NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Neratinib is an orally available tyrosine kinase receptor inhibitor that is used in the extended adjuvant therapy of early stage breast cancer. Neratinib is associated with a low rate of transient elevations in serum aminotransferase levels during therapy, but has not been convincingly linked to cases of clinically apparent liver injury with jaundice.
Background
Neratinib (ne ra' ti nib) is a small molecule tyrosine kinase receptor inhibitor with potent activity against human epidermal growth factor receptor 2 (HER2 or ErbB2). These tyrosine kinase receptors are often mutated and over expressed in tumor tissue and cause unregulated cell growth and proliferation. This mutation is present in 20% of breast cancers and is particularly overexpressed in early stage cancers. Inhibition of the unregulated receptor can lead to reversal of progression of the cancer, although clinical responses are sometimes limited by the development of tumor resistance caused by further mutations in the receptor gene. In several large controlled trials, adjuvant therapy with neratinib was shown to improve progression free survival in patients with early stage, HER2-positive breast cancer. Neratinib received approval for use in the United States in 2017. Current indications are as extended adjuvant therapy for early stage, HER2-positive breast cancer following trastuzumab treatment. Neratinib is available in tablets of 40 mg under the brand name Nerlynx. The recommended dose is 240 mg (6 tablets) once daily for one year or until intolerable toxicity occurs. Side effects are common and particularly diarrhea [95%], which can be severe and dose limiting and for which prophylaxis with antidiarrheal agents is recommended. Other frequent side effects include nausea, abdominal pain, anorexia, weight loss, abdominal distension, fatigue, rash, stomatitis, dry skin, paronychia, muscle spasms and urinary treat infections. Uncommon, but potentially severe side effects include severe diarrhea leading to dehydration and renal failure, and embryo-fetal toxicity.
Hepatotoxicity
Elevations in serum aminotransferase levels are not uncommon during neratinib therapy occurring in up to 10% of patients, but rising above 5 times the upper limit of the normal range in only 1% to 2%. In prelicensure studies, there were no instances of neratinib related clinically apparent liver injury and serum enzyme elevations were typically mild and self-limited and not associated with symptoms or jaundice. Hepatotoxicity may be a class effect among protein kinase inhibitors of HER2, although the frequency and severity vary among the different agents. Specific details of the liver injury associated with neratinib such as latency, serum enzyme pattern, clinical features and course, have not been published. Other tyrosine kinase receptor inhibitors typically cause liver injury arising within days or weeks of starting therapy and presenting abruptly with hepatocellular enzyme elevations and a moderate-to-severe course. Immunoallergic and autoimmune features are not common. The rate of clinically significant liver injury and hepatic failure is increased in patients with preexisting cirrhosis or hepatic impairment due to liver tumor burden. Nevertheless, neratinib has not been convincingly linked to instances of clinically apparent liver injury.
Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury).
Mechanism of Injury
The abrupt and severe nature of the clinically apparent liver injury attributed to EGF receptor inhibitors suggests that it is immunologically mediated. On the other hand, the transient serum enzyme elevations that are not uncommon during therapy suggest a direct, intrinsic hepatotoxicity, perhaps caused by inhibition of critical tyrosine kinase receptors in hepatocytes. Neratinib is metabolized in the liver largely by the cytochrome P450 system and is susceptible to drug-drug interactions with inhibitors or inducers of CYP 3A4.
Outcome and Management
Liver injury due to neratinib has largely been in the form of asymptomatic, transient serum enzyme elevations. Monitoring of liver tests during neratinib therapy is recommended before treatment, monthly for the first 3 months and every 3 months thereafter or as clinically indicated. Serum aminotransferase elevations above 5 times the upper limit of normal should lead to temporary discontinuation, which should be permanent if laboratory values worsen or do not resolve or improve significantly within a few weeks, or if symptoms or jaundice arises. Restarting therapy is usually, but not always followed by recurrence of the serum enzyme elevations. There does not appear to be cross reactivity with other tyrosine kinase receptor inhibitors and, in some situations, switching to another protein kinase inhibitor may be appropriate.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Neratinib – Nerlynx®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
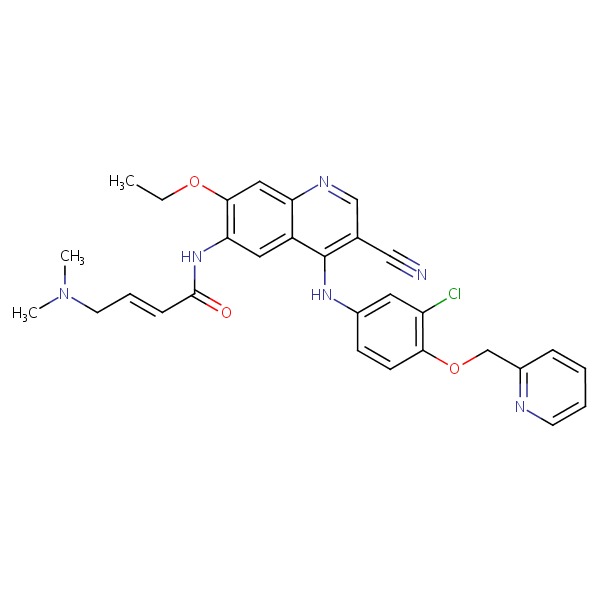
| Neratinib | 698387-09-6 | C26-H23-N7-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2018
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of tyrosine kinase receptor inhibitors).
- DeLeve LD. Erlotinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents; mentions that tyrosine kinase inhibitors have been linked to cases of clinically apparent liver injury, some of which were fatal).
- Chabner BA, Barnes J, Neal J, Olson E, Mujagic H, Sequist L, Wilson W, et al. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and cytokines. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1731-54.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy). - Martin M, Bonneterre J, Geyer CE Jr, Ito Y, Ro J, Lang I, Kim SB, et al. A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2+ advanced breast cancer. Eur J Cancer 2013; 49: 3763-72. [PubMed: 23953056](Among 233 women with HER2 positive breast cancer and previous trastuzumab treatment, median overall survival was 20 months in those receiving neratinib vs 24 months in those on lapatinib with capecitabine, while side effects, dose reductions and discontinuations were less with neratinib including ALT elevations [7% vs 13%]; there were no liver related serious adverse events or deaths in either group).
- Saura C, Garcia-Saenz JA, Xu B, Harb W, Moroose R, Pluard T, Cortés J, et al. Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2014; 32: 3626-33. [PubMed: 25287822](Among 104 women with HER2 positive breast cancer treated with varying doses of neratinib and capecitabine for up to 99 weeks, ALT elevations arose in 3 [3%], but there were no cases of ALT elevations with jaundice).
- Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2015; 88: 74-9. [PubMed: 25704957](In a pooled analysis of 21 trials of tyrosine kinase inhibitors of EGFR found higher reported rates of "grade 3" hepatotoxicity with gefitinib [18%] than with erlotinib [5.4%] and afatinib [1.7%], and hepatotoxicity was listed as the cause of drug discontinuation in 25% of cases).
- Awada A, Colomer R, Inoue K, Bondarenko I, Badwe RA, Demetriou G, Lee SC, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: The NEfERT-T Randomized Clinical Trial. JAMA Oncol 2016; 2: 1557-64. [PubMed: 27078022](Among 479 women with metastatic HER2 positive breast cancer treated with paclitaxel and either neratinib or trastuzumab, progression free survival was similar in the two groups while adverse events were more frequent with neratinib including diarrhea [93% vs 33%], nausea [44% vs 30%], weight loss [16% vs 5%] and ALT elevations [any elevation: 13% vs 11%; above 5 times ULN: 5% vs 2%]).
- Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, Robert NJ, et al.; ExteNET Study Group. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016; 17: 367-77. [PubMed: 26874901](Among 2840 women with HER2 positive breast cancer after trastuzumab adjuvant therapy, invasive disease free survival was greater with a one year course of neratinib than with placebo therapy [94% vs 92%], but was also associated with a higher rate of adverse events including diarrhea [40% vs 1.6%] and ALT elevations above 5 times ULN [1.1% vs 0.3%], but there were no cases of clinically apparent liver injury).
- Park JW, Liu MC, Yee D, Yau C, van 't Veer LJ, Symmans WF, Paoloni M, et al.; I-SPY 2 Investigators. Adaptive randomization of neratinib in early breast cancer. N Engl J Med 2016; 375: 11-22. [PMC free article: PMC5259558] [PubMed: 27406346](Among 193 women with early breast cancer treated with standard neoadjuvant chemotherapy with or without neratinib, adverse events were more frequent with neratinib including diarrhea [96% vs 50%] and ALT elevations [37% vs 12%], which were above 5 times ULN in 11% vs 1%, although there were no liver related severe adverse events in either group).
- Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, von Minckwitz G, et al.; ExteNET Study Group. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 1688-1700. [PubMed: 29146401](A 5 year follow up on 2840 women with HER2 positive breast cancer treated with a one year course of neratinib vs placebo [Chan 2016] found continued improved invasive disease free survival [90% vs 88%] and no new safety signals).
- Ding PN, Lord SJ, Gebski V, Links M, Bray V, Gralla RJ, Yang JC, et al. Risk of treatment-related toxicities from EGFR tyrosine kinase inhibitors: a meta-analysis of clinical trials of gefitinib, erlotinib, and afatinib in advanced EGFR-mutated non-small cell lung cancer. J Thorac Oncol 2017; 12: 633-43. [PubMed: 28007626](In a systematic review of 16 trials of EGFR tyrosine kinase inhibitors, liver enzyme elevations were highest for gefitinib [62%] vs afatinib [20%] and erlotinib [18%]; does not mention neratinib).
- Singh H, Walker AJ, Amiri-Kordestani L, Cheng J, Tang S, Balcazar P, Barnett-Ringgold K, et al. U.S. Food and Drug Administration approval: neratinib for the extended adjuvant treatment of early stage HER2-positive breast cancer. Clin Cancer Res 2018; 24: 3486-91. [PubMed: 29523624](Summary of evidence for the safety and efficacy of neratinib that led to its approval for the extended adjuvant treatment of women with early stage, HER2 positive breast cancer; mentions but does not discuss ALT and AST elevations during therapy).
- Neratinib (Nerlynx) for HER2-positive breast cancer. Med Lett Drugs Ther 2018; 60 (1539): 23. [PubMed: 29364199](Concise review of the mechanism of action, clinical efficacy, safety and costs of neratinib shortly after its approval in the US for therapy of breast cancer; mentions that diarrhea is the most common side effect and that ALT and AST elevations occurred in 5-9% of treated patients versus 3-4% of controls).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Position Paper on the Value of Extended Adjuvant Therapy with Neratinib for Early HER2+/HR+ Breast Cancer.[Breast Care (Basel). 2021]Review Position Paper on the Value of Extended Adjuvant Therapy with Neratinib for Early HER2+/HR+ Breast Cancer.Balic M, Rinnerthaler G, Bartsch R. Breast Care (Basel). 2021 Dec; 16(6):664-676. Epub 2021 Oct 29.
- Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial.[Lancet Oncol. 2017]Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial.Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, von Minckwitz G, Chia SKL, Mansi J, Barrios CH, et al. Lancet Oncol. 2017 Dec; 18(12):1688-1700. Epub 2017 Nov 13.
- Review Neratinib as adjuvant therapy in patients with HER2 positive breast cancer: expert opinion.[Future Oncol. 2023]Review Neratinib as adjuvant therapy in patients with HER2 positive breast cancer: expert opinion.Caputo R, Buono G, Di Lauro V, Cianniello D, Von Arx C, Pensabene M, Pagliuca M, Pacilio C, Di Rella F, Verrazzo A, et al. Future Oncol. 2023 Aug; 19(24):1695-1708. Epub 2023 Aug 22.
- Neratinib after trastuzumab-based adjuvant therapy in patients from Asia with early stage HER2-positive breast cancer.[Future Oncol. 2019]Neratinib after trastuzumab-based adjuvant therapy in patients from Asia with early stage HER2-positive breast cancer.Iwata H, Masuda N, Kim SB, Inoue K, Rai Y, Fujita T, Chiu J, Ohtani S, Takahashi M, Miyaki T, et al. Future Oncol. 2019 Jul; 15(21):2489-2501. Epub 2019 May 29.
- Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial.[Lancet Oncol. 2016]Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial.Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, Robert NJ, Silovski T, Gokmen E, von Minckwitz G, et al. Lancet Oncol. 2016 Mar; 17(3):367-377. Epub 2016 Feb 10.
- Neratinib - LiverToxNeratinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...