NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Penbutolol is a nonselective beta-adrenergic receptor blocker (beta-blocker) used for the therapy of hypertension. Penbutolol has yet to be convincingly associated with clinically apparent liver injury.
Background
Penbutolol (pen bue' toe lol) is a nonselective beta-blocker, acting on both beta-1 and beta-2 adrenergic receptors. Beta-1 adrenergic blockade reduces the heart rate and myocardial contractility by slowing the AV conduction and suppressing automaticity. Beta-2 blockade affects peripheral vascular resistance and can cause bronchospasm and hypoglycemia. Penbutolol also has mild sympatheticomimetic activity acting as a partial beta-adrenergic receptor agonist. Penbutolol is indicated for the management of hypertension and was approved for use in the United States in 1987. Penbutolol is available in tablets of 20 mg under the trade name Levatol. The typical initial oral dose of penbutolol in adults is 20 mg once daily, with subsequent dose modification based upon clinical response and tolerance, the average total daily maintenance dose being 20 to 60 mg. Common side effects of penbutolol include bradycardia, hypotension, fatigue, dizziness, depression, memory loss, impotence, cold limbs and, less commonly, severe hypotension, heart failure and bronchospasm. Sudden withdrawal can trigger rebound hypertension. Beta-blockers are contraindicated in patients with asthma, bradycardia and heart failure and should be used cautiously in the elderly and in patients with diabetes.
Hepatotoxicity
Mild-to-moderate elevations in serum aminotransferase levels occur in less than 2% of patients on penbutolol and are usually transient and asymptomatic, resolving even with continuation of therapy. Despite its wide spread use, penbutolol has not been convincingly linked to instances of clinically apparent liver injury. Other beta-blockers have been linked to rare instances of acute liver injury with a latency to onset of 4 to 24 weeks, a hepatocellular pattern of serum enzyme elevations and a rather mild, self-limiting course without evidence of hypersensitivity or autoimmune reactions.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Penbutolol undergoes extensive metabolism by the liver and is excreted in the urine as inactive metabolites. The reason why penbutolol rarely causes liver injury is unknown; other beta-blockers with similar chemical structures have been linked to cases of clinically apparent, idiosyncratic liver injury.
References to the safety and potential hepatotoxicity of penbutolol are provided in the overview on Beta-Adrenergic Receptor Antagonists, last updated in June 2019.
Drug Class: Beta-Adrenergic Receptor Antagonists
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Penbutolol – Levatol®
DRUG CLASS
Beta-Adrenergic Receptor Antagonists
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
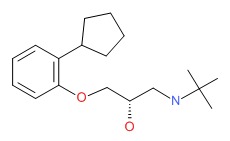
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Penbutolol | 38363-40-5 | C18-H29-N-O2 |
 |
- PubChem SubstanceRelated PubChem Substances
- Review Penbutolol: a new beta-adrenergic blocking agent.[DICP. 1990]Review Penbutolol: a new beta-adrenergic blocking agent.Schlanz KD, Thomas RL. DICP. 1990 Apr; 24(4):403-8.
- [On the pharmacology of the beta-receptor blocker penbutolol (author's transl)].[Arzneimittelforschung. 1980][On the pharmacology of the beta-receptor blocker penbutolol (author's transl)].Kaiser J, Härtfelder G, Lindner E, Schölkens B. Arzneimittelforschung. 1980; 30(3):420-7.
- Review Penbutolol and carteolol: two new beta-adrenergic blockers with partial agonism.[J Clin Pharmacol. 1990]Review Penbutolol and carteolol: two new beta-adrenergic blockers with partial agonism.Frishman WH, Covey S. J Clin Pharmacol. 1990 May; 30(5):412-21.
- DNA strand breaks and mutations caused by penbutolol, a beta blocker.[Mutat Res. 1988]DNA strand breaks and mutations caused by penbutolol, a beta blocker.Hussain SS, Al-Dakan AA, Aboul-Enein HY, Hannan MA. Mutat Res. 1988 Apr; 204(4):675-82.
- Effect of oral penbutolol on renal haemodynamics of hypertensive patients with renal insufficiency.[N Z Med J. 1985]Effect of oral penbutolol on renal haemodynamics of hypertensive patients with renal insufficiency.Bailey RR, Carlson RV, Walker RJ, Swainson CP. N Z Med J. 1985 Aug 28; 98(785):683-5.
- Penbutolol - LiverToxPenbutolol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...