NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Nitazoxanide is an antimicrobial with activity against several parasitic worms and protozoa that is used predominantly in the United States in treatment of giardiasis and cryptosporidiosis. Nitazoxanide therapy has not been reported to cause serum aminotransferase elevations during therapy or clinically apparent liver injury.
Background
Nitazoxanide (nye" ta zox' a nide) is a thiazolide with a broad spectrum of activity against several nematodes (Ascaris, Trichuris, Ancylostoma), cestodes (Taenia) and trematodes (Fasciola, Schistosoma), as well as protozoan parasites such as Giardia, Cryptosporidium and Entamoeba. The thiazolides are believed to act by interference with pyruvate ferredoxin oxidoreductase, an enzyme important in anaerobic metabolism. Nitazoxanide may also have antiviral activity in vitro against hepatitis B and C and influenza viruses, the basis of which is not known. Nitazoxanide was approved for use in the United States in 2004 and current indications are for diarrhea due to infection with Giardia lamblia or Cryptosporidium parvum. Nitazoxanide is available in tablets 500 mg and as an oral suspension (100 mg/5 mL) under the brand name Alinia. The typical dose for treating giardiasis and cryptosporidiosis in adults is 500 mg orally every 12 hours for 3 days. Nitazoxanide is generally well tolerated; side effects are usually mild and can include diarrhea, gastrointestinal upset, headaches and hair loss.
Hepatotoxicity
Nitazoxanide therapy has not been associated with elevations in serum aminotransferase levels nor with clinically apparent acute liver injury. However, there have been few studies of long term therapy with nitazoxanide and most controlled trials of this agent used short term courses without serum aminotransferase monitoring. Nitazoxanide has been used as adjunctive therapy for chronic hepatitis C, usually in combination with peginterferon with or without ribavirin; in these studies, most patients had improvements in serum aminotransferase levels, and no instances of acute exacerbation of hepatitis or jaundice were reported.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Nitazoxanide acts by interference with enzymatic activity important in anaerobic metabolism in parasitic worms and protozoa, which is not present in mammals. Hypersensitivity reactions to nitazoxanide have been reported and mild hepatic injury could potentially arise as a part of these immunoallergic reactions.
Outcome and Management
Nitazoxanide is usually well tolerated and liver injury has not been reported with its use.
Drug Class: Anthelmintic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nitazoxanide – Alinia®
DRUG CLASS
Anthelmintic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
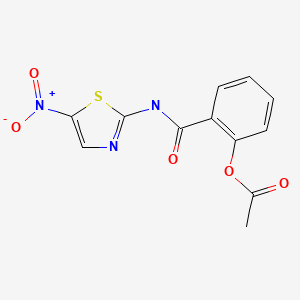
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Nitazoxanide | 55981-09-4 | C12-H9-N3-O5-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 30 April 2020
- Zimmerman HJ. Antihelminthics. Hepatic injury from antimicrobial agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 626-8.(Expert review of hepatotoxicity of anthelmintics written in 1999; nitazoxanide is not discussed).
- Wetzel DM, Phillips MA. Chemotherapy of protozoal infections: amebiasis, giardiasis, trichomoniasis, trypanosomiasis, leishmaniasis, and other protozoal infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 987-999-61.(Textbook of pharmacology and therapeutics).
- Rossignol JF, Hidalgo H, Feregrino M, Higuera F, Gomez WH, Romero JL, Padierna J, et al. A double-'blind' placebo-controlled study of nitazoxanide in the treatment of cryptosporidial diarrhoea in AIDS patients in Mexico. Trans R Soc Trop Med Hyg. 1998;92:663–6. [PubMed: 10326116](66 patients with AIDs and cryptosporidial diarrhea were enrolled in a controlled trial of nitazoxanide in two doses vs placebo for 14 days; no adverse event was attributed to therapy, but 1 patient developed jaundice that was “possibly related"; no further details given).
- Rossignol JF, Abaza H, Friedman H. Successful treatment of human fascioliasis with nitazoxanide. Trans R Soc Trop Med Hyg. 1998;92:103–4. [PubMed: 9692168](Case report of clinical response in a 14 year old with severe hepatic fascioliasis and description of outcome of treatment of 137 Egyptian patients with F. hepatica treated with 6 days of nitazoxanide, reporting that there were no significant changes in serum chemistry results in treated patients).
- Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Giardia intestinalis and Entamoeba histolytica or E. dispar: a randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis. 2001;184:381–4. [PubMed: 11443569](Among 89 Egyptian patients with diarrhea due to protozoa randomized to placebo vs nitazoxanide [1 g daily for 3 days], there were no clinically apparent hepatic side effects).
- Ortiz JJ, Ayoub A, Gargala G, Chegne NL, Favennec L. Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from Northern Peru. Aliment Pharmacol Ther. 2001;15:1409–15. [PubMed: 11552913](Among 110 children with giardiasis, response rates were similar with 3 days of nitazoxanide and 5 days of metronidazole, with only mild side effects to both and no jaundice or hepatitis reported).
- Parashar A, Arya R. Nitazoxanide. Indian Pediatr. 2005;42:1161–5. [PubMed: 16340060](Review of the pharmacology, clinical indications and adverse side effects of nitazoxanide).
- Fox LM, Saravoltaz LD. Nitazoxanide: a new thiazolide antiparasitic agent. Clin Infect Dis. 2005;40:1173–80. [PubMed: 15791519](Nitazoxanide is a benzamide with broad spectrum of activity against many protozoa and helminths, usually given orally twice daily for 3 days, it is generally well tolerated with mild and transient gastrointestinal complaints only; mentions that elevated ALT levels have been reported).
- Rossignol JF, Abu-Zekry M, Hussein A, Santoro MG. Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: randomised double-blind placebo-controlled trial. Lancet. 2006;368:124–9. [PubMed: 16829296](Among 38 Egyptian children with severe rotavirus diarrhea enrolled in a controlled trial, duration of diarrhea was shorter with nitazoxanide [median time 31 hours] than placebo [75 hours], and “there were no significant adverse events”).
- Rossignol JF, Kabil SM, el-Gohary Y, Younis AM. Effect of nitazoxanide in diarrhea and enteritis caused by Cryptosporidium species. Clin Gastroenterol Hepatol. 2006;4:320–4. [PubMed: 16527695](Among 90 patients with diarrhea due to cryptosporidiosis, response rates were greater with 3 days of nitazoxanide than placebo [96% vs 41%] and side effects were mild and transient, no jaundice or hepatitis reported).
- Hemphill A, Mueller J, Esposito M. Nitazoxanide, a broad-spectrum thiazolide anti-infective agent for the treatment of gastrointestinal infections. Expert Opin Pharmacother. 2006;7:953–64. [PubMed: 16634717](Review of the chemistry, mechanism of action, spectrum of activity and clinical efficacy of nitazoxanide, a medication used to treat nematodes, trematodes and protozoa and has recently been found to have antiviral activity against hepatitis B and C virus. Nitazoxanide is well tolerated and there are no reports of discontinuation due to adverse events).
- Rossignol JF, Elfert A, El-Gohary Y, Keeffe EB. Improved virologic response in chronic hepatitis C genotype 4 treated with nitazoxanide, peginterferon, and ribavirin. Gastroenterology. 2009;136:856–62. [PubMed: 19135998](In a controlled trial of various dose regimens of nitazoxanide combined with peginterferon with or without ribavirin for 48 weeks, adverse events where no greater with the addition of nitazoxanide and no jaundice or worsening of hepatitis was reported).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease collected in the US between 2003 and 2008, none were attributed to an anthelmintic agent).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; none were attributed to an anthelmintic agent).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children; no anthelmintic agent was among to 40 most common causes reported).
- Drugs for parasitic infections. Treat Guidel Med Lett. 2013;11 Suppl:e1–31.(Brief description of drugs for parasitic infections in adults and children as well as a table of their major side effects; nitazoxanide is the drug of choice for cryptosporidiosis and a possible secondary agent for giardiasis).
- Speich B, Ame SM, Ali SM, Alles R, Hattendorf J, Utzinger J, Albonico M, et al. Efficacy and safety of nitazoxanide, albendazole, and nitazoxanide-albendazole against Trichuris trichiura infection: a randomized controlled trial. PLoS Negl Trop Dis. 2012;6:e1685. [PMC free article: PMC3367984] [PubMed: 22679525](Controlled trial comparing 2 day courses of nitazoxanide vs albendazole vs the combination in 577 Tanzanian school children with Trichuris trichiura [whipworm] infection found cure rates of 6.6%, 14.5% and 16% and more side effects [mostly abdominal cramps and headaches; no mention of liver injury] with nitazoxanide, suggesting poor efficacy and need for better anthelmitics).
- Haffizulla J, Hartman A, Hoppers M, Resnick H, Samudrala S, Ginocchio C, Bardin M, et al. US Nitazoxanide Influenza Clinical Study Group. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2014;14:609–18. [PMC free article: PMC7164783] [PubMed: 24852376](Among 624 patients with suspected influenza treated with nitazoxanide [600 or 1200 mg daily] or placebo for 5 days, time to resolution of symptoms was shorter with the higher dose of nitazoxanide in those with confirmed influenza infection [n=257] and adverse events including changes in laboratory values were similar in the 3 groups).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, one was attributed to an anthelmintic [mebendazole] but none to nitazoxanide).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to nitazoxanide or other antiprotozoal agents).
- Macías J, López-Cortés LF, Téllez F, Recio E, Ojeda-Burgos G, Ríos MJ, Rivero-Juárez A, et al. Low efficacy of pegylated interferon plus ribavirin plus nitazoxanide for HCV genotype 4 and HIV coinfection. PLoS One. 2015;10:e0143492. [PMC free article: PMC4671604] [PubMed: 26640956](Among 21 Spanish patients with HIV and chronic hepatitis C, genotype 4, who were treated with peginterferon, ribavirin and nitazoxanide for 48 weeks, only 2 [10%] had a sustained virological response and adverse events were frequent leading to early discontinuation in 2 patients; no mention of ALT elevations or hepatotoxicity during therapy).
- Kohla MA, El-Said H, El-Fert A, Ehsan N, Ezzat S, Taha H. Impact of nitazoxanide on sustained virologic response in Egyptian patients with chronic hepatitis C genotype 4: a double-blind placebo-controlled trial. Eur J Gastroenterol Hepatol. 2016;28:42–7. [PubMed: 26473300](Among 195 patients with chronic hepatitis C, genotype 4 treated with 48 weeks of peginterferon and ribavirin with or without nitazoxanide, the sustained virologic response rate were similar in the two groups [58% vs 61%] as were adverse event rates, no patient requiring discontinuation).
- Abd-Elsalam S, El-Kalla F, Elwan N, Badawi R, Hawash N, Soliman S, Soliman S, et al. A randomized controlled trial comparing nitazoxanide plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. J Clin Gastroenterol. 2019;53:226–30. [PubMed: 29668561](Among 120 patients with cirrhosis and hepatic encephalopathy treated with lactulose and either nitazoxanide or placebo, encephalopathy scores improved in both groups but more with nitazoxanide; no mention of changes in ALT values with treatment or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Nitazoxanide treatment for giardiasis and cryptosporidiosis in children.[Ann Pharmacother. 2004]Review Nitazoxanide treatment for giardiasis and cryptosporidiosis in children.Bailey JM, Erramouspe J. Ann Pharmacother. 2004 Apr; 38(4):634-40. Epub 2004 Feb 27.
- Nitazoxanide in the treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa.[Am J Trop Med Hyg. 1997]Nitazoxanide in the treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa.Doumbo O, Rossignol JF, Pichard E, Traore HA, Dembele TM, Diakite M, Traore F, Diallo DA. Am J Trop Med Hyg. 1997 Jun; 56(6):637-9.
- Review Nitazoxanide: a new broad spectrum antiparasitic agent.[Expert Rev Anti Infect Ther. 2...]Review Nitazoxanide: a new broad spectrum antiparasitic agent.White CA Jr. Expert Rev Anti Infect Ther. 2004 Feb; 2(1):43-9.
- Cryptosporidium and Giardia: treatment options and prospects for new drugs.[Exp Parasitol. 2010]Cryptosporidium and Giardia: treatment options and prospects for new drugs.Rossignol JF. Exp Parasitol. 2010 Jan; 124(1):45-53. Epub 2009 Jul 24.
- In vitro effect of nitazoxanide against Entamoeba histolytica, Giardia intestinalis and Trichomonas vaginalis trophozoites.[J Eukaryot Microbiol. 2002]In vitro effect of nitazoxanide against Entamoeba histolytica, Giardia intestinalis and Trichomonas vaginalis trophozoites.Cedillo-Rivera R, Chávez B, González-Robles A, Tapia A, Yépez-Mulia L. J Eukaryot Microbiol. 2002 May-Jun; 49(3):201-8.
- Nitazoxanide - LiverToxNitazoxanide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...