NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Methimazole is an antithyroid medication which is now considered the first line agent for medical therapy of hyperthyroidism and Graves disease. Methimazole has been linked to serum aminotransferase elevations during therapy as well as to a clinically apparent, idiosyncratic liver injury that is typically cholestatic and self-limited in course.
Background
Methimazole (meth im' a zole), which is also known as thiamazole, is a thioamide and a thyroid hormone antagonist which acts by inhibiting the incorporation of iodine into tyrosyl residues of thyroglobulin and, thus, lowering thyroid hormone levels. Methimazole resembles propylthiouracil both in chemical structure and activity. Methimazole was introduced into use in 1954 and is still widely used for the temporary amelioration of hyperthyroidism in Graves disease, particularly in patients with mild or self-limited hyperthyroidism or who wish to avoid thyroidectomy or radiation therapy. Because of the hepatotoxicity of propylthiouracil which can be fatal, methimazole is now considered the first line treatment for hyperthyroidism when there is a need to avoid surgery or radioiodine therapy. Methimazole is available in generic forms and under the brand name of Tapazole as tablets of 5 and 10 mg. The usual dose in adults is 15 to 60 mg daily in three divided doses until the patient is euthyroid, followed by a maintenance dose of 5 to 15 mg daily. Common side effects include gastrointestinal upset, headache, drowsiness, arthralgias, paresthesias, hair loss and rash. Rare complications of methimazole (<1%) include agranulocytosis, aplastic anemia, nephritis and hepatitis.
Hepatotoxicity
Methimazole has been associated with transient, asymptomatic elevations in serum aminotransferase levels, typically during the first 3 months after starting during high dose, induction therapy. These elevations are rarely clinically significant and usually resolve even with continuation of therapy. Methimazole is also capable of causing clinically apparent, idiosyncratic liver injury. The onset of hepatotoxicity is usually within 2 to 12 weeks of starting and the pattern of enzyme elevations is typically cholestatic or mixed, although hepatocellular patterns have also been described. The cholestatic hepatitis caused by methimazole can be prolonged, but fatalities are rare and symptoms and jaundice usually clear within 2 to 8 weeks of stopping therapy. Rare instances of prolonged cholestasis have been described, but no instance of vanishing bile duct syndrome.
Complicating the assessment of the role of methimazole or propylthiouracil in causing liver injury is the fact that hyperthyroidism by itself can cause liver test abnormalities and even jaundice. Indeed, more than half of patients with untreated hyperthyroidism have serum enzyme abnormalities (usually less than 5 times the upper limit of the normal range) and a small proportion are jaundiced and present with cholestatic hepatitis. The liver test elevations are most frequent in patients with high output heart failure. The abnormalities resolve rapidly with treatment of hyperthyroidism either with surgery, radioactive iodine or antithyroid medications.
Likelihood score: A (well known but rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which methimazole causes acute liver injury is unknown, but is likely due to an immunological reaction to a metabolic product of its metabolism.
Outcome and Management
The severity of methimazole induced liver injury varies from mild, transient serum aminotransferase elevations to moderately severe cholestatic hepatitis. Fatal cases are rare. Some cases have features of autoimmunity or immunoallergic hepatitis and have been treated with corticosteroids, but without proven evidence of benefit. Recovery is usually rapid once methimazole is stopped, and the first priority should be immediate discontinuation of antithyroid therapy at the first sign of clinically apparent liver disease. The presence of hyperthyroidism may play a role in worsening liver function, and temporary management with beta-blockers or other approaches may be necessary even during the course of the acute liver injury. In several instances, patients with methimazole induced liver injury have been switched to propylthiouracil without evidence of recurrence, but in at least one case, recurrent jaundice appeared. In severe cases, however, more definitive therapy of the hyperthyroidism with radioactive iodide or surgery may be more appropriate.
Drug Class: Antithyroid Agents
Other Drugs in the Class: Propylthiouracil
CASE REPORT
Case 1. Mixed cholestatic-hepatocellular injury due to methimazole.(1)
A 43 year old woman with Graves disease developed pruritus and jaundice one month after starting therapy with methimazole (10 mg) and propranolol (20 mg), both given three times daily. She did not have abdominal pain, nausea, fever or rash. She continued taking methimazole for 4 days after the appearance of jaundice and presented to the hospital two weeks later because of persistent jaundice and pruritus. She had no history of liver disease or alcohol abuse and no risk factors for viral hepatitis. She had been clinically hyperthyroid with palpitations, tremor and elevated serum T4 levels [30.7 μg/dL] before therapy and routine liver tests were mildly abnormal (Table). On presentation, she was jaundiced but had no signs of chronic liver disease. Laboratory testing showed elevations in serum direct and total bilirubin and a cholestatic pattern of enzyme elevations. CT imaging of the abdomen showed no evidence of biliary obstruction. Tests for hepatitis A, B and C and autoantibodies were negative. Propranolol therapy was restarted but methimazole was held. She improved and jaundice resolved within 4 weeks and other liver test abnormalities within 8 weeks. After recovery from the liver injury, her hyperthyroidism was treated successfully with radioactive iodine.
Key Points
| Medication: | Methimazole (30 mg daily) |
|---|---|
| Pattern: | Cholestatic (R=0.6) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 1 month |
| Recovery: | 2 months |
| Other medications: | Amlodipine, propranolol (both continued) |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | 134 | 201 | 0.8 | ||
| 6 weeks | 14 days | 104 | 289 | 16.7 | Admission |
| 15 days | 91 | 264 | 14.9 | ||
| 16 days | 13.3 | ||||
| 18 days | 130 | 233 | 12.6 | ||
| 7 weeks | 20 days | 269 | 235 | 11.4 | |
| 22 days | 248 | 209 | 8.8 | Discharge | |
| 8 weeks | 26 days | 151 | 150 | 4.7 | |
| 12 weeks | 8 weeks | 63 | 154 | 1.6 | |
| 5 months | 4 months | 66 | 111 | 0.6 | |
| Normal Values | <75 | <116 | <1.2 | ||
Comment
Typical cholestatic hepatitis arising one month after starting therapy with methimazole. The patient was ill for almost two months but recovery was otherwise uncomplicated.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Methimazole – Tapazole®
DRUG CLASS
Antithyroid Agents
Product labeling at DailyMed, National Library of Medicine, NIH
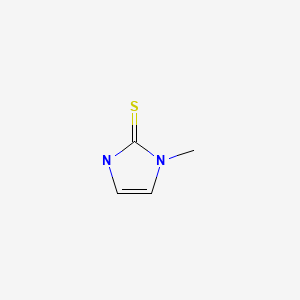
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Methimazole | 60-56-0 | C4-H6-N2-S |
|
CITED REFERENCE
- 1.
- Mikhail NE. Methimazole-induced cholestatic jaundice. South Med J. 2004;97:178–82. [PubMed: 14982270]
ANNOTATED BIBLIOGRAPHY
References updated: 10 February 2020
- Zimmerman HJ. Antithyroid drugs. Hormonal derivatives and related drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 579-81.(Expert review of hepatotoxicity of antithyroid medications published in 1999; mentions 35 recorded cases of jaundice attributed to propylthiouracil [usually hepatocellular] and 15 to methimazole [usually cholestatic]).
- Chitturi S, Farrell GC. Antithyroid drugs. Adverse effects of hormones and hormone antagonists on the liver. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 614-5.(Review of hepatotoxicity of antithyroid agents mentions that methimazole typically causes a cholestatic hepatitis arising within 2-12 weeks of starting and with "uneventful recovery").
- Brent GA, Koenig RJ. Thyroid and anti-thyroid drugs. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp.787-802.(Textbook of pharmacology and therapeutics).
- Specht NW, Boehme EJ. Death due to agranulocytosis induced by methimazole therapy. JAMA. 1952;149:1010–1. [PubMed: 14938092](67 year old woman developed fever 4 weeks after starting methimazole with agranulocytosis and jaundice, dying within 1-2 days; liver at autopsy showing “central congestion”).
- Rosenbaum H, Reveno WS. Agranulocytosis and toxic hepatitis from methimazole. JAMA. 1953;152:27. [PubMed: 13034518](62 year old woman developed jaundice 7 weeks after starting methimazole followed by agranulocytosis, resolving rapidly with stopping methimazole; had tolerated propylthiouracil).
- Shipp JC. Jaundice during methimazole (‘Tapazole’) administration. Ann Intern Med. 1955;42:701–6. [PubMed: 14350490](63 year old developed pruritus followed by jaundice 2 weeks after starting methimazole [bilirubin 8.7 mg/dL, Alk P 3 times ULN], with subsequent worsening of jaundice and agranulocytosis responding to antibiotics; resolution of jaundice in 10 weeks).
- Tennenbaum JI, Dreskin OH. Toxic hepatitis during treatment with methimazole (Tapazole). Report of a case with apparent recovery. Ohio Med J. 1962;58:306–7. [PubMed: 13920238](38 year old woman developed rash, jaundice and pruritus ~4 weeks after starting methimazole [bilirubin 6.2 mg/dL, ALT 545 U/L, Alk P ~2 times ULN, 12% eosinophils], slowly resolving on stopping therapy).
- Martinez-Lopez JI, Greenberg SE, Kling RR. Drug-induced hepatic injury during methimazole therapy. Gastroenterology. 1962;43:84–7. [PubMed: 14470520](36 year old woman developed jaundice and pruritus 1 month after starting methimazole [bilirubin 18 m/dL, Alk P 3 times ULN, AST 400 U/L, protime 18 sec]; she delayed in stopping methimazole and jaundice persisted for 10 weeks).
- Greenberger NJ, Milligan FD, DeGroot LJ, Isselbacher KJ. Jaundice and thyrotoxicosis in the absence of congestive heart failure. A study of four cases. Am J Med. 1964;36:840–6. [PubMed: 14162890](Description of 4 patients with jaundice and hyperthyroidism with congestive heart failure, but not on therapy and with no other known cause of liver disease [bilirubin 1.3-6.4 mg/dL, Alk P 1-3 times ULN, AST 13-40 U/L], jaundice resolving with successful therapy of hyperthyroidism).
- Sambe K. Liver injury due to drugs. Acta Hepatol (Japan). 1965;6:69.(Review of pathology of 19 cases of drug induced liver disease, one due to methylthioracil [similar to propylthiouracil, not used in US] and one to mercazol [carbimazole]; little clinical information given).
- Becker CE, Gorden P, Robbins J. Hepatitis from methimazole during adrenal steroid therapy for malignant exophthalmos. JAMA. 1968;26:1787–9. [PubMed: 4177058](54 year old woman on corticosteroids for severe exophthalmos developed jaundice 4 weeks after starting methimazole [bilirubin 5.1 mg/dL, ALT 414 U/L, Alk P 1.5 times ULN], switched to propylthiouracil and recovered promptly).
- Fischer MG, Nayer HR, Miller A. Methimazole-induced jaundice. JAMA. 1973;223:1028–9. [PubMed: 4739295](74 year old woman developed jaundice 2 weeks after starting methimazole [bilirubin 13.6 mg/dL, Alk P 270 U/L, ALT 96 U/L], jaundice lasted 2 months after stopping even with prednisone therapy).
- Ishizuki Y. Horumon To Rinsho. 1974;22:1083–5. [2 cases of liver diseases caused by thyroid antagonists] Japanese. [PubMed: 4473289](Two cases, ages 62 and 75 years, had onset of jaundice 3 and 5 weeks after starting antithyroid medications, with prolonged cholestasis in patient in whom methimazole was continued).
- Kimura T, Shindo T. Nippon Naika Gakkai Zasshi. 1982;71:685–91. [A case of insulin autoimmune syndrome with cholestatic hepatitis induced by methimazole and propylthiouracil] Japanese. [PubMed: 7130811]
- Cooper DS. Antithyroid drugs. N Engl J Med. 1984;311:1353–62. [PubMed: 6387489](Extensive review of mechanism of action, efficacy and safety of propylthiouracil and methimazole in treating hyperthyroidism; side effects occur in 1-5% of patients, including fever, rash, urticaria, transient leucopenia, and arthralgias particularly with higher doses; severe side effects include agranulocytosis, vasculitis, aplastic anemia, thrombocytopenia and nephritic syndrome).
- Vitug AC, Goldman JM. Hepatotoxicity from antithyroid drugs. Horm Res. 1985;21:229–34. [PubMed: 4007783](Review of literature identified 29 cases of hepatic injury due to propylthiouracil [n=17], methimazole [n=10] and carbimazole [n=3]; propylthiouracil cases were predominantly hepatocellular with onset in 10 days to 5 months; liver injury from other agents was primarily cholestatic arising in 10 days to 8 weeks).
- Schmidt G, Boerach G, Mueller KM, Wegener M. Methimazole associated cholestatic liver injury: case report and brief literature review. Hepato-gastroenterol. 1986;33:244–6. [PubMed: 3804181](58 year old woman developed abdominal pain 18 days after starting methimazole [bilirubin 1.2 mg/dL, ALT 93 U/L, Alk P 572 U/L], with improvement on stopping and recurrent rise in Alk P with rechallenge).
- Yao JD, Gross JB Jr, Ludwig J, Purnell DC. Cholestatic jaundice in hyperthyroidism. Am J Med. 1989;86:619–20. [PubMed: 2712072](42 year old man presented with jaundice and weight loss [bilirubin 16.7 rising to 36.0 mg/dL, ALT 76 U/L, Alk P 252 U/L] on no medications, liver biopsy showed intrahepatic cholestasis and he was found to be hyperthyroid, jaundice resolving after radioactive iodine therapy).
- Baker B, Shapiro B, Fig LM, Woodbury D, Sisson JC, Beierwaltes WH. Unusual complications of antithyroid drug therapy: four case reports and review of literature. Thyroidology. 1989;1:17–26. [PubMed: 2484903](Three cases of hepatotoxicity from thyroid medications; 34 year old woman developed jaundice 6 months after starting propylthiouracil [bilirubin 20.6 mg/dL, ALT 957 U/L, Alk P 176 U/L], with delayed withdrawal and slow recovery; 9 year old girl developed jaundice 3 months after starting propylthiouracil [bilirubin 9.0 mg/dL, ALT 1407 U/L, Alk P 848 U/L], resolving rapidly upon stopping; 20 year old woman developed jaundice 8 months after starting methimazole [bilirubin 27 mg/dL, ALT 2040 U/L, Alk P 389 U/L], progressing to hepatic failure and death and autopsy showed massive necrosis).
- Werner MC, Romaldini JH, Bromberg N, Werner RS, Farah CS. Adverse effects related to thioamide drugs and their dose regimen. Am J Med Sci. 1989;297:716–9. [PubMed: 2523194](Among 389 treated patients with Graves disease, 5 had hepatotoxicity, 4 of 131 [2%] on propylthiouracil and 1 of 258 [0.5%] on methimazole, all recovered; mostly on high dose therapy).
- Kang H, Choi JD, Jung IG, Kim DW, Kim TB, Shin HK, et al. A case of methimazole-induced acute hepatic failure in a patient with chronic hepatitis B carrier. Korean J Intern Med. 1990;5:69–73. [PMC free article: PMC4535001] [PubMed: 2271514](43 year old man with HBsAg carrier state developed jaundice 7 months after starting methimazole [bilirubin 5.0 mg/dL, ALT 180 U/L, Alk P 848 U/L, no detectable HBV DNA], developed worsening hepatic failure and died 30 days after admission, autopsy showed cirrhosis with severe cholestasis).
- Di Gregorio C, Ghini F, Rivasi F. Granulomatous hepatitis in a patient receiving methimazole. Ital J Gastroenterol. 1990;22:75–7. [PubMed: 2131935](56 year old woman developed abnormal liver tests 11 years after starting methimazole and not resolving when drug was stopped, biopsy showing active granulomas with giant cells suggestive of sarcoidosis [bilirubin normal, ALT 51-138 U/L, Alk P 484-652 U/L]).
- Findor J, Bruch Igartúa E, Sorda J, Jury R. Acta Gastroenterol Latinoam. 1991;21:115–9. [Jaundice caused by methimazole] Spanish. [PubMed: 1687933]
- Sola J, Pardo-Mindán FJ, Zozaya J, Quiroga J, Sangro B, Prieto J. Liver changes in patients with hyperthyroidism. Liver. 1991;11:193–7. [PubMed: 1943501](Four patients with thyrotoxicosis and “cholestasis” [bilirubin 0.8-1.0 mg/dL, Alk P 300-548 U/L, GGT 17-166 U/L], biopsies showing intrahepatic cholestasis).
- Peter SA. Propylthiouracil-associated hepatitis. J Natl Med Assoc. 1991;83:75–7. [PMC free article: PMC2627005] [PubMed: 1994070](43 year old woman developed jaundice 10 weeks after starting propylthiouracil [bilirubin 6.4 mg/dL, AST 926 U/L, Alk P 292 U/L], worsening for 10 days and then resolving within 10 weeks of stopping).
- Liaw YF, Huang MJ, Fan KD, Li KL, Wu SS, Chen TJ. Hepatic injury during propylthiouracil therapy in patients with hyperthyroidism. A cohort study. Ann Intern Med. 1993;118:424–8. [PubMed: 8439116](60 patients with hyperthyroidism and normal baseline ALT levels were monitored on propylthiouracil, 28% developed ALT elevations [40-231 U/L] all within 2 months of starting initial high dosage [300 mg/day], falling to normal with continuation [100 mg/day]; no symptoms, jaundice or Alk P elevations; liver biopsies in 3 patients showed spotty necrosis and ill defined granulomas).
- Sadoul JL, Canivet B, Freychet P. Toxic hepatitis induced by antithyroid drugs: four cases including one with cross-reactivity between carbimazole and benzylthiouracil. Eur J Med. 1993;2:473–7. [PubMed: 7504976](Retrospective analysis of 236 patients with hyperthyroidism treated at one center found 4 cases [1.7%] with hepatotoxicity due to carbimazole; only one with jaundice [bilirubin 3.5 mg/dL, ALT 162 U/L, Alk P 318 U/L], resolution in 4 weeks of stopping; other cases anicteric and associated with drug fever or rash, largely cholestatic enzyme patterns).
- Huang MJ, Li KL, Wei JS, Wu SS, Fan KD, Liaw YF. Sequential liver and bone biochemical changes in hyperthyroidism: prospective controlled follow-up study. Am J Gastroenterol. 1994;89:1071–6. [PubMed: 7912472](Prospective study of 95 patients with hyperthyroidism treated with propylthiouracil; 76% had at least one liver test abnormality before therapy, 37% in ALT [peak 169 U/L] and 64% in Alk P [peak 337 U/L]; ALT levels often decreased with treatment, but rose further in 38%, one developing jaundice [ALT 1490 U/L], resolving with stopping therapy).
- Mamianetti A, Muñoz A, Ronchetti RD, Maccione E, Poggi U, Mugnolo R, et al. Medicina (B Aires). 1995;55:693–6. [Acquired sideroblastic anemia and cholestasis in a hyperthyroid patient treated with methimazole and atenolol] Spanish. [PubMed: 8731582](62 year old woman developed jaundice and pruritus within 10 days of starting methimazole [bilirubin 16 rising to 39 mg/dL, ALT 65 U/L, Alk P 670 U/L], with prolonged jaundice and sideroblastic anemia, resolving slowly with normal tests 14 months later).
- Arab DM, Malatjalian DA, Rittmaster RS. Severe cholestatic jaundice in uncomplicated hyperthyroidism treated with methimazole. J Clin Endocrinol Metab. 1995;80:1083–5. [PubMed: 7714072](48 year old man with hyperthyroidism and mild liver enzyme abnormalities developed jaundice and worsening pruritus 1 month after starting methimazole [bilirubin 30.1 mg/dL, AST 40 U/L, Alk P 475 U/L], improving with stopping methimazole and achieving euthyroidism with radioactive iodide).
- Schwab GP, Wetscher GJ, Vogl W, Redmond E. Methimazole-induced cholestatic liver injury, mimicking sclerosing cholangitis. Langenbecks Arch Chir. 1996;381:225–7. [PubMed: 8817448](68 year old man developed jaundice and pruritus 2 months after starting methimazole [bilirubin 3.1 rising to 12.2 mg/dL, ALT 61 U/L, Alk P 530 U/L], resolving within 3 months of stopping and with thyroidectomy).
- Deidiker R, deMello DE. Propylthiouracil-induced fulminant hepatitis: case report and review of the literature. Pediatr Pathol Lab Med. 1996;16:845–52. [PubMed: 9025882](13 year old girl developed jaundice 4 months after starting propylthiouracil [bilirubin 13.8 mg/dL, ALT 1716 U/L, Alk P not given, ANA 1:20], worsening and undergoing liver transplantation within 7 days, but dying postoperatively, explant showing massive necrosis and collapse).
- Gürlek A, Cobankara V, Bayraktar M. Liver tests in hyperthyroidism: effect of antithyroid therapy. J Clin Gastroenterol. 1997;24:180–3. [PubMed: 9179740](At least one liver test abnormality was found in 60% of patients with hyperthyroidism before therapy; Alk P in 44% and ALT in 23%; often improving on propylthiouracil therapy, but 15% developed de novo ALT elevations by 6 weeks, although none were symptomatic, jaundiced or required dose modification).
- Waseem M, Seshadri KG, Kabadi UM. Successful outcome with methimazole and lithium combination therapy for propylthiouracil-induced hepatotoxicity. Endocr Pract. 1998;4:197–200. [PubMed: 15251733](49 year old man developed nausea 2 months after starting propylthiouracil; at 4 months bilirubin was 20.4 mg/dL, ALT 1043 U/L, Alk P 186 U/L, values worsening for 1 month despite stopping and then slowly returning towards normal despite use of methimazole).
- Hung YT, Yu WK, Chow E. Delayed cholestatic hepatitis due to methimazole. Hong Kong Med J. 1999;5:200–1. [PubMed: 11821593](71 year old woman developed jaundice a few weeks after stopping a 5 month course of methimazole with prolonged jaundice [bilirubin 40 mg/dL, ALT 40 U/L, Alk P 600 U/L], with slow recovery over more than 6 months).
- Babini G, Gurioli L, Rizzi R, Bertello P. Appearance of severe jaundice after radiometabolical treatment of thyrotoxicosis. J Endocrinol Invest. 1999;22:209–11. [PubMed: 10219889](63 year old man developed jaundice 2 weeks after receiving radioactive iodine for toxic goiter [bilirubin 6.8 mg/dL, ALT 86, Alk P 426 U/L], worsening for 2 weeks and then slowly improving with methimazole therapy of the hyperthyroidism).
- Woeber KA. Methimazole-induced hepatotoxicity. Endocr Pract. 2002;8:222–4. [PubMed: 12467281](36 year old woman developed pruritus and jaundice 3 weeks after starting methimazole [bilirubin 12.1 rising to 25.8 mg/dL, ALT 127 U/L, Alk P 265 U/L], with slow recovery and Alk P abnormalities for ~12 months).
- Kontoleon P, Ilias I, Koutras DA, Kontogiannis D, Papapetrou PD. Successful treatment with carbimazole of a hyperthyroid pregnancy with hepatic impairment after propylthiouracil administration: a case report. Clin Exp Obstet Gynecol. 2002;29:304–5. [PubMed: 12635752](27 year old woman developed elevations in ALT [151 U/L], with normal bilirubin [0.5 mg/dL] after 12th week of pregnancy while on propylthiouracil, resolving within 10 days of switching to carbimazole).
- Russo MV, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among 2291 liver transplants for acute liver failure done in the US between 1990 and 2002, 357 were attributed to medications, 53% to acetaminophen; in remaining 137 cases, most common agents were isoniazid [24; 17.5%], propylthiouracil [13; 9.5%], phenytoin [10; 7.3%], valproate [10; 7.3%], nitrofurantoin [7; 5%], herbals [7; 5%], ketoconazole [6; 4%] and disulfiram [6; 4%]; none due to methimazole).
- Mikhail NE. Methimazole-induced cholestatic jaundice. South Med J. 2004;97:178–82. [PubMed: 14982270](43 year old woman developed jaundice and pruritus one month after starting methimazole [bilirubin 16.7 mg/dL, ALT 104 U/L, Alk P 289 U/L], resolving 4 months after stopping; review of literature found 20 cases, 18 in women, onset in 3 days to 3 months, largely cholestatic, no fatalities from liver disease, recurrence on rechallenge with methimazole or carbimazole: Case 1).
- Piñero Madrona A, Pons Miñano JA, Madrid Conesa J, Parrilla Paricio P. Rev Clin Esp. 2004;204:388. [Methimazole hepatitis] Spanish. [PubMed: 15274789](46 year old woman with subclinical hyperthyroidism developed arthralgias and malaise 6 days after starting methimazole [ALT 1280 U/L, Alk P 520 U/L with normal bilirubin], resolving within 2 weeks of stopping).
- Casallo Blanco S, Valero MA, Marcos Sánchez F, de Matías Salces L, Blanco González JJ, Martín Barranco MJ. Gastroenterol Hepatol. 2007;30:268–70. [Methimazole and propylthiouracil induced acute toxic hepatitis] Spanish. [PubMed: 17493435](79 year old woman developed jaundice 1 month after starting methimazole [bilirubin 3.2 mg/dL, ALT 184 U/L, Alk P 574 U/L], with prompt improvement on stopping; then, 2 weeks after starting propylthiouracil, she redeveloped jaundice [bilirubin 5.5 mg/dL, ALT 448 U/L, Alk P 279 U/L], values normalizing within 2 months of stopping and with concurrent prednisone therapy).
- Ramos-Bonner LS, Goldberg TH, Moyer S, Anastasopoulou C. Methimazole-induced cholestatic jaundice in an elderly hyperthyroid patient. Am J Geriatr Pharmacother. 2007;5:236–40. [PubMed: 17996663](76 year old woman developed jaundice and pruritus 6 weeks after starting methimazole [bilirubin 25.4 mg/dL, ALT 676 U/L, Alk P 620 U/L, INR 1.2], worsening for 5 days and then resolving slowly).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to propylthiouracil or methimazole).
- Cooper DS, Rivkees SA. Putting propylthiouracil in perspective. J Clin Endocrinol Metab. 2009;94:1881–2. [PubMed: 19401361](Editorial summarizing issues of hepatotoxicity of propylthiouracil, 33 publications of hepatotoxicity in adults and 14 in children with UNOS reporting 16 liver transplants for acute liver failure due to propylthiouracil in adults and 7 in children between 1990 and 2007. In contrast, methimazole can cause liver injury but fatalities are rare; these factors led to recommendations that methimazole be used instead of propylthiouracil, except in first trimester of pregnancy [or for intolerance], and when surgery or radioiodine are not an option).
- Bahn RS, Burch HS, Cooper DS, Garber JF, Greenlee CM, Klein IL, et al. The role of propylthiouracil in the management of Graves’ disease in adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid. 2009;19:673–4. [PubMed: 19583480](In 2008, propylthiouracil was prescribed for 101,000 persons in the US, while UNOS has reported 16 liver deaths in adults and 7 in children from propylthiouracil since 1990; making the estimated fatality rate from acute liver failure due to propylthiouracil 1:10,000 in adults and as high as 1:2000 in children; for these reasons, propylthiouracil should not be considered the “first line” of treatment of Graves disease, methimazole being preferred except in first trimester of pregnancy).
- Rivkees SA, Mattison DR. Propylthiouracil (PTU) hepatotoxicity in children and recommendations for discontinuation of use. Int J Pediatr Endocrinol. 2009;2009:132041. [PMC free article: PMC2777303] [PubMed: 19946400](Review of propylthiouracil induced liver injury; 29 cases reported in literature, 14 in children, 9 resulting in death [3 in children] and 3 in liver transplantation; in contrast, fatality or transplantation due to methimazole induced liver disease has not been reported; concluded that propylthiouracil should not be used as treatment of Graves disease in children).
- Gallelli L, Staltari O, Palleria C, De Sarro G, Ferraro M. Hepatotoxicity induced by methimazole in a previously healthy patient. Curr Drug Saf. 2009;4:204–6. [PubMed: 19534646](54 year old man developed fever, rash and then jaundice arising 14 days after starting methimazole [bilirubin 4.4 mg/dL, ALT 55 U/L, Alk P 374 U/L, GGT 627 U/L], resolving rapidly with stopping methimazole).
- Zhang M, Zhou H, He R, Di F, Yang L, Yang T. Steroids for the treatment of methimazole-induced severe cholestatic jaundice in a 74-year-old woman with type 2 diabetes. Endocrine. 2010;37:241–3. [PubMed: 20960257](74 year old woman developed jaundice and pruritus 1 month after starting methimazole [bilirubin 14.9 mg/dL, ALT 92 U/L, Alk P 301 U/L], resolving slowly and with 8 weeks of prednisone).
- Livadas S, Xyrafis X, Economou F, Boutzios G, Christou M, Zerva A, Karachalios A, Palioura H, Palimeri S, Diamanti-Kandarakis E. Liver failure due to antithyroid drugs: report of a case and literature review. Endocrine. 2010;38:24–8. [PubMed: 20960098](34 year old woman developed pruritus 20 days after starting methimazole [bilirubin 2.4 rising to 3.6 mg/dL, ALT 141 to 1125 U/L, Alk P 189 to 396 U/L], resolving within 4 weeks of stopping).
- Shen C, Zhao CY, Liu F, Wang YD, Yu J. Acute-on-chronic liver failure due to thiamazole in a patient with hyperthyroidism and trilogy of Fallot: case report. BMC Gastroenterol. 2010;10:93. [PMC free article: PMC2928759] [PubMed: 20707932](24 year old man developed jaundice 1 year after starting methimazole [bilirubin 34.3 mg/dL, ALT 75 U/L, Alk P 133 U/L, INR 2.3], with progression to hepatic failure and death 27 days after presentation).
- Alvarez MP, Cano RL, Fernández CP, Méndez LF, García RG. Endocrinol Nutr. 2010;57:451–3. [Acute toxic hepatitis induced by methimazole: two cases] Spanish. [PubMed: 20675204](Two cases; 71 and 52 year old women developed enzyme elevations 6 weeks after starting methimazole [bilirubin 0.8 and 0.9 mg/dL, ALT 102 and 546 U/L, Alk P 889 and 999 U/L], resolving within 2 and 4 months of stopping).
- Rivkees SA, Szarfman A. Dissimilar hepatotoxicity profiles of propylthiouracil and methimazole in children. J Clin Endocrinol Metab. 2010;95:3260–7. [PubMed: 20427502](Analysis of FDA adverse event reports between 1969 and 2008 showed a higher rate of severe liver injury due to propylthiouracil than methimazole in children; “We are unaware of reports of death and liver failure in children and adolescents taking methimazole”).
- Malozowski S, Chiesa A. Propylthiouracil-induced hepatotoxicity and death. Hopefully, never more. J Clin Endocrinol Metab. 2010;95:3161–3. [PMC free article: PMC2928912] [PubMed: 20610609](Editorial in response to Rivkees [2010] summarizing the factors that led to the recommendation that propylthiouracil be avoided in children and be considered a second line drug for treating hyperthyroidism in adults).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 5 due to propylthiouracil, but none to methimazole).
- Sato H, Minagawa M, Sasaki N, Sugihara S, Kazukawa I, Minamitani K, Wataki K, et al. Comparison of methimazole and propylthiouracil in the management of children and adolescents with Graves' disease: efficacy and adverse reactions during initial treatment and long-term outcome. J Pediatr Endocrinol Metab. 2011;24:257–63. [PubMed: 21823520](Retrospective analysis of safety and efficacy of methimazole in 64 and propylthiouracil in 69 children with hyperthyroidism found similar response rates, but more frequent adverse events with initial high doses of propylthiouracil).
- Yang J, Zhong J, Zhou LZ, Hong T, Xiao XH, Wen GB. Sudden onset agranulocytosis and hepatotoxicity after taking methimazole. Intern Med. 2012;51:2189–92. [PubMed: 22892501](38 year old woman with hyperthyroidism developed agranulocytosis and liver test abnormalities within a week of starting methimazole [bilirubin 1.5 mg/dL, ALT 226 U/L, Alk P 143], resolving within a week of stopping methimazole and starting G-CSF).
- Hackmon R, Blichowski M, Koren G. The safety of methimazole and propylthiouracil in pregnancy: a systematic review. J Obstet Gynaecol Can. 2012;34:1077–86. [PubMed: 23231846](Systematic review of safety of propylthiouracil and methimazole during pregnancy concludes that propylthiouracil "has been associated with a rare but serious form of hepatic failure").
- Otsuka F, Noh JY, Chino T, Shimizu T, Mukasa K, Ito K, Ito K, Taniyama M. Hepatotoxicity and cutaneous reactions after antithyroid drug administration. Clin Endocrinol (Oxf). 2012;77:310–5. [PubMed: 22332800](Among 391 patients with hyperthyroidism, ALT levels >2 times ULN occurred in 9% on methimazole vs 26% on propylthiouracil arising after 12 to 60 days, but "...no serious liver failure was observed").
- Kwon H, Lee SH, Kim SE, Lee JH, Jee YK, Kang HR, Park BJ, Park JW, Hong CS. Spontaneously reported hepatic adverse drug events in Korea: multicenter study. J Korean Med Sci. 2012;27:268–73. [PMC free article: PMC3286773] [PubMed: 22379337](Summary of 2 years of adverse event reporting in Korea; of 9360 reports, 567 were liver related, including two attributed to propylthiouracil, but none to methimazole).
- Maciá-Bobes C, Ronzón-Fernández A, Macías-Robles MD, Fau-Cubero C. Farm Hosp. 2012;36:431–2. [Methimazole-induced liver injury] Spanish. [PubMed: 22857866](75 year old woman with toxic nodular goiter and multiple drug allergies developed liver test abnormalities 6 weeks after starting methimazole [bilirubin normal, ALT 454 U/L, GGT 268 U/L, Alk P normal], resolving within a month of switching to carbimazole).
- Regelmann MO, Miloh T, Arnon R, Morotti R, Kerkar N, Rapaport R. Graves disease presenting with severe cholestasis. Thyroid. 2012;22:437–9. [PubMed: 22458973](17 year old girl with acute hepatitis A and Graves disease developed marked cholestasis, which improved with therapy of hyperthyroidism).
- Yoshihara A, Noh J, Yamaguchi T, Ohye H, Sato S, Sekiya K, Kosuga Y, et al. Treatment of graves' disease with antithyroid drugs in the first trimester of pregnancy and the prevalence of congenital malformation. J Clin Endocrinol Metab. 2012;97:2396–403. [PubMed: 22547422](Among 5997 infants of Japanese women with thyrotoxicosis during the first trimester, congenital malformations were found in 4.1% of infants of 1231 methimazole treated, 1.9% of 1399 propylthiouracil treated and 2.1% of 1906 untreated mothers).
- de Campos Mazo DF, de Vasconcelos GB, Pereira MA, de Mello ES, Bacchella T, Carrilho FJ, et al. Clinical spectrum and therapeutic approach to hepatocellular injury in patients with hyperthyroidism. Clin Exp Gastroenterol. 2013;6:9–17. [PMC free article: PMC3579408] [PubMed: 23550044](Among 7 patients with hyperthyroidism found to have liver disease, 2 were attributed to propylthiouracil hepatotoxicity, 2 to autoimmune hepatitis and 3 to the underlying hyperthyroidism, but none to methimazole).
- Korelitz JJ, McNally DL, Masters MN, Li SX, Xu Y, Rivkees SA. Prevalence of thyrotoxicosis, antithyroid medication use, and complications among pregnant women in the United States. Thyroid. 2013;23:758–65. [PMC free article: PMC3675839] [PubMed: 23194469](Analysis of 4902 15-44 year old women with thyrotoxicosis during pregnancy in a health insurance database [2005-2009] found no increase in liver abnormalities or congenital defects associated with either propylthiouracil or methimazole therapy).
- Andersen SL, Olsen J, Wu CS, Laurberg P. Birth defects after early pregnancy use of antithyroid drugs: a Danish nationwide study. J Clin Endocrinol Metab. 2013;98:4373–81. [PubMed: 24151287](In a population based cohort study from Denmark, exposure to either methimazole or propylthiouracil during pregnancy was associated with an increased rate of birth defects, but not use of these agents before or after pregnancy).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to antithyroid medications).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, five [including 2 deaths] were attributed to propylthiouracil but none to methimazole).
- Wang MT, Lee WJ, Huang TY, Chu CL, Hsieh CH. Antithyroid drug-related hepatotoxicity in hyperthyroidism patients: a population-based cohort study. Br J Clin Pharmacol. 2014;78:619–29. [PMC free article: PMC4243912] [PubMed: 25279406](Among 71,379 patients with hyperthyroidism initiating medical therapy between 2004 and 2009 identified in the Taiwanese National Health Insurance Research Database, methimazole/carbimazole had a higher rate of clinically apparent liver injury than propylthiouracil [3.2 vs 1.2] but a lower rate of acute liver failure [0.32 vs o.68: both per 1000-patient years]).
- Akmal A, Kung J. Propylthiouracil, and methimazole, and carbimazole-related hepatotoxicity. Expert Opin Drug Saf. 2014;13:1397–406. [PubMed: 25156887](Review of the hepatotoxicity of antithyroid medications concludes that methimazole and carbimazole are more likely to cause cholestatic liver injury, while propylthiouracil is more likely to cause acute liver failure for which reason its use should be limited to use in adults and in the first trimester of pregnancy and thyroid storm)
- Heidari R, Niknahad H, Jamshidzadeh A, Abdoli N. Factors affecting drug-induced liver injury: antithyroid drugs as instances. Clin Mol Hepatol. 2014;20:237–48. [PMC free article: PMC4197171] [PubMed: 25320726](Review of risk factors associated with liver injury from methimazole and propylthiouracil including age, gender, alcohol use and co-morbidities, none of which appear to play a very major role).
- Heidari R, Niknahad H, Jamshidzadeh A, Eghbal MA, Abdoli N. An overview on the proposed mechanisms of antithyroid drugs-induced liver injury. Adv Pharm Bull. 2015;5:1–11. [PMC free article: PMC4352210] [PubMed: 25789213](Review of the possible mechanisms of liver injury due to methimazole and propylthiouracil).
- Papachristos DA, Huynh J, Grossman M, MacIsaac RJ. Liver dysfunction and anti-thyroid therapy. SAGE Open Med Case Rep 2015; 3: 2050313X14568335. [PMC free article: PMC4857332] [PubMed: 27489677](49 year old man with Graves disease developed jaundice 2 weeks after starting carbimazole [bilirubin 34 mg/dL, ALT 104 U/L, Alk P 147 U/L], which resolved within 3 months of stopping and after radioactive iodine treatment making it difficult to assess whether the injury was due to the medication versus hyperthyroidism).
- Yang J, Li LF, Xu Q, Zhang J, Weng WW, Zhu YJ, Dong MJ. Analysis of 90 cases of antithyroid drug-induced severe hepatotoxicity over 13 years in China. Thyroid. 2015;25:278–83. [PubMed: 25384184](Among 90 patients with hyperthyroidism and drug induced liver injury due to antithyroid therapy seen at a single medical center over a 13 year period, 51 were attributed to methimazole [1 fatal] and 39 to propylthiouracil [none fatal]; both had similar latencies to onset [80% within 3 months, 60% within 1 month] and methimazole cases were more likely to be cholestatic [35% vs 18%] and less likely hepatocellular [43% vs 56%]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 6 [0.7%] were attributed to antithyroid drugs including 3 to methimazole [thiamazole], all of which were cholestatic or mixed and self-limited in course, and 3 to propylthiouracil, one of which was fatal and one occurring in a woman treated during pregnancy).
- Ji H, Yue F, Song J, Zhou X. A rare case of methimazole-induced cholestatic jaundice in an elderly man of Asian ethnicity with hyperthyroidism: A case report. Medicine (Baltimore). 2017;96(49):e9093. [PMC free article: PMC5728948] [PubMed: 29245333](69 year old Asian man developed jaundice 3 weeks after starting methimazole for hyperthyroidism [bilirubin 21.6 mg/dL, ALT 59 U/L, Alk P 631 U/L], improving rapidly upon stopping).
- Abramavicius S, Velickiene D, Kadusevicius E. Methimazole-induced liver injury overshadowed by methylprednisolone pulse therapy: Case report. Medicine (Baltimore). 2017;96:e8159. [PMC free article: PMC5626305] [PubMed: 28953662](74 year old woman with Graves disease and ophthalmopathy on methimazole therapy developed elevated ALT and AST levels after a 5th weekly infusion of methylprednisolone, which gradually resolved despite continuing both drugs).
- Forner D, Kulai T, Arnason T, E, Gruchy S, MacLeod M. Ramipril-associated cholestasis in the setting of recurrent drug-induced liver injury. Gastroenterol Hepatol Bed Bench. 2017;10:143–6. [PMC free article: PMC5495903] [PubMed: 28702139](67 year old man had two episodes of suspected drug induced liver injury, one due to methimazole and another 20 years later to ramipril, both being cholestatic and self-limited in course).
- Jin S, Li X, Fan Y, Fan X, Dai Y, Lin H, Cai W, et al. Association between genetic polymorphisms of SLCO1B1 and susceptibility to methimazole-induced liver injury. Basic Clin Pharmacol Toxicol. 2019;125:508–17. [PubMed: 31240859](Several polymorphisms of SLCOB1 [the gene that encodes the organic anion-transporting peptide B1] were found more frequent among 44 patients with Graves disease who developed methimazole induced liver injury than in 118 Graves disease patients who did not).
- Li X, Jin S, Fan Y, Fan X, Tang Z, Cai W, Yang J, et al. Association of HLA-C*03:02 with methimazole-induced liver injury in Graves' disease patients. Biomed Pharmacother. 2019;117:109095. [PubMed: 31202168](Analysis of HLA class I and II alleles in patients with Graves disease who developed methimazole hepatotoxicity [n=40] or not [n=118], the allele frequency of HLA-C*03.02 was 6.7% in patients with methimazole hepatotoxicity compared to 0.4% of those who tolerated the drug without liver injury).
- Suzuki N, Noh JY, Hiruma M, Kawaguchi A, Morisaki M, Ohye H, Suzuki M, et al. Analysis of antithyroid drug-induced severe liver injury in 18,558 newly diagnosed patients with Graves' disease in Japan. Thyroid. 2019;29:1390–8. [PubMed: 31573408](Among 18,558 Japanese patients initiating medical therapy of Graves disease between 2005 and 2017, 416 [2.5%] developed evidence of liver injury [ALT above 8 times ULN or bilirubin above 3 times ULN], 1.4% on methimazole and 6.3% of propylthiouracil, 92% were women, onset usually within 30 days, rates being age-dependent and all patients recovered).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Antithyroid Agents.[LiverTox: Clinical and Researc...]Review Antithyroid Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Radioiodine therapy versus antithyroid medications for Graves' disease.[Cochrane Database Syst Rev. 2016]Review Radioiodine therapy versus antithyroid medications for Graves' disease.Ma C, Xie J, Wang H, Li J, Chen S. Cochrane Database Syst Rev. 2016 Feb 18; 2(2):CD010094. Epub 2016 Feb 18.
- Is there a methimazole dose effect on remission rate in Graves' disease? Results from a long-term prospective study. The European Multicentre Trial Group of the Treatment of Hyperthyroidism with Antithyroid Drugs.[Clin Endocrinol (Oxf). 1998]Is there a methimazole dose effect on remission rate in Graves' disease? Results from a long-term prospective study. The European Multicentre Trial Group of the Treatment of Hyperthyroidism with Antithyroid Drugs.Benker G, Reinwein D, Kahaly G, Tegler L, Alexander WD, Fassbinder J, Hirche H. Clin Endocrinol (Oxf). 1998 Oct; 49(4):451-7.
- Review Propylthiouracil.[LiverTox: Clinical and Researc...]Review Propylthiouracil.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- [Methimazole-induced cholestatic jaundice in a hyperthyroid patient].[Acta Gastroenterol Latinoam. 2...][Methimazole-induced cholestatic jaundice in a hyperthyroid patient].López-P Rdel P, Forero JD, Sierra F. Acta Gastroenterol Latinoam. 2014 Mar; 44(1):52-8.
- Methimazole - LiverToxMethimazole - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...