NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Piroxicam is a commonly used nonsteroidal antiinflammatory drug (NSAID) that is available by prescription only and is used in therapy of chronic arthritis. Piroxicam can cause mild serum aminotransferase elevations and, in rare instances, leads to clinically apparent acute liver injury that can be severe and even fatal.
Background
Piroxicam (pir ox' i kam) belongs to the oxicam family, which is a class of enolic acids structurally unrelated to other NSAIDs. Piroxicam, like other NSAIDs, acts through inhibition of tissue cyclooxygenases (Cox-1 and -2) leading to a decrease in synthesis of pro-inflammatory prostaglandins, which are potent mediators of pain and inflammation. Piroxicam has analgesic as well as antipyretic and antiinflammatory activities. Piroxicam was approved for use in the United States in 1982 and is still widely used, with several million prescriptions filled yearly. Current indications include rheumatoid arthritis and osteoarthritis. Piroxicam is available as capsules of 10 and 20 mg in several generic forms as well as under brand names such as Feldene, Novo-Pirocam and Nu-Pirox. The recommended dose is 10 to 20 mg orally once daily. Piroxicam is available by prescription only. Other oxicam NSAIDs include meloxicam, tenoxicam, and droxicam, the latter two being available in other countries, but not the United States. As with other NSAIDs, piroxicam is generally well tolerated, but side effects can include headache, dizziness, somnolence, dyspepsia, abdominal discomfort, diarrhea, peripheral edema and hypersensitivity reactions. Rare but serious adverse events from NSAIDs include gastrointestinal ulceration and bleeding, increased risk for cardiovascular disease, renal dysfunction and hypersensitivity reactions including anaphylaxis, exfoliative dermatitis and Stevens Johnson syndrome.
Hepatotoxicity
Elevated serum aminotransferase levels have been reported in 3% to 18% of patients taking piroxicam, but symptomatic liver disease with jaundice is rare (estimated at 1 to 5 cases per 100,000 prescriptions). The latency to onset of symptoms of clinically apparent liver injury due to piroxicam is variable from a few days to several months, but is generally within the first 1 to 6 weeks of treatment. The pattern of injury is predominantly cholestatic, although cases presenting with mixed or hepatocellular patterns have been reported (Case 1). Eosinophilia, rash and fever can occur, but are not always present and are usually not prominent. Autoantibodies are rarely found. The injury is usually self-limited and recovery occurs within 1 to 2 months. Rare cases of acute liver failure have been reported.
Likelihood score: B (rare but likely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of piroxicam induced liver injury is not known, but may be due to a toxic metabolic intermediate of piroxicam metabolism, which occurs largely in the liver. Cases with allergic manifestations (fever, rash, eosinophilia) may also have a component of hypersensitivity.
Outcome and Management
Severity of the liver injury from piroxicam ranges from asymptomatic elevations in serum aminotransferase levels to severe hepatitis with acute liver failure. Several instances of chronic vanishing bile duct syndrome have been attributed to other oxicam NSAIDs, but not specifically to piroxicam. In most instances, however, complete recovery is expected after stopping the drug and usually takes one to two months. Cross sensitivity to liver injury among the various NSAIDs has not been well studied or described. Due to the wide availability of alternative medications, rechallenge with piroxicam and other oxicam forms of NSAIDs (meloxicam, droxicam, tenoxicam) should be avoided.
Drug Class: Nonsteroidal Antiinflammatory Drugs, see also Meloxicam
CASE REPORT
Case 1. Acute cholestatic hepatitis arising 30 days after starting piroxicam therapy.(1)
A 74 year old woman with chronic arthritis was treated for 30 days with piroxicam and presented 2 days later with pruritus and dark urine followed by jaundice. She had no history of liver disease or exposures to viral hepatitis and did not drink alcohol. She had hypertension and had been treated with atenolol and hydrochlorothiazide chronically. She had no fever or rash, but was jaundiced and had mild hepatic tenderness. Laboratory tests showed a total bilirubin of 6.5 mg/dL and prominent elevations in both ALT and alkaline phosphatase (Table) and eosinophilia. Tests for hepatitis A and B were negative. Ultrasound of the abdomen was normal. A liver biopsy showed intrahepatic cholestasis with minimal portal inflammation suggestive of drug induced liver disease. Her symptoms and jaundice cleared over the next month, and on follow up 3 months later, all liver tests were normal.
Key Points
| Medication: | Piroxicam (20 mg daily) |
|---|---|
| Pattern: | Mixed→cholestatic (R=3.5→0.8) |
| Severity: | 2+ (jaundice but never hospitalized) |
| Latency: | 30 days to onset of symptoms |
| Recovery: | Complete recovery between 1 and 3 months |
| Other medications: | Atenolol and hydrochlorothiazide, chronically |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 0 | Piroxicam started for arthritis | ||||
| 30 days | Piroxicam stopped | ||||
| 32 days | 0 | 694 | 575 | 6.5 | Itching, jaundice |
| 34 days | 6 days | 270 | 736 | 6.2 | |
| 40 days | 8 days | 358 | 1688 | 3.8 | Liver biopsy |
| 54 days | 23 days | 159 | 1240 | 1.5 | |
| 60 days | 29 days | 50 | 630 | 0.6 | |
| 2 months | 1 month | 61 | 291 | 0.6 | |
| 4 months | 3 months | 36 | 103 | 0.5 | |
| Normal Values | <40 | <280 | <1.2 | ||
Comment
This patient had the onset of itching after a 30 day course of piroxicam. While the “R” value at the onset of injury (3.5) indicated a “mixed” hepatocellular-cholestatic pattern, the course was clearly cholestatic, with prominence of pruritus, further elevations in alkaline phosphatase (and rapid decreases in ALT yielding R values of <2), and a liver biopsy showing intrahepatic cholestasis with only mild inflammation and hepatocellular necrosis. Immunoallergic features were minimal: there was no rash or fever, but "discrete eosinophilia" was said to be present. Other causes of acute liver injury were effectively ruled out, and she recovered steadily once therapy was stopped. Application of the RUCAM causality system to this case gives a score of 8, which suggests that the likelihood that piroxicam is the cause of the injury is highly probable. As in this case, NSAID related hepatotoxicity may be more common in the elderly and among women.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Piroxicam – Generic, Feldene®
DRUG CLASS
Nonsteroidal Antiinflammatory Drugs
Product labeling at DailyMed, National Library of Medicine, NIH
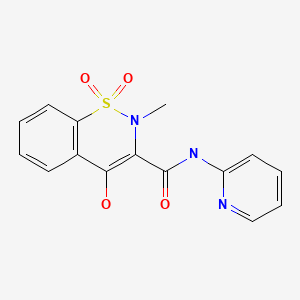
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Piroxicam | 36322-90-4 | C15-H13-N3-O4-S |
|
CITED REFERENCES
- 1.
- Caballeria E, Masso RM, Arago JV, Sanchis A. Piroxicam hepatotoxicity. Am J Gastroenterol. 1990;85:898–9. [PubMed: 2371992]
ANNOTATED BIBLIOGRAPHY
References updated: 20 March 2020
Abbreviations: NSAIDs, nonsteroidal antiinflammatory drugs.
- Zimmerman HJ. Drugs used to treat rheumatic and musculospastic disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 517-53.(Expert review of hepatotoxicity published in 1999; piroxicam has been implicated in several instances of liver injury including some with fatal outcomes or requiring liver transplantation).
- Lewis JH, Stine JG. Nonsteroidal anti-inflammatory drugs and leukotriene receptor antagonists: pathology and clinical presentation of hepatotoxicity. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013. pp. 370-402.(Expert review of liver injury caused by NSAIDs mentions that piroxicam has been reported to cause rare instances of severe hepatic necrosis and cholestatic hepatitis as have other oxicam NSAIDs, two [sudoxicam and isoxicam] having been withdrawn because of hepatotoxicity).
- Grossner T, Smyth EM, Fitzgerald GA. Pharmacotherapy of inflammation, fever, pain, and gout. In, Brunton LL, Hilal-Dandan R, Knollman BC. Goodman & Gilman’s The pharmacological basis of therapeutics, 13th ed. New York: McGraw-Hill, 2018. pp. 685-709.(Textbook of pharmacology and therapeutics).
- Brogden RN, Heel RC, Speight TM, Avery GS. Piroxicam: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1981;22:165–87. [PubMed: 7021122](Thorough review of pharmacology, structure, indications, efficacy and safety of piroxicam; most common side effects are gastrointestinal; no mention of hepatotoxicity or ALT elevations during treatment).
- Zarski JP, Aubert H, Dentant L, Rachail M. Gastroenterol Clin Biol. 1984;8:980–1. [Hepatitis due to non-steroidal anti-inflammatory agents: a new involved molecule, piroxicam (Feldène) ?] French. [PubMed: 6519409](73 year old man developed weakness 1 month and jaundice 2 months after starting piroxicam [bilirubin 2.8 mg/dL, ALT 2 times ULN, Alk P 3 times ULN], resolving within 2 months of stopping).
- Hartmann H, Fischer G, Janning G. Prolonged cholestatic jaundice and leukopenia associated with piroxicam. Z Gastroenterol. 1984;22:343–5. [PubMed: 6485437](60 year old woman developed jaundice 2 months after starting piroxicam [bilirubin 8.2 mg/dL, ALT 24 U/L, Alk P 340 U/L with leucopenia, but no eosinophilia], resolving slowly over next 10 weeks).
- Coscojuela C, Charro L, Guallar A, Ferrer-Dufoll MA, Carapeto FJ. Med Cutan Ibero Lat Am. 1985;13:291–300. [Toxic epidermal necrolysis (Lyell's syndrome) caused by piroxicam, with fatal outcome from disseminated aspergillosis] Spanish. [PubMed: 3912630](71 year old man developed toxic epidermal necrolysis within days of starting piroxicam [AST 220 U/L, GGT 270 U/L without jaundice], resolving within 2 weeks of stopping; nevertheless, the patient died during convalescence of disseminated aspergillosis).
- Lee SM, O'Brien CJ, Williams R, Whitaker S, Gould SR. Subacute hepatic necrosis induced by piroxicam. Br Med J (Clin Res Ed). 1986;293:540–1. [PMC free article: PMC1341314] [PubMed: 3092908](66 year old woman developed jaundice 3 days after starting piroxicam [bilirubin rising to 19.0 mg/dL, AST 430 U/L, Alk P 190 U/L, protime 16 seconds], with progression to subacute hepatic failure and death; ANA positive 1:160 and hyperglobulinemia).
- Haye OL. Piroxicam and pancreatitis. Ann Intern Med. 1986;104:895. [PubMed: 3706949](42 year old woman developed pancreatitis after taking piroxicam for undefined period [amylase 1048 U/L, liver tests normal], and had rapid recurrence of abdominal pain upon reexposure).
- Bismuth H, Samuel D, Gugenheim J, Castaing D, Bernuau J, Rueff B, et al. Emergency liver transplantation for fulminant hepatitis. Ann Intern Med. 1987;107:337–41. [PubMed: 3304049](Description of 17 cases of liver transplantation for fulminant hepatitis including 5 due to drugs – amiodarone, quinidine, pirprofen, clometacine and piroxicam; piroxicam case had bilirubin 26.3 mg/dL, ALT 20 times ULN, and survived with graft).
- Hannequin JR, Doffoel M, Schmutz G. Rev Rhum Mal Osteoartic. 1988;55:983–8. [Hepatitis secondary to current non-steroidal anti-inflammatory agents] French. [PubMed: 3070713](Report of 83 cases of acute hepatitis due to NSAIDs published in the literature: six cases attributed to piroxicam arising after 1-7 weeks, some with nephritis as well, suspected to be immunoallergic, two cases were fatal).
- Smolinske SC, Hall AH, Vandenberg SA, Spoerke DG, McBride PV. Toxic effects of nonsteroidal anti-inflammatory drugs in overdose. An overview of recent evidence on clinical effects and dose-response relationships. Drug Saf. 1990;5:252–74. [PubMed: 2198051](Review of literature on overdose of NSAIDS: piroxicam overdose typically causes drowsiness, stupor and even coma with mild gastrointestinal upset, but ALT levels may be elevated without jaundice and with rapid return to normal).
- Caballeria E, Masso RM, Arago JV, Sanchis A. Piroxicam hepatotoxicity. Am J Gastroenterol. 1990;85:898–9. [PubMed: 2371992](74 year old woman developed jaundice 30 days after starting piroxicam [bilirubin 6.5 mg/dL, ALT 694 U/L, Alk P 575 U/L, eosinophilia but no rash], resolving within 3 months of stopping: Case 1).
- Planas R, De Leon R, Quer JC, Barranco C, Bruguera M, Gassull MA. Fatal submassive necrosis of the liver associated with piroxicam. Am J Gastroenterol. 1990;85:468–70. [PubMed: 2327391](64 year old woman developed rash 5 days and jaundice 20 days after starting piroxicam [bilirubin 10.9 mg/dL, AST 774 U/L, Alk P 467 U/L], which progressed to liver failure and death 53 days after onset).
- Hepps KS, Maliha GM, Estrada R, Goodgame RW. Severe cholestatic jaundice associated with piroxicam. Gastroenterology. 1991;101:1737–40. [PubMed: 1955140](61 year old man developed jaundice 6 weeks after starting piroxicam which was continued until 12 weeks [bilirubin 15.2 rising to 22 mg/dL, ALT 74 U/L, Alk P 230 U/L], resolving 4 months after stopping).
- Sherman KE, Jones C. Hepatotoxicity associated with piroxicam use. Gastroenterology. 1992;103:354–5. [PubMed: 1612350](61 year old HBsAg-positive woman developed jaundice 12 days after starting piroxicam [bilirubin rising from 1.0 to 20 mg/dL, ALT 1800 U/L, IgM anti-HBc negative, Alk P and HBV DNA not reported], resolving within 3 months of stopping).
- Paterson D, Kerlin P, Walker N, Lynch S, Strong R. Piroxicam induced submassive necrosis of the liver. Gut. 1992;33:1436–8. [PMC free article: PMC1379622] [PubMed: 1446877](Two case reports; 68 year old woman developed symptomatic hepatitis after 15 months of piroxicam therapy [bilirubin 2.1 mg/dL, ALT 1610 U/L, Alk P 235 U/L, 10% eosinophils], progressing to subacute hepatic failure and death within 3 months of onset; 48 year old man developed jaundice 6 weeks after starting piroxicam [bilirubin 14.2 mg/dL, AST 1860 U/L, Alk P 87, INR 2.4], progressing rapidly to acute liver failure with successful liver transplantation 2 weeks after presentation).
- Jick H, Derby LE, Garcia Rodriguez LA, Jick SS, Dean AD. Liver disease associated with diclofenac, naproxen, and piroxicam. Pharmacotherapy. 1992;12:207–12. [PubMed: 1608854](Analysis of cases of liver injury arising after ~153,000 prescriptions of diclofenac, ~65,000 of piroxicam, ~138,000 of naproxen: 6 cases attributed to naproxen [1 in 20,000 persons], 3 to diclofenac [1 in 50,000], none to piroxicam [less than 1 in 50,000]).
- Morillas Ariño J, Sánchez de la Fuente MF, García-Cano Lizcano J, Jiménez Sánchez F, Rosa Herranz C, Razquin Murillo J, et al. Rev Esp Enferm Dig. 1993;83:197–201. [Hepatotoxicity induced by Droxicam: presentation of 4 cases] Spanish. [PubMed: 8489815](4 cases of liver injury arising on droxicam, a prodrug of piroxicam not available in the US, with onset after 10-32 days of therapy [bilirubin 2.0, 23.2, 2.1, and 14.3 mg/dL; ALT 406-708 U/L; Alk P 991-2082 U/L], resolving within 2-10 months of stopping, 2 had mild eosinophilia; one had long term Alk P elevations).
- Jobard JM, Ory JP, Cleau D, Debieuvre D. Gastroenterol Clin Biol. 1993;17:614–5. [Symptomatic hepatic disorders after ingestion of tenoxicam] French. [PubMed: 8253331](32 year old man developed severe fatigue 7 days after starting tenoxicam [no jaundice, ALT 592 U/L, Alk P 643 U/L], resolving rapidly with stopping, all tests being normal 3 months later).
- García Rodríguez LA, Williams R, Derby LE, Dean AD, Jick H. Acute liver injury associated with nonsteroidal anti-inflammatory drugs and the role of risk factors. Arch Intern Med. 1994;154:311–6. [PubMed: 8297198](Retrospective cohort study of cases of acute liver injury in England after exposure to NSAIDs; 23 cases identified , none fatal, including 5 attributed to ibuprofen, 4 diclofenac, 4 naproxen, 2 mefenamic acid, 3 ketoprofen, 2 piroxicam, 2 fenbuten and 3 sulindac).
- Sungur C, Ates B, Akbas O. Acute hepatitis caused by tenoxicam. Ann Pharmacother. 1994;28:1309. [PubMed: 7849359](77 year old woman developed abdominal pain and jaundice 3 days after starting tenoxicam [an oxicam NSAID not available in the US] for back pain [bilirubin 3.0 mg/dL, ALT 406 U/L, Alk P 292 U/L], resolving within 2 weeks of stopping).
- Pascual AF, Jimenez Martos F, Rodriguez Moreno C. Aten Primaria. 1995;15:329. [Cholestatic hepatitis associated with tenoxicam] Spanish. [PubMed: 7734692](84 year old woman developed abdominal pain and itching 6 months after starting tenoxicam [ALT 141 U/L, Alk P 310 U/L, bilirubin not mentioned], resolving within 30 days of stopping).
- Walker AM. Quantitative studies of the risk of serious hepatic injury in persons using nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1997;40:201–8. [PubMed: 9041931](Review of population based studies of NSAID use and hepatic injury; estimated frequency of piroxicam hepatotoxicity varied from 9 to 14 per 100,000 person-years of use).
- Katsinelos P, Katsos I, Patsiaoura K, Xiarchos P, Goulis I, Eugenidis N. Tenoxicam-associated hepatic injury: a case report and review. Eur J Gastroenterol Hepatol. 1997;9:403–6. [PubMed: 9160206](51 year old woman developed jaundice within 4 days of starting tenoxicam [bilirubin 3.0 mg/dL, ALT 157 U/L, Alk P 365 U/L], resolving within 7 weeks of stopping).
- Poniachik J, Guerrero J, Calderón P, Smok G, Morales A, Muñoz G, et al. Rev Med Chil. 1998;126:548–52. [Cholestatic hepatitis associated with piroxicam use. Case report] Spanish. [PubMed: 9731437](22 year old woman developed jaundice several months after starting piroxicam [bilirubin 7.8 mg/dL, ALT 387 U/L, Alk P 784 U/L], resolving within 8 weeks of stopping).
- Gutiérrez A, Enriquez R, Amorós F, Sillero C, Reyes A. Rev Esp Enferm Dig. 2002;94:169–70. [Acute hepatic and renal failure due to piroxicam use] Spanish. [PubMed: 12185885](33 year old man developed fever and jaundice 12 months after starting intermittent piroxicam [bilirubin 3.2 mg/dL, ALT 4828 U/L, Alk P normal], also developing renal insufficiency [creatinine 8.2 mg/dL]; recovery within 2 weeks).
- Trak-Smayra V, Cazals-Hatem D, Asselah T, Duchatelle V, Degott C. Prolonged cholestasis and ductopenia associated with tenoxicam. J Hepatol. 2003;39:125–8. [PubMed: 12821054](36 year old man developed fever and jaundice 7 days after starting tenoxicam [an oxicam NSAID available in Europe], with bilirubin 30.9 mg/dL, ALT 1159 U/L, Alk P 2710 U/L and prolonged jaundice and biopsy showing cholangitis and intrahepatic cholestasis, very slow recovery marked by persistence of Alk P >1000 U/L, and repeat liver biopsies over 3 years showing progressive ductopenia).
- Lacroix I, Lapeyre-Mestre M, Bagheri H, Pathak A, Montastruc JL. Club de Reflexion des cabinets de Groupe de Gastro-Enterologie(CREGG); General Practitioner Networks. Nonsteroidal anti-inflammatory drug-induced liver injury: a case-control study in primary care. Fundam Clin Pharmacol. 2004;18:201–6. [PubMed: 15066135](Case controlled study of patients presenting with suspected drug induced liver injury in a general practice context in Southern France found 88 cases which were matched with 178 controls; 22 cases vs 16 controls were exposed to NSAIDs; 5 diclofenac, 4 ibuprofen, 4 ketoprofen, 2 niflumic acid, 1 flurbiprofen and 1 meloxicam, the rest to salicylates which was just as commonly used in controls; piroxicam not mentioned).
- de Abajo FJ, Montero D, Madurga M, García Rodríguez LA. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol. 2004;58:71–80. [PMC free article: PMC1884531] [PubMed: 15206996](Population based study of 1.6 million persons in UK found 128 suspected cases of drug induced liver disease, calculated odds ratios for liver injury to be 4.1 for diclofenac, but 0.4 for all other NSAIDs [2 cases only, both due to naproxen]).
- Andrade RJ, Lucena MI, Fernández MC, et al. Spanish Group for the Study of Drug-Induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21. [PubMed: 16083708](Reports to a Spanish network found 570 cases of drug induced liver disease with ibuprofen listed as 4th major cause; 18 cases in all: half mixed, half hepatocellular; eosinophilia in 2; acute liver failure in 2, one death and one liver transplant; piroxicam not mentioned).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](Summary of 25 years of adverse drug reaction reporting in Sweden identified 103 cases of drug induced acute liver failure; diclofenac and naproxen mentioned in top 20 causes; ibuprofen listed as associated with one case, but piroxicam not mentioned).
- Lapeyre-Mestre M, de Castro AM, Bareille MP, Del Pozo JG, Requejo AA, Arias LM, et al. Non-steroidal anti-inflammatory drug-related hepatic damage in France and Spain: analysis from national spontaneous reporting systems. Fundam Clin Pharmacol. 2006;20:391–5. [PubMed: 16867024](Analysis of reports of liver injury from NSAIDs from France and Spain from 1982-2001; relative risk definitely raised for droxicam, sulindac, nimesulide, and clometacin; minimally raised for naproxen, diclofenac, piroxicam and tenoxicam; 33 hepatic adverse events due to piroxicam reported from Spain and 378 from France).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, NSAIDs were implicated as a sole agent in 8 cases [4 diclofenac, 2 celecoxib, 1 meloxicam, 1 oxaprozin] and as one of several agents in 3 cases [1 diclofenac, 1 celecoxib, 1 ibuprofen], but none attributed to piroxicam).
- Bessone F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J Gastroenterol. 2010;16:5651–61. [PMC free article: PMC2997980] [PubMed: 21128314](Review of estimated frequency of drug induced liver injury due to NSAIDs from large published epidemiological studies; mentions personal experience with two cases of piroxicam induced liver injury, one a 42 year old woman who developed acute liver failure 28 days after starting drug who eventually recovered, and a 42 year old man with severe and prolonged cholestasis which arose after 58 days of piroxicam use).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which 7 were due to NSAIDs, including 4 attributed to bromfenac, 2 to diclofenac and 1 to etodolac, but none to piroxicam).
- Lapeyre-Mestre M, Grolleau S, Montastruc JL., Adsociation Française des Centres Régionaux de Pharmacovigilance (CRPV). Adverse drug reactions associated with the use of NSAIDs: a case/noncase analysis of spontaneous reports from the French pharmacovigilance database 2002-2006. Fundam Clin Pharmacol. 2013;27:223–30. [PubMed: 21929527](Analysis of spontaneous reporting of 42,389 adverse events to the French Pharmacovigilance database for 8 NSAIDs between 2002 and 2006; hepatic events per million daily doses was highest for nimesulide [0.15], compared to diclofenac [0.09], ketoprofen [0.09], naproxen [0.04], meloxicam [0.03], tenoxicam [0.03] and piroxicam [0.06]).
- Suzuki A, Andrade RJ, Björnsson E, Lucena MI, Lee WM, Yuen NA, Hunt CM, et al. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in VigiBase: unified list based on international collaborative work. Drug Saf. 2010;33:503–22. [PubMed: 20486732](The combination of several large data sources identified 385 different drugs to be linked to liver injury and 107 to acute liver failure, the most commonly implicated NSAIDs being diclofenac, ibuprofen, naproxen, nimesulide, piroxicam, and sulindac).
- Zhou Y, Yang L, Liao Z, He X, Zhou Y, Guo H. Epidemiology of drug-induced liver injury in China: a systematic analysis of the Chinese literature including 21 789 patients. Eur J Gastroenterol Hepatol. 2013;25:825–9. [PubMed: 23510965](Search of 3 electronic databases of the Chinese medical literature from 1994-2011 identified 279 reports on a total of 24,111 patients with drug induced liver injury, the most commonly implicated being antituberculosis agents [31%], HDS products [19%], antibiotics [10%] and NSAIDs [7.6%], and the most frequent individual NSAIDs being acetaminophen, ibuprofen, indomethacin, aspirin and phenylbutazone).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](Prospective analysis of all cases of drug induced liver injury in Iceland between 2010-11 identified 97 cases [19 per 100,000 inhabitants], 6 of which were attributed to diclofenac; no other NSAID mentioned).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, the most common class of implicated agents being NSAIDs [n=62, 32%], and specific agents were nimesulide [n=53], piroxicam [5], diclofenac [2], gold salts [1], and naproxen [1]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 28 were attributed to NSAIDs [Schmeltzer 2016]).
- Schmeltzer PA, Kosinski AS, Kleiner DE, Hoofnagle JH, Stolz A, Fontana RJ, Russo MW., Drug-Induced Liver Injury Network (DILIN). Liver injury from nonsteroidal anti-inflammatory drugs in the United States. Liver Int. 2016;36:603–9. [PMC free article: PMC5035108] [PubMed: 26601797](Among 1221 cases of drug induced liver injury enrolled in a prospective, US database between 2004 and 2014, 30 cases [2.5%] were attributed to NSAIDs, most commonly diclofenac [n=16], but also celecoxib [3], meloxicam [3], etodolac [2], ibuprofen [2], oxaprozin [2], valdecoxib [1] and sulindac [1]).
- Donati M, Conforti A, Lenti MC, Capuano A, Bortolami O, Motola D, Moretti U, et al. DILI-IT Study Group. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case-control study in Italy. Br J Clin Pharmacol. 2016;82:238–48. [PMC free article: PMC4917796] [PubMed: 26991794](Among 179 cases of acute liver injury and 1770 controls admitted to 9 Italian hospitals between 2010 and 2014, NSAIDs used more frequently in cases compared to controls included nimesulide [17% vs 10%: odds ratio 1.88] and ibuprofen [14% vs 10%: odds ratio 1.59] and risk was higher in those taking higher doses).
- Zoubek ME, González-Jimenez A, Medina-Cáliz I, Robles-Díaz M, Hernandez N, Romero-Gómez M, Bessone F, et al. High Prevalence of ibuprofen drug-induced Liver injury in Spanish and Latin-American registries. Clin Gastroenterol Hepatol. 2018;16:292–4. [PubMed: 28782674](Analysis of a Spanish and Latin-American registries identified 73 cases of NSAID induced liver injury, the most common agents being nimesulide [38%], diclofenac [34%] and ibuprofen [17%]; other agents not mentioned).
- Tujios SR, Lee WM. Acute liver failure induced by idiosyncratic reaction to drugs: challenges in diagnosis and therapy. Liver Int. 2018;38:6–14. [PMC free article: PMC5741491] [PubMed: 28771932](Review of acute liver failure and the contribution of drug induced liver injury, of which 5% were due to NSAIDs, most commonly diclofenac and etodolac).
- Meunier L, Larrey D. Recent advances in hepatotoxicity of non-steroidal anti-inflammatory drugs. Ann Hepatol. 2018;17:187–91. [PubMed: 29469052](Review of the hepatotoxicity of NSAIDS mentions the most commonly implicated are diclofenac, nimesulide, sulindac, ibuprofen, piroxicam, naproxen and aspirin).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Tolmetin.[LiverTox: Clinical and Researc...]Review Tolmetin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Diclofenac.[LiverTox: Clinical and Researc...]Review Diclofenac.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Flurbiprofen.[LiverTox: Clinical and Researc...]Review Flurbiprofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Ketoprofen.[LiverTox: Clinical and Researc...]Review Ketoprofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Fenoprofen.[LiverTox: Clinical and Researc...]Review Fenoprofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Piroxicam - LiverToxPiroxicam - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...