NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The organic nitrates, including nitroglycerin, isosorbide dinitrate and isosorbide mononitrate, act as prodrugs for nitric oxide and are used to treat or prevent acute attacks of angina pectoris. These agents have been in wide scale use for many decades and have not been implicated in causing serum enzyme elevations or clinically apparent liver injury.
Background
The organic nitrates, including nitroglycerin, isosorbide dinitrate and isosorbide mononitrate, act as prodrugs for nitric oxide (NO) which is a potent vasodilator gas. Nitric oxide activates guanylyl-cyclase in vascular endothelial cells, which increases intracellular cyclic GMP and thereby reduces intracellular calcium concentrates and dephosphorylates myosin light chains causing relaxation of smooth muscle cells. The effect of nitrates is largely on vascular endothelium, both arteries and veins, but predominantly veins, causing venous pooling and a decrease venous return to the heart, thus decreasing cardiac preload. Arteriolar relaxation reduces systemic vascular resistance and systolic blood pressure decreasing cardiac afterload. Nitrates also cause increase in coronary blood flow. These combined effects decrease myocardial oxygen demand and relieves ischemia. Nitrates are typically given sublingually and have a rapid onset of action and somewhat short duration of action. Long acting formulations and transdermal, oral, intravenous, aerosol spray and other formulations are available that can be used chronically and for prevention of angina attacks. Nitroglycerin is typically used to treat an acute attack of angina (or in anticipation of an acute attack as with exercise or stress). Isosorbide dinitrate and mononitrate are typically given chronically to prevent angina attacks. However, nitrate tolerance develops rapidly with persistent exposure and these agents are given in a way that allows for an 8 to 12 hour nitrate free period to restore efficacy. The three forms of organic nitrates are described separately in this document, but their mechanism of action is similar as are their clinical effects and adverse event profiles.
Nitroglycerin (nye" troe glis' er in) is a propanetriol trinitrate, a nitric oxide prodrug that has been in common use as a treatment for acute angina pectoris for more than 100 years. Current indications include treatment and prevention of angina attacks and management of acute coronary syndromes. Nitroglycerin is highly volatile and is formulated in a matrix that stabilizes the molecule. However, it is still susceptible to inactivation and is generally provided in a light-proof bottle with a short shelf life (6 months). Nitroglycerin is well absorbed orally but has a high first pass clearance by the liver, for which reason it is typically given sublingually or by transdermal routes. However, nitroglycerin is available generically in many forms, sublingual tablets, topical ointments, transdermal patches, transmucosal ointments, aerosolized sprays, extended release oral tablets and liquid solutions for intravenous use. The typical sublingual dose is 0.3 to 0.6 mg at the onset of angina pain or in anticipation of angina. The dose can be repeated every 5 minutes for 15 to 30 minutes for a maximum of 3 doses. The onset of action is within 2 to 5 minutes. The oral and transdermal forms are used largely for prevention of angina and long term therapy. Side effects of nitroglycerin include headache, dizziness, weakness, flushing, syncope, tachycardia, palpitations and postural hypotension. Dermal forms can cause local rash and irritation.
Isosorbide dinitrate (eye" soe sor' bide dye nye' trate) is a prodrug of nitric oxide used to treat as well as prevent attacks of angina pectoris. Isosorbide dinitrate is well absorbed orally, but undergoes extensive first pass metabolism by the liver for which reason it is typically given sublingually. Isosorbide dinitrate was approved for use in the United States in 1966 and is still in wide scale use. It is available in sublingual as well as oral extended release tablets and capsules generically and under several brand names such as Isordil, Dilatrate-SR and Sorbitrate. The sublingual forms for treatment of acute angina attacks are generally given in doses of 2.5 to 5 mg, repeated at 5 to 10 minute intervals for a maximum of 3 doses. The oral extended release forms are given in doses of 4 to 40 mg two to four times daily. A daily, 8 to 10 hour nitrate free period is recommended as diminished efficacy can occur due to nitrate tolerance with continuous use. Side effects of isosorbide dinitrate include headache, dizziness, weakness, flushing, syncope, tachycardia, palpitations and postural hypotension.
Isosorbide mononitrate (eye" soe sor' bide mon" oh nye' trate) is a prodrug of nitric oxide used to prevent (but not to treat) attacks of angina pectoris. Isosorbide mononitrate is well absorbed orally but, unlike nitroglycerin, does not undergo first pass metabolism by the liver and can be given in typical oral forms. Isosorbide mononitrate was approved for use in the United States in 1991 for prophylaxis of angina. It is available in generically and under the brand name ISMO and Monoket in tablets of 10 and 20 mg. The recommended dose regimen is 20 mg twice daily given 7 hours apart (thus with a 10 hour rest period to avoid nitrate tolerance). Isosorbide mononitrate is also available in extended release forms generically and under the brand name Imdur in 30, 60 and 120 mg tablets, the recommended initial dose being 30 to 60 mg once daily, gradually increasing to a maximum of 240 once daily. Side effects of isosorbide dinitrate include headache, dizziness, weakness, nausea and vomiting, peripheral edema, flushing, syncope, tachycardia, palpitations and postural hypotension.
Hepatotoxicity
Despite many decades of wide scale use, nitroglycerin, isosorbide dinitrate and isosorbide mononitrate have not been implicated in causing serum enzyme elevations or clinically apparent liver injury.
Likelihood score: E (unlikely causes of clinically apparent acute liver injury).
Mechanism of Injury
The organic nitrates are rapidly metabolized to release nitric oxide in many tissues and are unlikely to have a direct toxic effect on the liver and also unlikely to cause idiosyncratic metabolic or immunoallergic injury.
Outcome and Management
No convincing instances of clinically apparent or severe acute liver injury have been linked to organic nitrates in the published literature.
Drug Class: Antiangina Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nitroglycerin – Generic
Isosorbide Dinitrate – Generic, Isordil®
Isosorbide Mononitrate – Generic, Imdur®, ISMO®, Monoket®
DRUG CLASS
Antiangina Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Nitroglycerin | 55-63-0 | C3-H5-N3-O9 |
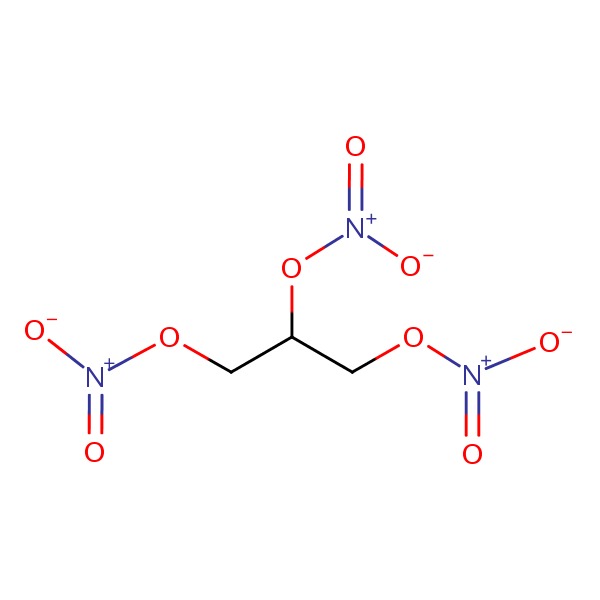 |
| Isosorbide Dinitrate | 87-33-2 | C6-H8-N2-O8 |
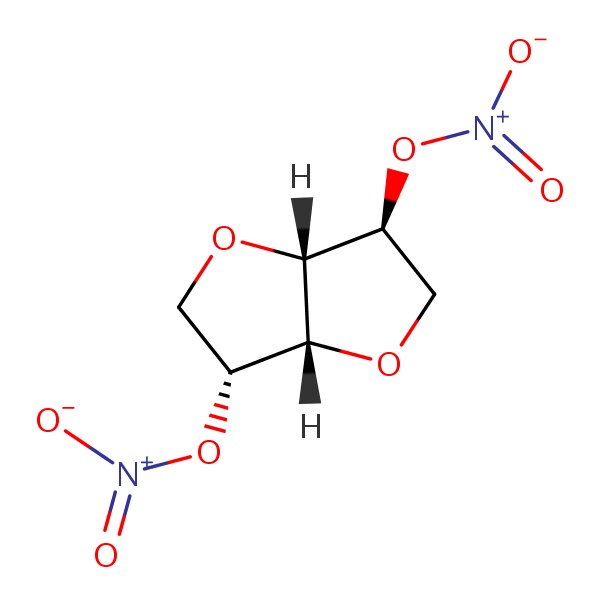 |
| Isosorbide Mononitrate | 16051-77-7 | C6-H9-N-O6 |
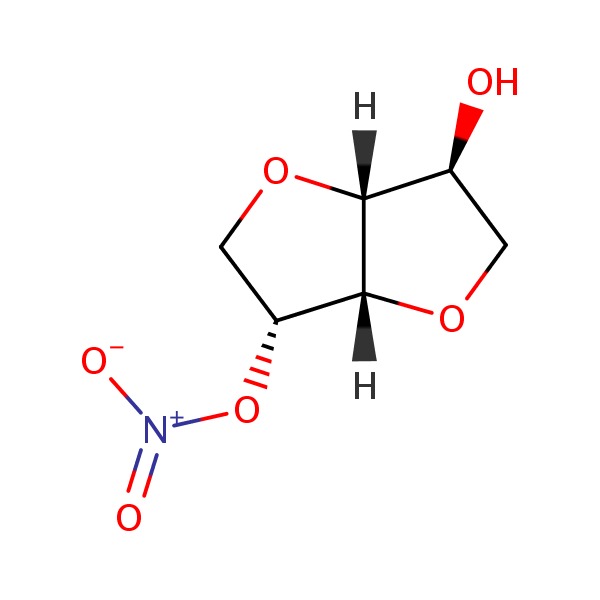 |
ANNOTATED BIBLIOGRAPHY
References updated: 27 April 2018
- Zimmerman HJ. Organic Nitrates. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 653-4.(Textbook of hepatotoxicity published in 1999; mentions that "there has been no hint, during all the years of use of nitrate for angina, of resultant hepatic injury").
- Michel T, Hoffman BB. Organic nitrates. Treatment of myocardial ischemia and hypertension. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 747-55.(Textbook of pharmacology and therapeutics).
- Raubach KH, Vlahov V, Wolter K, Bussmann WD. Double-blind randomized multicenter study on the efficacy of trapidil versus isosorbide dinitrate in stable angina pectoris. Clin Cardiol 1997; 20: 483-8. [PMC free article: PMC6655814] [PubMed: 9134282](Among 95 patients with angina pectoris treated with isosorbide dinitrate or trapidil [a phosphodiesterase inhibitor], "tolerability was good" and there were no "clinically relevant alterations" in standard laboratory tests in either group).
- Villanueva C, Balanzó J, Novella MT, Soriano G, Sáinz S, Torras X, Cussó X, et al. Nadolol plus isosorbide mononitrate compared with sclerotherapy for the prevention of variceal rebleeding. N Engl J Med 1996 Jun 20; 334 (25): 1624-9. [PubMed: 8628357](Among 86 patients with cirrhosis and variceal hemorrhage treated with sclerotherapy or nadolol and isosorbide dinitrate, recurrent bleeding was lower in the medically treated subjects and adverse events were less; no mention of worsening of liver tests or acute liver injury).
- Chrysant SG, Glasser SP, Bittar N, Shahidi FE, Danisa K, Ibrahim R, Watts LE, et al. Efficacy and safety of extended-release isosorbide mononitrate for stable effort angina pectoris. Am J Cardiol 1993; 72: 1249-56. [PubMed: 8256699](Among 313 patients with angina pectoris treated with isosorbide mononitrate [30, 60, 120 or 240 mg daily] or placebo for 42 days, exercise tolerance was improved 4 and 12 hours after dosing, but not at 24 hours; common side effects were headache [38-75% vs 17%], dizziness, fatigue, and nausea: "Analyses of laboratory data did not reveal any finding consistent with drug-related toxicity").
- Hall R, Chong C. A double-blind, parallel-group study of amlodipine versus long-acting nitrate in the management of elderly patients with stable angina. Cardiology 2001; 96 (2): 72-7. [PubMed: 11740135](Among 97 elderly patients with angina treated with amlodipine or isosorbide mononitrate [25-50 mg once daily] for 28 weeks, exercise times improved more with amlodipine than nitrates, but adverse event rates were similar and there "were no clinically relevant changes in... clinical chemistry parameters").
- Wakai A, McCabe A, Kidney R, Brooks SC, Seupaul RA, Diercks DB, Salter N, et al. Nitrates for acute heart failure syndromes. Cochrane Database Syst Rev 2013; (8): CD005151. [PMC free article: PMC8101690] [PubMed: 23922186](Systematic review of controlled trials of nitrates for acute heart failure syndromes concluded that "nitrates appeared to be well tolerated", headaches being the most common side effect; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. PubMed Citation. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to organic nitrates or agents for therapy of angina pectoris).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Nitrates: why and how should they be used today? Current status of the clinical usefulness of nitroglycerin, isosorbide dinitrate and isosorbide-5-mononitrate.[Eur J Clin Pharmacol. 1990]Review Nitrates: why and how should they be used today? Current status of the clinical usefulness of nitroglycerin, isosorbide dinitrate and isosorbide-5-mononitrate.Silber S. Eur J Clin Pharmacol. 1990; 38 Suppl 1:S35-51.
- Review Nitrate tolerance, rebound, and their clinical relevance in stable angina pectoris, unstable angina, and heart failure.[Cardiovasc Drugs Ther. 1997]Review Nitrate tolerance, rebound, and their clinical relevance in stable angina pectoris, unstable angina, and heart failure.Thadani U. Cardiovasc Drugs Ther. 1997 Jan; 10(6):735-42.
- [Selection of therapy with nitrates in patients with stable effort angina: results of comparative study of common isosorbide dinitrate and long acting preparation of isosorbide-5-mononitrate].[Kardiologiia. 2005][Selection of therapy with nitrates in patients with stable effort angina: results of comparative study of common isosorbide dinitrate and long acting preparation of isosorbide-5-mononitrate].Martsevich SIu, Semenova IuE, Alimova EV, Dmitrieva nA, Kozyreva MP, Koniakhina IP, Lukina IuV, Egorov VA. Kardiologiia. 2005; 45(11):42-5.
- Review Higher than recommend dosage of sublingual isosorbide dinitrate for treating angina pectoris: a case report and review of the literature.[Pan Afr Med J. 2021]Review Higher than recommend dosage of sublingual isosorbide dinitrate for treating angina pectoris: a case report and review of the literature.Cao HY, Wu HD, Song ZK, Tang ML, Yang S, Liu Y, Qin L. Pan Afr Med J. 2021; 39:28. Epub 2021 May 11.
- Treatment of coronary heart disease with isosorbide mononitrate ('Elantan' 20): a multi-centre study in hospital and general practice.[Curr Med Res Opin. 1984]Treatment of coronary heart disease with isosorbide mononitrate ('Elantan' 20): a multi-centre study in hospital and general practice.Marten W, Weiss M, Haase W. Curr Med Res Opin. 1984; 9(2):96-106.
- Organic Nitrates - LiverToxOrganic Nitrates - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...