NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Perampanel is glutamate receptor antagonist that is used as an anticonvulsant in the therapy of partial onset seizures. Perampanel has not been associated with serum aminotransferase elevations during therapy, and clinically apparent liver injury from perampanel has yet to be reported and must be rare, if it occurs at all.
Background
Perampanel (per am' pan el) is a glutamate receptor antagonist that is used to treat severe or refractory partial onset seizures. Perampanel binds to the alpha-amino-3-hydroxyl-5-methyl-4-isooxazole-propionate receptor (AMPAR) for glutamate, a major central nervous system excitatory neurotransmitter. The inhibition of the AMPAR leads to reduction in seizure activity in animal models as well as in patients with difficult to control seizures. In several large, prelicensure randomized controlled trials, addition of perampanel to conventional anticonvulsant medications led to improved control of epilepsy attributed to partial onset seizures. Perampanel was approved for use in the United States in 2012 as an anticonvulsant to be used in combination with other agents as adjunctive therapy of partial onset seizures. Perampanel is available in tablets of 4, 6, 8, 10 and 12 mg under the brand name Fycompa. The recommended initial dose is 2 mg once daily, which can be increased subsequently based upon tolerance and efficacy to a dose of 4 to 12 mg once daily. The dose should be increased and tapered gradually. The most common side effects include dizziness, somnolence, impaired concentration, vertigo, ataxia, nervousness, irritability, anger, aggression, blurred vision, headache, weight gain, fatigue, nausea and weakness. Rare, but potentially severe side effects include serious psychiatric and behavioral reactions including homicidal ideation and threats. Perampanel can cause euphoria for which reason it is classified as a scheduled III controlled substance, meaning that it has accepted medical uses, but also has a moderate or low potential for physical or psychological dependence.
Hepatotoxicity
Limited data are available on the hepatotoxicity of perampanel. In clinical trials, therapy with perampanel was not associated with an increased frequency of serum aminotransferase elevations as compared to placebo treatment, and there were no reported instances of clinically apparent liver injury. No individual case reports of perampanel hepatotoxicity have been published since its general clinical availability. Perampanel has been implicated in rare instances of severe cutaneous hypersensitivity reactions including DRESS syndrome, which can be associated with variable degrees of liver injury. Thus, clinically apparent liver injury due to perampanel may occur, but must be very rare.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which perampanel might cause liver injury is unknown. It is partially metabolized by the liver, largely by CYP 3A4 and is susceptible to drug interactions with agents that induce or inhibit this microsomal enzyme.
Outcome and Management
Liver injury from perampanel is rare, if it occurs at all. There is no reason to suspect cross sensitivity to hepatotoxicity between perampanel and other anticonvulsants such as phenytoin or carbamazepine with which it shares no structural similarity.
Drug Class: Anticonvulsants
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Perampanel – Fycompa®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Perampanel | 380917-97-5 | C23-H15-N3-O |
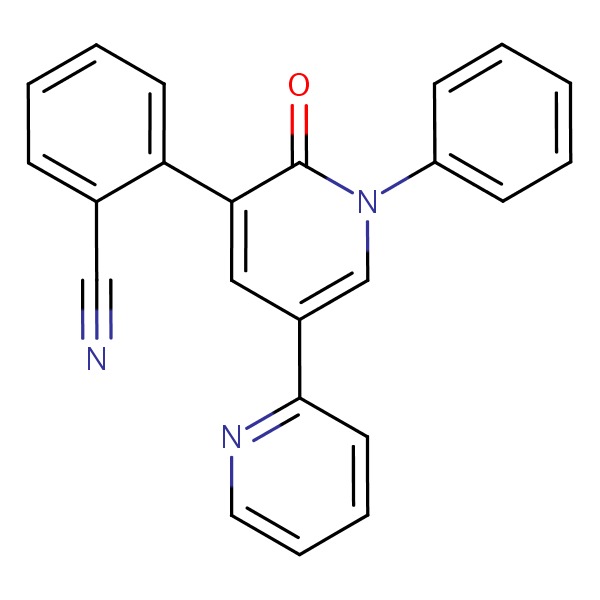 |
ANNOTATED BIBLIOGRAPHY
References updated: 04 May 2016
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999 before the availability of perampanel).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-42.(Review of anticonvulsant induced liver injury; perampanel is not discussed).
- McNamara JO. Pharmacology of the epilepsies. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 583-607.(Textbook of pharmacology and therapeutics).
- Krauss GL, Bar M, Biton V, Klapper JA, Rektor I, Vaiciene-Magistris N, Squillacote D, et al. Tolerability and safety of perampanel: two randomized dose-escalation studies. Acta Neurol Scand 2012; 125: 8-15. [PubMed: 21883097](Among 201 adults with refractory partial onset seizures enrolled in two studies of different doses of perampanel vs placebo for 6 to 7 weeks, there were no serious liver related adverse events and “no clinically significant differences” in laboratory values between placebo and perampanel treated subjects).
- Rektor I, Krauss GL, Bar M, Biton V, Klapper JA, Vaiciene-Magistris N, Kuba R, et al. Perampanel Study 207: long-term open-label evaluation in patients with epilepsy. Acta Neurol Scand 2012; 126: 263-9. [PubMed: 22913800](Among 130 patients with refractory partial onset seizures who continued on perampanel after participation in controlled trials, 57 had at least 3 years and 18 had 4 years exposure; no new adverse events were uncovered, although one patient developed an ALT elevation above 5 times ULN at 2 years, but no details given).
- French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, Kumar D, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology 2012; 79: 589-96. [PMC free article: PMC3413761] [PubMed: 22843280](Among 388 patients with refractory partial onset seizures treated with adjunctive perampanel [8 or 12 mg daily] or placebo for 19 weeks, serious adverse events occurred in 6.0 and 6.7% of perampanel vs 5.0% of placebo recipients, and there were no “clinically important mean changes in laboratory values”).
- Hsu WW, Sing CW, He Y, Worsley AJ, Wong IC, Chan EW. Systematic review and meta-analysis of the efficacy and safety of perampanel in the treatment of partial-onset epilepsy. CNS Drugs 2013; 27: 817-27. [PMC free article: PMC3784051] [PubMed: 23918722](Systematic review of efficacy and safety of perampanel concludes that it resulted in a statistically significant decrease in seizure frequency and common side effects are dizziness and somnolence; no mention of ALT elevations or hepatotoxicity).
- Steinhoff BJ, Ben-Menachem E, Ryvlin P, Shorvon S, Kramer L, Satlin A, Squillacote D, et al. Efficacy and safety of adjunctive perampanel for the treatment of refractory partial seizures: a pooled analysis of three phase III studies. Epilepsia 2013; 54: 1481-9. [PubMed: 23663001](Pooled analysis of results of three controlled trials of perampanel in patients with refractory partial onset seizures, mentions that there were no deaths and that serious adverse events occurred in 5.0% of placebo and 5.5% of perampanel recipients, none of which were liver related and that there were “no clinically important mean changes in laboratory values”).
- French JA, Krauss GL, Steinhoff BJ, Squillacote D, Yang H, Kumar D, Laurenza A. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia 2013; 54: 117-25. [PubMed: 22905857](Among 386 patients with refractory partial onset seizures treated with perampanel [8 or 12 mg] or placebo for 19 weeks, the most common side effects were dizziness, somnolence, fatigue and headache, and there were no liver related serious adverse events and “no clinically important changes from baseline in mean laboratory values”).
- Drugs for epilepsy. Treat Guidel Med Lett 2013; 11: 9-18. [PubMed: 23348233](Concise review of indications and side effects of anticonvulsants; perampanel is approved only as adjunctive treatment for partial onset seizures in patients 12 years old or older; no mention of adverse effects on the liver).
- Gaitatzis A, Sander JW. The long-term safety of antiepileptic drugs. CNS Drugs 2013; 27 (6): 435-55. [PubMed: 23673774](Review of the adverse effects with long term anticonvulsant therapy including liver failure, nonalcoholic fatty liver, osteoporosis, skin and hair changes, reproductive health, cancer, heart, renal, lung, pancreas, gastrointestinal and psychiatric conditions; perampanel is mentioned, but not discussed).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none of the 96 were attributed to perampanel).
- Faulkner MA. Perampanel: a new agent for adjunctive treatment of partial seizures. Am J Health Syst Pharm 2014; 71: 191-8. [PubMed: 24429011](Review of the mechanism of action, pharmacology, clinical efficacy and safety of perampanel does not mention any liver related serious adverse events and states that ALT and AST elevations above 3 times ULN occurred in fewer than 1% of recipients).
- Krauss GL, Perucca E, Ben-Menachem E, Kwan P, Shih JJ, Clément JF, Wang X, et al. Long-term safety of perampanel and seizure outcomes in refractory partial-onset seizures and secondarily generalized seizures: results from phase III extension study 307. Epilepsia 2014; 55: 1058-68. [PMC free article: PMC4283992] [PubMed: 24867391](Summary of continued follow up on 1216 patients with refractory seizures who were enrolled in open label long term extension studies of perampanel including more than 300 followed for at least 2 years, ALT elevations above 3 times ULN occurred in less than 1% of patients; no specific mention of liver related serious adverse events).
- Rugg-Gunn F. Adverse effects and safety profile of perampanel: a review of pooled data. Epilepsia. 2014 Jan; 55 Suppl 1: 13-5. [PubMed: 24400692](Review of adverse events reported in three controlled trials of perampanel mentions that there were “no clinically important changes” in biochemical parameters).
- Perampanel (Fycompa) for epilepsy. Med Lett Drugs Ther 2014; 56 (1435): 9-10. [PubMed: 24662975](Concise review of the mechanism of action, clinical efficacy, adverse events, drug interactions, dosage and cost of perampanel shortly after its approval for use in epilepsy in the US does not mention hepatotoxicity or ALT elevations).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were attributed to anticonvulsants, but none to perampanel).
- Laurenza A, Yang H, Williams B, Zhou S, Ferry J. Absence of liver toxicity in perampanel-treated subjects: pooled results from partial seizure phase III perampanel clinical studies. Epilepsy Res 2015; 113: 76-85. [PubMed: 25986193](Among 1038 patients with refractory partial onset seizures treated with perampanel in 3 controlled trials, de novo ALT elevations occurred in 2.6% of placebo and 1.7% of perampanel treated subjects, and hepatobiliary adverse events occurred in only 4 perampanel and no placebo treated subjects which were cholelithiasis in 3 and transiently elevated liver tests without jaundice in 1 [<0.2%]).
- Rosenfeld W, Conry J, Lagae L, Rozentals G, Yang H, Fain R, Williams B, et al. Efficacy and safety of perampanel in adolescent patients with drug-resistant partial seizures in three double-blind, placebo-controlled, phase III randomized clinical studies and a combined extension study. Eur J Paediatr Neurol 2015; 19: 435-45. [PubMed: 25823975](Summary of 3 controlled trials and an extension study of perampanel in 143 adolescents [ages 12-17] with refractory partial onset seizures, serious adverse events were rare, and none were liver related; there were “no clinically significant changes from baseline in clinical laboratory values”).
- French JA, Krauss GL, Wechsler RT, Wang XF, DiVentura B, Brandt C, Trinka E, et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy: A randomized trial. Neurology 2015; 85: 950-7. [PMC free article: PMC4567458] [PubMed: 26296511](Among 164 patients with refractory tonic-clonic seizures treated with adjunctive perampanel or placebo for 17 weeks, there were no liver related serious adverse events and “no significant laboratory abnormalities”).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Perampanel: What is its Place in the Management of Partial Onset Epilepsy?[Neurol Ther. 2013]Review Perampanel: What is its Place in the Management of Partial Onset Epilepsy?Ledingham DR, Patsalos PN. Neurol Ther. 2013 Dec; 2(1-2):13-24. Epub 2013 Aug 30.
- Efficacy and safety of adjunctive perampanel in adolescent patients with epilepsy: Post hoc analysis of six randomized studies.[Epilepsy Behav. 2020]Efficacy and safety of adjunctive perampanel in adolescent patients with epilepsy: Post hoc analysis of six randomized studies.Piña-Garza JE, Rosenfeld W, Saeki K, Villanueva V, Yoshinaga H, Patten A, Williams B, Malhotra M. Epilepsy Behav. 2020 Mar; 104(Pt A):106876. Epub 2020 Jan 16.
- Review Perampanel: as adjunctive therapy in patients with partial-onset seizures.[CNS Drugs. 2012]Review Perampanel: as adjunctive therapy in patients with partial-onset seizures.Plosker GL. CNS Drugs. 2012 Dec; 26(12):1085-96.
- Adjunctive perampanel in adolescents with inadequately controlled partial-onset seizures: A randomized study evaluating behavior, efficacy, and safety.[Epilepsia. 2016]Adjunctive perampanel in adolescents with inadequately controlled partial-onset seizures: A randomized study evaluating behavior, efficacy, and safety.Lagae L, Villanueva V, Meador KJ, Bagul M, Laurenza A, Kumar D, Yang H. Epilepsia. 2016 Jul; 57(7):1120-9. Epub 2016 May 25.
- Pharmacokinetic/pharmacodynamic analysis of adjunctive perampanel in subjects with partial-onset seizures.[Acta Neurol Scand. 2018]Pharmacokinetic/pharmacodynamic analysis of adjunctive perampanel in subjects with partial-onset seizures.Takenaka O, Ferry J, Saeki K, Laurenza A. Acta Neurol Scand. 2018 Apr; 137(4):400-408. Epub 2017 Nov 23.
- Perampanel - LiverToxPerampanel - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...