NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Plecanatide is a minimally absorbed agonist of guanylate cyclase C receptors in the intestine and is used for treatment of chronic constipation and irritable bowel syndrome. Plecanatide has not been linked to serum enzyme elevations during treatment or to episodes of clinically apparent liver injury.
Background
Plecanatide (ple kan’ a tide) is synthetic analogue of uroguanylin, the endogenous guanylate cyclase-C receptor agonist which acts by increasing intracellular concentrations of cyclic guanosine monophosphate (cGMP) which activates the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel. The CFTR ion channel stimulates secretion of chloride and bicarbonate into the lumen of the intestine which increases intraluminal fluid and speeds intestinal transit. In several prelicensure clinical trials, plecanatide therapy was associated with increases in the number of spontaneous bowel movements, improved stool consistency and alleviation of symptoms of chronic constipation. Plecanatide was approved for therapy of chronic idiopathic constipation in adults in 2017, the second guanylate cyclase agonist approved for this indication in the United States, the first being linaclotide. Plecanatide is available in tablets of 3 mg under the brand name Trulance. The recommended dose for chronic idiopathic constipation is 3 mg once daily. It is contraindicated in children below the age of 6 years and not recommended for those below 18 years of age. Side effects include diarrhea (~5%) which is severe in less than 1%. Other adverse events include abdominal pain, bloating, flatulence and headache.
Hepatotoxicity
In prelicensure clinical trials, plecanatide was associated with low rates of serum enzyme elevations during treatment with some degree of elevation in serum ALT or AST in 1% to 2% of treated subjects. Values rose above 5 times the upper limit of normal in only 0.2%. In these studies, there were no episodes of clinically apparent liver injury with jaundice that could be linked to plecanatide therapy. Since its approval and marketing, there have been no published reports of serum aminotransferase elevations or clinically apparent liver injury attributable to plecanatide. Thus, liver injury from plecanatide must be rare if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Plecanatide is largely active on the epithelial cells in the intestinal tract and has minimal absorption. The lack of systemic exposure and the low total daily dose (3 mg) may account for its lack of liver injury.
Drug Classes: Gastrointestinal Agents, Agents for Constipation, Irritable Bowel Syndrome Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Plecanatide – Trulance®
DRUG CLASS
Gastrointestinal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Plecanatide | 467426-54-6 | C65-H104-N18-O26-S4 |
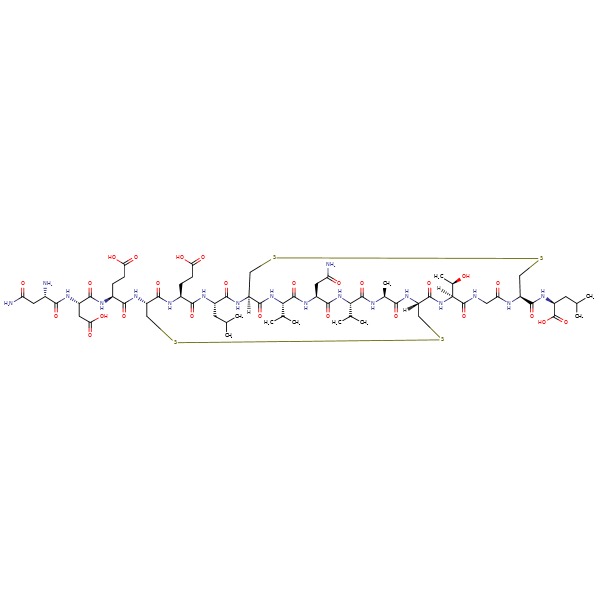 |
ANNOTATED BIBLIOGRAPHY
References updated: 16 April 2019
- Zimmerman HJ. Laxatives. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 721-3.(Expert review of hepatotoxicity of laxatives published in 1999 with discussion of oxyphenisatin and other agents, but not plecanatide).
- Sharkey KA, McNaughton WK. Gastrointestinal motility and water flux: emesis, and biliary and pancreatic disease. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 921-44.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2017/208745Orig1s000TOC.cfm . (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions that ALT elevations above 5 times ULN occurred in 0.1% of 1673 plecanatide vs none of 841 placebo recipients and two subjects had jaundice accompanied by ALT elevations, both of whom, however, had other more likely reasons for acute liver injury). - Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to agents used for constipation or irritable bowel syndrome [IBS]).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to gastrointestinal agents, laxatives or drugs for IBS).
- Shah E, Kim S, Chong K, Lembo A, Pimentel M. Evaluation of harm in the pharmacotherapy of irritable bowel syndrome. Am J Med 2012; 125: 381-93. [PubMed: 22444104](Systematic review of adverse side effects of drugs used to treat irritable bowel syndrome including lubiprostone, but not linaclotide or plecanatide).
- Camilleri M. Guanylate cyclase C agonists: emerging gastrointestinal therapies and actions. Gastroenterology 2015; 148: 483-7. [PubMed: 25576859](Review of the role of guanylate cyclase C in intestinal physiology and the effects of agonists [bacterial enterotoxins, linaclotide and plecanatide] on intercellular signaling, enterocyte chloride secretion and intestinal peristalsis, as well as their clinical effects and role in management of chronic idiopathic constipation and constipation predominant irritable bowel syndrome).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was attributed to metoclopramide, but none to other prokinetic agents or drugs for chronic idiopathic constipation).
- DeMicco M, Barrow L, Hickey B, Shailubhai K, Griffin P. Randomized clinical trial: efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Therap Adv Gastroenterol 2017; 10: 837-51. [PMC free article: PMC5673020] [PubMed: 29147135](Among 1337 patients with chronic idiopathic constipation treated with plecanatide [3 or 6 mg] vs placebo once daily for 12 weeks, plecanatide improved stool frequency and consistency and adverse events were uncommon, the most frequent being diarrhea which arose in 3.2% and 4.5% on plecanatide vs 1.3% on placebo and “laboratory findings…were all unremarkable, with low incidence of any clinically important change”).
- Miner PB Jr, Koltun WD, Wiener GJ, De La Portilla M, Prieto B, Shailubhai K, Layton MB, et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol 2017; 112: 613-21. [PMC free article: PMC5415706] [PubMed: 28169285](Among 1394 patients with chronic idiopathic constipation treated with plecanatide [3 or 6 mg] or placebo once daily for 12 weeks, spontaneous bowel movements increased by 3.1 to 3.2 per week with plecanatide vs 1.2 on placebo and adverse events were uncommon, diarrhea arose in 5.9% and 5.7% vs 1.3% of patients and “laboratory findings...were all unremarkable, with low incidence of any clinical important changes”).
- Al-Salama ZT, Syed YY. Plecanatide: first global approval. Drugs 2017; 77: 593-8. [PubMed: 28255961](Review of mechanism of action, history of development, pharmacology, clinical efficacy and safety; adverse events more frequent with plecanatide than placebo included diarrhea [5% vs 1%], abdominal distension, abdominal tenderness and increase in serum aminotransferase levels above 5 times ULN [ALT 0.2%, AST 0.3%]).
- Plecanatide (Trulance) for chronic idiopathic constipation. Med Lett Drugs Ther 2017; 59 (1519): 66-8. [PubMed: 28419073](Concise review of the mechanism of action, clinical efficacy, safety and costs of plecanatide shortly after its approval for use in chronic idiopathic constipation in the United States; discusses diarrhea as the most frequent adverse event, but also mentions the adverse event reaction of liver enzyme elevations).
- Brenner DM, Fogel R, Dorn SD, Krause R, Eng P, Kirshoff R, Nguyen A, et al. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: results of two phase 3 randomized clinical trials. Am J Gastroenterol 2018; 113: 735-45. [PubMed: 29545635](Among 2189 persons with constipation predominant irritable bowel syndrome enrolled in 2 clinical trials of plecanatide [3 or 6 mg] vs placebo once daily for 12 weeks, clinical responses were more frequent with plecanatide and the most frequent side effect was diarrhea [4.0% and 4.3% vs 1%] and discontinuations were uncommon [1.2% and 1.4% vs none]; no mention of ALT elevations or hepatotoxicity).
- Black CJ, Ford AC. Chronic idiopathic constipation in adults: epidemiology, pathophysiology, diagnosis and clinical management. Med J Aust 2018; 209: 86-91. [PubMed: 29996755](Review of the role of guanylate cyclase C in gastrointestinal physiology and the biologic basis for the use of guanylate cyclase receptor agonists as therapy of gastrointestinal disorders).
- Waldman SA, Camilleri M. Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders. Gut 2018; 67: 1543-52. [PMC free article: PMC6204952] [PubMed: 29563144](Review of the definition, diagnosis, epidemiology, pathophysiology, clinical features and management and drug therapy of chronic idiopathic constipation).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Plecanatide for Treatment of Chronic Constipation and Irritable Bowel Syndrome.[Am J Med. 2019]Review Plecanatide for Treatment of Chronic Constipation and Irritable Bowel Syndrome.Love BL. Am J Med. 2019 May; 132(5):572-575. Epub 2018 Dec 11.
- Efficacy and Tolerability of Guanylate Cyclase-C Agonists for Irritable Bowel Syndrome with Constipation and Chronic Idiopathic Constipation: A Systematic Review and Meta-Analysis.[Am J Gastroenterol. 2018]Efficacy and Tolerability of Guanylate Cyclase-C Agonists for Irritable Bowel Syndrome with Constipation and Chronic Idiopathic Constipation: A Systematic Review and Meta-Analysis.Shah ED, Kim HM, Schoenfeld P. Am J Gastroenterol. 2018 Mar; 113(3):329-338. Epub 2018 Jan 30.
- Review Plecanatide for the treatment of constipation-predominant irritable bowel syndrome.[Expert Rev Gastroenterol Hepat...]Review Plecanatide for the treatment of constipation-predominant irritable bowel syndrome.Miner PB. Expert Rev Gastroenterol Hepatol. 2020 Feb; 14(2):71-84. Epub 2020 Feb 5.
- Review Efficacy and safety of plecanatide in treating constipation predominant irritable bowel syndrome.[Expert Opin Pharmacother. 2018]Review Efficacy and safety of plecanatide in treating constipation predominant irritable bowel syndrome.Miner PB Jr. Expert Opin Pharmacother. 2018 Feb; 19(2):177-183. Epub 2018 Jan 29.
- Plecanatide for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation: Post hoc analyses of placebo-controlled trials in adults with severe constipation.[Neurogastroenterol Motil. 2023]Plecanatide for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation: Post hoc analyses of placebo-controlled trials in adults with severe constipation.Cash BD, Sharma A, Walker A, Laitman AP, Chang L. Neurogastroenterol Motil. 2023 Sep; 35(9):e14632. Epub 2023 Jun 18.
- Plecanatide - LiverToxPlecanatide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...