80 and 160 mg per day. Am J Cardiol. 2003;91:878–81. [PubMed: 12667578](Two placebo controlled trials of higher doses of pravastatin [40 and 160 mg/day for 6 weeks]; no ALT or AST elevations above 3 times the ULN in either study).
- Parra JL, Reddy KR. Hepatotoxicity of hypolipidemic drugs. Clin Liver Dis. 2003;7:415–33. [PubMed: 12879992](Review and discussion of individual agents; rate of serum ALT elevations with pravastatin has been similar to that with placebo; mentions that single case report of cholestatic hepatitis due to pravastatin has appeared in the literature).
- de Denus S, Spinler SA, Miller K, Peterson AM. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584–91. [PubMed: 15162892](Systematic review of 13 large controlled trials of statins with at least 48 weeks of therapy in 43,390 patients; overall odds ratio for liver test abnormalities with statins versus placebo was 1.26; lovastatin 1.78; simvastatin 1.06; pravastatin 1.00, and fluvastatin, 3.54).
- Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis. 2004;174:193–6. [PubMed: 15135271](5 patients with nonalcoholic steatohepatitis were treated with pravastatin [20 mg daily for 6 months], ALT levels became normal in all five patients and histology improved in some, but not fibrosis scores).
- Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94:1140–6. [PubMed: 15518608](Pharmacokinetic studies that demonstrate that drugs that inhibit CYP 3A4, the major P450 drug metabolizing enzyme [itraconazole, clarithromycin, verapamil], cause increases in blood levels of simvastatin and atorvastatin, but have little effect on pravastatin levels).
- Nissen SE. Effect of intensive lipid lowering on progression of coronary atherosclerosis: evidence for an early benefit from the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) trial. Am J Cardiol. 2005;96(5A):61F–68F. [PubMed: 16126025](Controlled trial comparing pravastatin [40 mg] to atorvastatin [80 mg] daily for 18 months in 654 patients; ALT elevations >3 times ULN occurred in 1.6% on pravastatin vs 2.3% on atorvastatin, but no instances of clinically apparent hepatitis).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, et al. Spanish Group for the Study of Drug-Induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21. [PubMed: 16083708](Among 461 cases of drug induced liver disease enrolled in a Spanish Registry between 1984 and 2004, 11 were attributed to statins, but no specific agent caused more than 4 cases).
- Alsheikh-Ali AA, Karas RH. Adverse events with concomitant amiodarone and statin therapy. Prev Cardiol. 2005;8:95–7. [PubMed: 15860984](Review of MedWatch adverse event reports for proportion that included combination with amiodarone; 1.0% for simvastatin, 0.7% atorvastatin and 0.4% pravastatin; 77% had muscle and 30% liver involvement).
- Conforti A, Magro L, Moretti U, Scotto S, Motola D, Salvo F, Ros B, et al. Fluvastatin and hepatic reactions: a signal from spontaneous reporting in Italy. Drug Safety. 2006;29:1163–72. [PubMed: 17147462](Italian Pharmacovigilance Group review of 35,757 adverse reaction reports: 1260 due to statins of which 178 were hepatic; 69 [36%] fluvastatin, 37 [21%] atorvastatin, 50 [28%] simvastatin, 16 [9%] pravastatin, 6 [3%] rosuvastatin; proportion reporting rate based on number of prescriptions was highest for fluvastatin [~9] compared to other agents [~2-3]; 26 fluvastatin cases described as “hepatitis”, but no details given except that most cases occurred within 90 days of starting).
- Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8A):52C–60C. [PubMed: 16581329](Review of safety of statins; 38 cases of acute liver failure attributed to statins submitted to MedWatch by end of 1999, which gives an estimated rate of 1 per million person years of use; rate of confirmed ALT elevations >3 times ULN is 0.1% with statins and 0.04% with placebo).
- Khorashadi S, Hasson NK, Cheung RC. Incidence of statin hepatotoxicity in patients with hepatitis C. Clin Gastroenterol Hepatol. 2006;4:902–7. [PubMed: 16697272](Electronic record review of rate of ALT elevations in patients with hepatitis C with or without statin therapy and controls on statin therapy found no differences between the three groups [20%, 24% and 17%]; severe abnormalities most frequent in patients with chronic hepatitis C not on statin [6.6% vs 1.2%]).
- Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clin Ther. 2006;28:26–35. [PubMed: 16490577](Metaanalysis of adverse event rates in 18 placebo controlled trials of six statins in 71,108 patients; ALT elevations >3 times ULN in 1.7% of statin vs 1.4% placebo recipients; event rates highest with atorvastatin, lowest with fluvastatin).
- Dale KM, White CM, Henyan NN, Kluger J, Coleman CI. Impact of statin dosing intensity on transaminase and creatine kinase. Am J Med. 2007;120:706–12. [PubMed: 17679130](Metaanalysis of rates of ALT and CPK elevations in 9 controlled studies comparing low vs high doses of statins; ALT elevations >3 times ULN occurred in 1.5% of high- and 0.4% of low-intensity statin groups, effect particularly seen with hydrophilic [pravastatin and atorvastatin] compared to lipophilic agents [simvastatin and lovastatin]).
- Alsheikh-Ali AA, Karas RH. Safety of lovastatin/extended release niacin compared with lovastatin alone, atorvastatin alone, pravastatin alone, and simvastatin alone (from the United States Food and Drug Administration adverse event reporting system). Am J Cardiol. 2007;99:379–81. [PubMed: 17261402](Analysis of MedWatch reports of adverse events found no excess in liver related adverse event reports per million prescription due to lovastatin alone [2.3] vs niacin alone [2.5] vs the combination [3.2], but slightly higher rates with atorvastatin [4.5], simvastatin [5.7] and pravastatin [4.9], but data relied upon spontaneous reporting).
- Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R., Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453–63. [PubMed: 17668878](Controlled trial of pravastatin [80 mg daily] vs placebo for 36 weeks in 326 patients with chronic liver disease and hypercholesterolemia [64% nonalcoholic steatohepatitis and 25% chronic hepatitis C]; cumulative incidence of ALT levels >twice baseline or ULN was 7.5% for pravastatin and 12.5% for placebo, and none had exacerbation of underlying liver disease or jaundice).
- Contreras AM, Monteón FJ, Flores MR, Mendoza-Sánchez F, Ruiz I. Drug-related hepatotoxicity in a renal transplant recipient with long-term survival and hepatitis C. Ann Hepatol. 2007;6:70–3. [PubMed: 17297434](26 year old man with a renal transplant had ALT elevations [peak 151 U/L] without jaundice after several years of pravastatin therapy, which did not improve with stopping pravastatin or azathioprine, and later found to be due to chronic hepatitis C).
- Bhardwah SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis. 2007;11:597–613. [PMC free article: PMC2048990] [PubMed: 17723922](Review of hepatotoxicity of statins reported rates of ALT or AST elevations >3 times ULN: atorvastatin 0.7%, fluvastatin 1.2%, lovastatin 0.6%, pravastatin 1.4%, rosuvastatin 0% and simvastatin 1.8%. Abnormalities are usually asymptomatic, individual case reports of autoimmune hepatitis have been published).
- Alsheikh-Ali AA, Maddukuri PV, Han H, Karas RH. Effect of the magnitude of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: insights from large randomized statin trials. J Am Coll Cardiol. 2007;50:409–18. [PubMed: 17662392](Systematic review of relationship between LDL cholesterol lowering effects and adverse events in 23 statin treatment arms representing 309,506 person years of therapy; positive and graded relationship between statin dose [simvastatin, lovastatin and atorvastatin] and rates of ALT elevations, but no independent relationship to degree of LDL cholesterol decrease).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug-Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, 3 cases were attributed to atorvastatin, 3 to simvastatin/ezetimibe, and one each to pravastatin, fluvastatin, and simvastatin, but most cases were mild or not clearly attributable to the statin therapy).
- Martin JE, Cavanaugh TM, Trumbull L, Bass M, Weber F Jr, Aranda-Michel J, Hanaway M, et al. Incidence of adverse events with HMG-CoA reductase inhibitors in liver transplant patients. Clin Transplant. 2008;22:113–9. [PubMed: 18217912](Retrospective review of adverse events associated with statin and fibrate use in 69 patients with liver transplants; myalgias problematic in 5, myopathy in 1, but none had significant ALT elevations or hepatitis related to medication).
- Neuvonen PJ, Backman JT, Niemi M. Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin. Clin Pharmacokinet. 2008;47:463–74. [PubMed: 18563955](Review of literature on pharmacokinetics of statins; simvastatin and lovastatin are metabolized extensively by the P450 system and levels are affected by inhibitors or inducers of CYP 3A4 [itraconazole, erythromycin, verapamil, diltiazem, cyclosporine], whereas fluvastatin and pravastatin are minimally if at all affected).
- Russo MW, Scobey M, Bonkovsky HL. Drug-induced liver injury associated with statins. Semin Liver Dis. 2009;29:412–22. [PubMed: 19826975](Case reports and review of literature; 52 year old woman who developed fatigue 12 weeks after starting fluvastatin [bilirubin 1.2 mg/dL, ALT 850 U/L, Alk P 215 U/L, ANA negative], resolving on stopping fluvastatin, but recurring within 11 weeks of starting atorvastatin [bilirubin 1.0 rising to 12.5 mg/dL, ALT 1750 U/L, Alk P 285 U/L, ANA 1:160], responding to prednisone and azathioprine therapy).
- Liu Y, Cheng Z, Ding L, Fang F, Cheng KA, Fang Q, Shi GP. Atorvastatin-induced acute elevation of hepatic enzymes and the absence of cross-toxicity of pravastatin. Int J Clin Pharmacol Ther. 2010;48:798–802. [PMC free article: PMC3070374] [PubMed: 21084035](Two men, ages 53 and 58, developed ALT elevations [peak 120 and 278 U/L] within a day of starting atorvastatin, resolving within 7 days of stopping and not recurring in either when pravastatin was started).
- Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197. [PMC free article: PMC2874131] [PubMed: 20488911](Among 225,922 new users of statins in a UK health care database, there was an increased risk of moderate or severe liver dysfunction [ALT >3 times ULN], usually within first 6 months and associated with higher doses of statins; relative risks were highest with fluvastatin [2.53 in women, 1.97 in men] and lowest with pravastatin [0.93 to 1.58]).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 2 due to atorvastatin, 2 simvastatin and 2 cerivastatin, but none to pravastatin).
- Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol. 2012;56:374–80. [PubMed: 21889469](Between 1988 and 2010, the Swedish registry received 217 adverse event reports possibly related to statins, 124 [57%] being liver related, 73 of which could be evaluated: 2 were fatal and one led to liver transplant; 3 had positive rechallenge; 43 [59%] were hepatocellular, 22 [30%] cholestatic and 8 [11%] mixed; 30 were due to atorvastatin, 28 simvastatin, 11 fluvastatin, 2 pravastatin and 2 rosuvastatin, arising after 30-248 days; atorvastatin injury was more likely to be cholestatic and was estimated to occur in 2.9 per 100,000 person years).
- Farnier M, Marcereuil D, De Niet S, Ducobu J, Steinmetz A, Retterstøl K, Bryniarski L, et al. Safety of a fixed-dose combination of fenofibrate/pravastatin 160 mg/40 mg in patients with mixed hyperlipidaemia: a pooled analysis from a database of clinical trials. Clin Drug Investig. 2012;32:281–91. [PubMed: 22350498](Analysis of fixed combination of pravastatin with fenofibrate vs each alone in 5 large trials found no case of drug induced liver injury or rhabdomyolysis; elevations in ALT >3 times ULN occurred in 1.6% [2/122] on fenofibrate, 0.2% [1/519] on statins and 1.0% [16/1566] on the fixed combination, but all were transient and not accompanied by jaundice).
- Sirtori CR, Mombelli G, Triolo M, Laaksonen R. Clinical response to statins: mechanism(s) of variable activity and adverse effects. Ann Med. 2012;44:419–32. [PubMed: 21623698](Review of the possible mechanisms for the beneficial and adverse effects of statins, including genetic variations in CYP enzymes, ABC transporters and HLA genes in causing adverse events, focused mostly upon myopathy and myalgias).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 2 attributed to atorvastatin and 1 to simvastatin, but none to pravastatin).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to statins or lipid lowering agents).
- Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, Chalasani N, et al. Spectrum of statin hepatotoxicity: Experience of the drug-induced liver injury network. Hepatology. 2014;60:679–86. [PMC free article: PMC4110177] [PubMed: 24700436](Among 1,188 cases of drug induced liver disease collected in the US between 2004 to 2012, 22 [2%] were attributed to statins, including atorvastatin [8], simvastatin [5], rosuvastatin [4], fluvastatin [2], pravastatin [2] and lovastatin [1]; median age was 60 years and 68% were women; 9 cases were cholestatic and 12 hepatocellular [6 with autoimmune features]; the latency ranged widely, from 1 month to 10 years; only one case was fatal [a man with preexisting cirrhosis presenting with acute-on-chronic liver failure]).
- Bays H, Cohen DE, Chalasani N, Harrison SA. An assessment by the Statin Liver Safety Task Force: 2014 update. J Clin Lipidol. 2014;8(3) Suppl:S47–57. [PubMed: 24793441](Review of the safety of statins including their use in patients with liver disease recommending that liver tests be obtained before therapy, but that routine monitoring is not necessary and that statins can be safety used in patients with nonalcoholic liver disease, and are probably safe in other forms of chronic liver disease and after liver transplantation).
- Ooba N, Sato T, Wakana A, Orii T, Kitamura M, Kokan A, Kurata H, et al. A prospective stratified case-cohort study on statins and multiple adverse events in Japan. PLoS One. 2014;9:e96919. [PMC free article: PMC4014577] [PubMed: 24810427](Among 6877 patients started on statins between 2008 and 2010, 139 developed an increase in ALT or AST deemed likely due to the drug with no significant differences among those treated with pra-, ator-, flu-, pita- or rosu-vastatin).
- Macedo AF, Taylor FC, Casas JP, Adler A, Prieto-Merino D, Ebrahim S. Unintended effects of statins from observational studies in the general population: systematic review and meta-analysis. BMC Med. 2014;12:51. [PMC free article: PMC3998050] [PubMed: 24655568](Systematic review of 90 studies of 48 different "unintended effects" of statins with evidence of an increased risk of myopathy [Odds Ratio: OR=2.6] and raised liver enzymes [OR=1.5]).
- Drugs for lipids. Treat Guidel Med Lett. 2014;12(137):1–6. [PubMed: 24419209](Concise recommendations on management of hyperlipidemia mentions that 1-2% of patients on high doses of statins develop ALT elevations [above 3 times ULN], but that there is not always cross sensitivity to this side effect and that patients with mild-to-moderate ALT elevations can tolerate statins; no discussion of clinically apparent liver).
- Perdices EV, Medina-Cáliz I, Hernando S, Ortega A, Martín-Ocaña F, Navarro JM, Peláez G, et al. Hepatotoxicity associated with statin use: analysis of the cases included in the Spanish Hepatotoxicity Registry. Rev Esp Enferm Dig. 2014;106:246–54. [PubMed: 25075655](Among 858 cases of drug induced liver injury enrolled in a Spanish Registry between 1994 and 2012, 47 [5.5%] were attributed to statins [16 atorvastatin, 13 simvastatin, 12 fluvastatin, 4 lovastatin and 2 pravastatin], usually with a hepatocellular pattern of injury, 8.5% with autoimmune features, chronic injury in 19%, and no liver related deaths).
- Chen GL, Hsiao FY, Dong YH, Shen LJ, Wu FL. Statins and the risk of liver injury: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2014;23:719–25. [PubMed: 24829162](Among 2165 Taiwanese patients hospitalized for liver injury between 2002 and 2009, use of statins was not more frequent than among 16,600 hospitalized controls, except for use of high doses of rosuvastatin [adjusted odds ratio of 2.29]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 31 cases [3.4%] were attributed to statins, including 8 to atorvastatin, 8 simvastatin, 7 rosuvastatin, 4 pravastatin, 2 fluvastatin and 2 lovastatin).
- Carrascosa MF, Salcines-Caviedes JR, Lucena MI, Andrade RJ. Acute liver failure following atorvastatin dose escalation: is there a threshold dose for idiosyncratic hepatotoxicity? J Hepatol. 2015;62:751–2. [PubMed: 25463547](85 year old woman on atorvastatin for more than a year developed jaundice after increasing dose from 40 to 80 mg daily [bilirubin 4.1 mg/dL, ALT 909 U/L, Alk P 415 U/L, CK 4753 U/L, INR 1.71], with transient worsening, but ultimate improvement and resolution).
- Chang CH, Chang YC, Lee YC, Liu YC, Chuang LM, Lin JW. Severe hepatic injury associated with different statins in patients with chronic liver disease: a nationwide population-based cohort study. J Gastroenterol Hepatol. 2015;30:155–62. [PubMed: 25041076](Among 37,929 Taiwanese persons with chronic liver disease started on statin therapy for hyperlipidemia between 2005 and 2009, there were 912 incident cases of hospitalization for liver injury, rates being similar for the 6 different statins used [1.94-2.95 per 100,000 person-days], but higher in those on high doses of atorvastatin [40 or 80 mg daily]).
- Kim HS, Lee SH, Kim H, Lee SH, Cho JH, Lee H, Yim HW, et al. Statin-related aminotransferase elevation according to baseline aminotransferases level in real practice in Korea. J Clin Pharm Ther. 2016;41:266–72. [PubMed: 27015878](Among 21,233 Korean patients starting statin therapy between 2009 and 2013, abnormal ALT or AST values above 3 times ULN were more frequent among those with mild baseline elevations).
- Björnsson ES. Hepatotoxicity of statins and other lipid-lowering agents. Liver Int. 2017;37:173–8. [PubMed: 27860156](Review of the hepatotoxicity of statins mentions that atorvastatin has been the most frequently implicated statin [accounting for 30-40% of cases] in drug induced liver injury estimated to arise in 1 in 17,000 users, cholestatic in 56% and with autoimmune features in 10% and rarely fatal).
- Wang LY, Huang YS, Perng CL, Huang B, Lin HC. Statin-induced liver injury in an area endemic for hepatitis B virus infection: risk factors and outcome analysis. Br J Clin Pharmacol. 2016;82:823–30. [PMC free article: PMC5338125] [PubMed: 27197051](Analysis of the Taipei Veterans Hospital database from 2008 to 2012 identified 108 patients with statin-associated liver injury [including 28 rosu-, 20 flu-, 17 sim-, 11 pra-, 8 lo-, and 8 pita-vastatin] most of which 75 [69%] were mild and only one fatal [80 year old on rosuvastatin], and there were no differences in disease features or peak enzyme or bilirubin levels between HBsAg positive vs negative subjects [n=16 vs 92]).
- Giugliano RP, Wiviott SD, Blazing MA, De Ferrari GM, Park JG, Murphy SA, White JA, et al. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT Trial. JAMA Cardiol. 2017;2:547–555. [PMC free article: PMC5814987] [PubMed: 28291866](Among 15,281 patients recovering from an acute cardiac syndrome treated with simvastatin [40 mg daily] with or without ezetimibe for up to 6 years, 6.4% achieved very low LDL-cholesterol levels [<30 mg/dL] and subsequently had low rates of cardiovascular events, but also no increase in rates of adverse events from statins such including ALT elevations above 3 times ULN [2.2% vs 1.8-2.1%]).
- Liang X, He Q, Zhao Q. Effect of stains on LDL reduction and liver safety: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:7092414. [PMC free article: PMC5859851] [PubMed: 29693013](In a systematic review of 16 controlled trials of statins in 74,078 patients, rates of liver test abnormalities were higher with statin therapy [odds ratio, OR=1.18] but this was significant only for fluvastatin [OR=3.5] and with higher doses [40-80 mg daily] [OD=3.6] and was not significant for statins used at low or moderate doses).
- Yebyo HG, Aschmann HE, Kaufmann M, Puhan MA. Comparative effectiveness and safety of statins as a class and of specific statins for primary prevention of cardiovascular disease: A systematic review, meta-analysis, and network meta-analysis of randomized trials with 94,283 participants. Am Heart J. 2019;210:18–28. [PubMed: 30716508](Metaanalyses of 40 trials of statins that enrolled 94,283 patients followed for a median of 1 year for efficacy and safety reported that statins as a class increased the risk of hepatic dysfunction by 6% with fluvastatin having the highest relative risk).
- Lipid-lowering drugs. Med Lett Drugs Ther. 2019;61(1565):17–24. [PubMed: 30845106](Concise review of the mechanism of action, relative efficacy, safety and costs of lipid lowering drugs including statins, ezetimibe, PCSK9 inhibitors, bile acid sequestrants, fibric acid derivatives niacin and fish oil, mentions that statin therapy is associated with ALT elevations above 3 times ULN in 1-3% of patients but “whether statins actually cause liver damage is unclear”).
- Simon TG. When less is more: dosing simvastatin in decompensated cirrhosis. Lancet Gastroenterol Hepatol. 2020;5:3–5. [PubMed: 31607676](Editorial in response to Pose et al [2020] discusses the possible beneficial effects of statins in patients with cirrhosis and the issue of increased rate of muscle toxicity with 40 vs to 20 mg daily).
- Hopewell JC, Offer A, Haynes R, Bowman L, Li J, Chen F, Bulbulia R, et al. Independent risk factors for simvastatin-related myopathy and relevance to different types of muscle symptom. Eur Heart J. 2020;41:3336–3342. [PMC free article: PMC7544537] [PubMed: 32702748](In a combined analysis of 3 large clinical trials in patients with cardiovascular disease treated with simvastatin for a mean of 3.4 years, 171 of 58,390 participants [0.1%] developed myopathy [muscle pain and CK levels above 10 times ULN], and risk was higher with higher doses, in Asian subjects, women, and persons with higher BMI and multiple comorbidities as well as with SLCO1B1 genotype).
- Balasubramanian R, Maideen NMP. HMG-CoA reductase inhibitors (statins) and their drug interactions involving CYP enzymes, P-glycoprotein and OATP transporters-an overview. Curr Drug Metab. 2021;22:328–341. [PubMed: 33459228](Systematic review of literature on drug-drug interactions with statins and their clinical significance mentions that toxicity can be enhanced by inhibitors of CYP3A4 [ator-, sim- and lo-vastatin] as well as by inhibitors of P glycoprotein and OATP1B1 [most statins including rosuvastatin] with specific recommendations for the most common inhibitors).
- Sung S, Al-Karaghouli M, Kalainy S, Cabrera Garcia L, Abraldes JG. A systematic review on pharmacokinetics, cardiovascular outcomes and safety profiles of statins in cirrhosis. BMC Gastroenterol. 2021;21:120. [PMC free article: PMC7967963] [PubMed: 33726685](Systematic review of literature suggests that rosuvastatin and pitavastatin pharmacokinetics are unchanged in patients with Child’s Class A cirrhosis as opposed to atorvastatin and pravastatin, although unlike rosuvastatin, simvastatin, atorvastatin and pravastatin have been assessed in clinical trials in cirrhotic patients).
- Cai T, Abel L, Langford O, Monaghan G, Aronson JK, Stevens RJ, Lay-Flurrie S, et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses. BMJ. 2021;374(n1537) [PMC free article: PMC8279037] [PubMed: 34261627](Systematic review of placebo controlled trials of statins for cardiovascular disease prevention identified 62 publications with 120,456 patients and found an increased risk of muscle symptoms, liver test abnormalities, renal insufficiency and eye conditions for all 7 statins, but not muscle disorders or diabetes; rosuvastatin having relatively high risk for muscle symptoms and renal abnormalities and also was also associated with eye conditions and diabetes while atorvastatin and lovastatin had highest risk for liver abnormalities).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Simvastatin.[LiverTox: Clinical and Researc...]Review Simvastatin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Lovastatin.[LiverTox: Clinical and Researc...]Review Lovastatin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Rosuvastatin.[LiverTox: Clinical and Researc...]Review Rosuvastatin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Fluvastatin.[LiverTox: Clinical and Researc...]Review Fluvastatin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Atorvastatin.[LiverTox: Clinical and Researc...]Review Atorvastatin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Pravastatin - LiverToxPravastatin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...