NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Oseltamivir is an inhibitor of the influenza neuraminidase enzyme and is used as therapy and prophylaxis against influenza A and B. Oseltamivir has not been associated with clinically apparent liver injury.
Background
Oseltamivir (oh" sel tam' i vir) phosphate is an ester prodrug of an antiviral enzyme inhibitor which, after absorption, is converted in the liver to oseltamivir carboxylase, the active intermediate. Oseltamivir carboxylase is a potent inhibitor of the enzyme neuraminidase of the influenza virus particle. Inhibition of this enzyme causes a decrease in viral replication, probably as a result of interference with particle formation and release. Oseltamivir is active against both influenza A and B virus, but has no activity against other common upper respiratory tract viruses. In addition, resistance to oseltamivir can develop rapidly. Oseltamivir is indicated for therapy or post-exposure prevention of influenza A and B in both children and adults. Oseltamivir was approved for in the United States in 1999 and is commonly used during influenza outbreaks. Oseltamivir is available as capsules of 30, 45 and 75 mg and as an oral suspension (6 mg/mL) generically and under the brand name of Tamiflu. The recommended oral dose for therapy in adults is 75 mg twice daily for 5 days; the usual prophylactic dose is 75 mg once daily for 10 days, starting within 2 days of close contact with an infected person. Side effects are uncommon and include mild nausea, gastrointestinal upset, dizziness and headache. Serious adverse events are rare but can include hypersensitivity reactions, Stevens Johnson syndrome and neuropsychiatric syndromes such as disorientation, hallucinations and mania.
Hepatotoxicity
In clinical trials of oseltamivir, serum aminotransferase elevations occurred in 2% of treated subjects, but were asymptomatic and transient in all and there were no reports of clinically apparent liver injury with jaundice. The rates of ALT elevations with oseltamivir were generally similar to those treated with placebo or with a comparative agents. Since its approval in 1999, oseltamivir has been widely used during influenza seasonal outbreaks. There have been a few, isolated reports of mild liver injury in patients receiving oseltamivir, but the relationship of the injury with oseltamivir has not always been very convincingly shown. There have been no reports of acute liver failure or chronic liver disease attributed to oseltamivir use. Furthermore, a proportion of patients with influenza have serum enzyme elevations and even mild jaundice during the acute illness, independent of any therapy.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
Oseltamivir is metabolized by the liver to the active intermediate oseltamivir carboxylate, but has little further hepatic metabolism and is excreted largely in the urine. The rapid onset of liver injury attributed to oseltamivir in case reports and the occurrence of hypersensitivity reactions such as Stevens Johnson syndrome suggest that immunoallergic factors are responsible. The typical course of oseltamivir is for 5 to 10 days only,
and the brief exposure and minimal hepatic metabolism may account for why hepatotoxicity is rare.
Management and Outcome
The case reports of liver injury associated with oseltamivir therapy have been mild and self-limited without specific therapy. Oseltamivir should be discontinued promptly if liver injury with symptoms or jaundice arise and reexposure should be avoided. There is no information on cross sensitivity to liver injury or hypersensitivity reactions among the various neuraminidase inhibitors that are used to treat influenza.
Drug Class: Antiviral Agents
Other Drugs in the Class for Influenza: Amantadine, Baloxavir, Peramivir, Rimantadine, Zanamivir
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Oseltamivir – Generic, Tamiflu®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
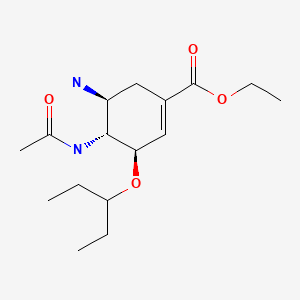
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Oseltamivir | 196618-13-0 | C16-H28-N2-O4 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 22 June 2020
- Zimmerman HJ. Antiviral agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 621-3.(Expert review of antiviral agents and liver injury published in 1999; amantadine and rimantadine have not caused "overt hepatic injury"; oseltamivir is not mentioned).
- Núñez M. Influenza virus treatments. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 513.(Review of hepatotoxicity of antiviral agents; oseltamivir is not discussed).
- Acosta EP. Antiviral agents (nonretroviral). In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1105-18.(Textbook of pharmacology and therapeutics).
- Nicholson KG, Aoki FY, Osterhaus AD, Trottier S, Carewicz O, Mercier CH, Rode A, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355:1845–50. [PubMed: 10866439](Controlled trial in 726 adults given oseltamivir in two doses or placebo for 5 days; laboratory results "did not differ significantly from baseline" for any group; no serious adverse events, and no hepatitis or jaundice mentioned).
- Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, Singh S, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–24. [PubMed: 10697061](Controlled trial in 629 adults given oseltamivir in two doses or placebo for 5 days; no drug related serious adverse events, and standard laboratory tests did not change significantly in any group).
- McNicholl IR, McNicholl JJ. Neuraminidase inhibitors: zanamivir and oseltamivir. Ann Pharmacother. 2001;35:57–70. [PubMed: 11197587](Review of efficacy and safety of both zanamivir and oseltamivir; no mention of hepatotoxicity or ALT elevations).
- Doucette KE, Aoki FY. Oseltamivir: a clinical and pharmacological perspective. Expert Opin Pharmacother. 2001;2:1671–83. [PubMed: 11825310](Review of structure, pharmacology, antiviral activity, efficacy and safety of oseltamivir; no mention of ALT elevations or hepatotoxicity).
- Dutkowski R, Thakrar B, Froehlich E, Suter P, Oo C, Ward P. Safety and pharmacology of oseltamivir in clinical use. Drug Saf. 2003;26:787–801. [PubMed: 12908848](More than 11,000 subjects evaluated in premarketing studies and more than 4 million prescriptions worldwide, 2300 spontaneous reports of adverse events received, largely for gastrointestinal upset and rash; no hepatic adverse events reported).
- Jones M, Del Mar C. Safety of neuraminidase inhibitors for influenza. Expert Opin Drug Saf. 2006;5:603–8. [PubMed: 16907649](Neuraminidase inhibitors include oseltamivir and zanamivir [given by inhalation]; nausea is the only side effect found to be more common with receipt of oseltamivir vs placebo).
- Jefferson T, Demicheli V, Rivetti D, Jones M, Di Pietrantonj C, Rivetti A. Antivirals for influenza in healthy adults: systematic review. Lancet. 2006;367:303–13. [PubMed: 16443037](Analysis of 51 reports of 52 controlled trials of antivirals for influenza; "Neuraminidase inhibitors are not associated with any adverse events when used as treatment as opposed to prophylaxis").
- Antiviral drugs for influenza. Med Lett Drugs Ther. 2009;51:89–92. [PubMed: 20220738](Review of status of antiviral agents for prevention and treatment of influenza A and B).
- Dutkowski R, Smith JR, Davies BE. Safety and pharmacokinetics of oseltamivir at standard and high dosages. Int J Antimicrob Agents. 2010;35:461–7. [PubMed: 20189775](Assessment of safety of higher doses [250-900 mg daily] of oseltamivir in 391 volunteers; headache, nausea and dizziness were common; laboratory data showed no significant changes from baseline or differences from placebo).
- Smith EV, Pynn MC, Blackford S, Leopold DJ. Stevens-Johnson syndrome secondary to oseltamivir (Tamiflu). Br J Gen Pract. 2010;60:133–4. [PMC free article: PMC2814276] [PubMed: 20132714](17 year old male developed rash and oral ulceration diagnosed as Stevens Johnson Syndrome 2 weeks after an influenza-like illness and treatment with oseltamivir and acetaminophen; no mention of liver test results).
- de Miguel-Bouzas JC, Castro-Tubío E, Freire-Vázquez MC. Hepatitis aguda en paciente a tratamiento con oseltamivir. Farm Hosp. 2010;34:261–2. [Acute hepatitis in a patient treated with oseltamivir] Spanish. [PubMed: 20171914](32 year old woman with suspected [but unconfirmed] influenza was found to have abnormal liver tests the day after starting oseltamivir and acetaminophen [bilirubin not given, ALT 139 rising to 834 U/L, Alk P 90 rising to 285 U/L], with peak of values at day 4 and rapid improvement once oseltamivir was stopped and near normal values by day 14).
- Yingying C. Abnormal liver chemistry in patients with influenza A H1N1. Liver Int. 2011;31:902. [PubMed: 21645222](Among 131 patients admitted to a hospital for influenza A H1N1, 13% had abnormal ALT and 5% abnormal Alk P elevations, but the range of values was not provided).
- Antiviral drugs for influenza 2013-2014. Med Lett Drugs Ther. 2014;56(1434):6–8. [PubMed: 24457560](Concise summary of safety and efficacy of medications for influenza appropriate for the 2013-14 season mentions that adverse effects of oseltamivir include nausea, vomiting and headache; no mention of liver injury).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to a drug used to treat influenza).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 cases [1.3%] were attributed to antiviral agents, but none to antivirals used to treat influenza).
- Marty FM, Vidal-Puigserver J, Clark C, Gupta SK, Merino E, Garot D, Chapman MJ, et al. Intravenous zanamivir or oral oseltamivir for hospitalized patients with influenza: an international, randomised, double-blind, double-dummy, phase 3 trial. Lancet Respir Med. 2017;5:135–46. [PubMed: 28094141](Among 626 patients with symptoms of influenza [488 confirmed] treated with zanamivir [300 or 600 mg] or oseltamivir twice daily for 5-10 days, time to clinical improvement was similar in all three groups as were adverse events including respiratory failure and death; ALT elevations arose in 1.2% of zanamivir vs 2.0% of oseltamivir treated subjects).
- Fang S, Qi L, Zhou N, Li C. Case report on alimentary tract hemorrhage and liver injury after therapy with oseltamivir: A case report. Medicine (Baltimore). 2018;97:e12497. [PMC free article: PMC6160054] [PubMed: 30235756](6 year old boy was treated for influenza like symptoms with ibuprofen [100 mg three times daily] and oseltamivir [60 mg twice daily] after the second dose of which he developed hematemesis and was found to have liver test abnormalities [bilirubin 0.4 rising to 4.9 mg/dL, ALT 80 rising to 193 U/L], resolving rapidly upon stopping both drugs).
- Antiviral drugs for treatment and prophylaxis of seasonal influenza. Med Lett Drugs Ther. 2019;61(1563):1–4. [PubMed: 30681660](Concise review of the drug therapy of influenza mentions that there are no data suggesting the superiority of one drug over another, they are all approved for treatment of uncomplicated influenza, should be started as soon as possible, and have been shown to shorten the duration of symptoms by one day in adults; no mention of ALT elevations or hepatotoxicity of any of the neuraminidase inhibitors).
- Abraham GM, Morton JB, Saravolatz LD. Baloxavir: a novel antiviral agent in the treatment of influenza. Clin Infect Dis. 2020 Feb 5; Epub ahead of print. [PubMed: 32020174](Review of the mechanism of action, microbiology, indications, clinical efficacy and toxicity of baloxavir mentions that adverse event rates are similar to those of oseltamivir and not much greater than placebo; no discussion of ALT elevations or hepatotoxicity).
- Yu Y, Nie X, Song Z, Xie Y, Zhang X, Du Z, Wei R, et al. Signal detection of potentially drug-induced liver injury in children Using electronic health records. Front Pediatr. 2020;8:171. [PMC free article: PMC7177017] [PubMed: 32373564](Using electronic medical records from over 200,000 Chinese children hospitalized between 2010 and 2018 with more than 49 million blood test results, the authors identified 12 drugs that were associated with ALT elevations in excess of expected, one of which was oseltamivir; no specific details given).
- Baker J, Block SL, Matharu B, Burleigh Macutkiewicz L, Wildum S, Dimonaco S, et al. Baloxavir marboxil single-dose treatment in influenza-infected children: a randomized, double-blind, active controlled phase 3 safety and efficacy trial (miniSTONE-2). Pediatr Infect Dis J. 2020 Jun 5; [PMC free article: PMC7360097] [PubMed: 32516282](Among 173 children less than 12 years old with influenza who were treated with a single dose of intravenous baloxavir or a 5 day course of oral oseltamivir, rates of clinical improvement were similar with the two regimens as were rates of adverse events [57% vs 46%] and there were no serious adverse events or deaths).
- Antiviral drugs for influenza. Med Lett Drugs Ther. 2020;62(1589):1–4. [PubMed: 31999661](Concise review of the drug therapy of influenza mentions that there is no evidence that one drug is more effective than any other in treating uncomplicated influenza infections but that oseltamivir is preferred for treatment of pregnant women, hospitalized patients and those with severe, complicated or progressive illness; common side effects of oseltamivir include nausea, vomiting and headache; no mention of ALT elevations or hepatic adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomised controlled trials.[BMJ. 2009]Review Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomised controlled trials.Shun-Shin M, Thompson M, Heneghan C, Perera R, Harnden A, Mant D. BMJ. 2009 Aug 10; 339:b3172. Epub 2009 Aug 10.
- Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment.[JAMA. 1999]Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment.Hayden FG, Treanor JJ, Fritz RS, Lobo M, Betts RF, Miller M, Kinnersley N, Mills RG, Ward P, Straus SE. JAMA. 1999 Oct 6; 282(13):1240-6.
- Review Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis.[BMJ. 2009]Review Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis.Jefferson T, Jones M, Doshi P, Del Mar C. BMJ. 2009 Dec 8; 339:b5106. Epub 2009 Dec 8.
- Review Early therapy with the neuraminidase inhibitor oseltamivir maximizes its efficacy in influenza treatment.[Med Microbiol Immunol. 2002]Review Early therapy with the neuraminidase inhibitor oseltamivir maximizes its efficacy in influenza treatment.Gillissen A, Höffken G. Med Microbiol Immunol. 2002 Dec; 191(3-4):165-8. Epub 2002 Sep 12.
- Review Oseltamivir.[J Postgrad Med. 2009]Review Oseltamivir.Tullu MS. J Postgrad Med. 2009 Jul-Sep; 55(3):225-30.
- Oseltamivir - LiverToxOseltamivir - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...