NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Mavyret is an oral, fixed combination of two antiviral agents, glecaprevir and pibrentasivir used to treat chronic hepatitis C virus (HCV) infection due to any genotype (1 through 6). This combination has not been linked to instances or worsening of serum enzymes during therapy or with de novo appearance of clinically apparent liver injury. However, like all effective direct acting antiviral agents for hepatitis C, Mavyret is considered capable of causing reactivation of hepatitis B in susceptible patients and transient hepatic decompensation in patients with cirrhosis.
Background
Mavyret is the commercial name for a fixed combination of oral antiviral agents used to treat chronic hepatitis C virus infection of all genotypes (1 through 6). The hepatitis C virus encodes several nonstructural (NS) polypeptides that are essential for its replication, including NS3/4 that has protease and helicase activities, NS5A that is a membrane bound polypeptide that is essential for viral replication and NS5B an HCV specific, RNA-dependent, RNA polymerase. These polypeptides are effective targets for antiviral therapy of hepatitis C. Mavyret is a fixed dose combination of glecaprevir (glek a' pre vir) which is a potent HCV NS3/4 protease inhibitor and pibrentasvir (pi brent' as vir) an HCV NS5A inhibitor. In cell culture, each of these agents has potent activity against all genotypes of HCV, but antiviral resistance can arise rapidly with continued exposure to a single agent. The combination of these two direct acting agents with different molecular targets allows for a sustained viral suppression while avoiding antiviral resistance. The combination of glecaprevir and pibrentasvir was shown to be very effective in suppressing HCV replication in patients infected with all 6 HCV genotypes and to result in sustained virological responses (SVR) and eradication of HCV in more than 95% of patients when given for 8 weeks or more. Mavyret was approved for use in the United States in 2017, the fifth all-oral antiviral combination to receive approval for chronic hepatitis C. It is available as tablets in a fixed dose combination of 100 mg of glecaprevir and 40 mg of pibrentasvir. The recommended dose in adults is 3 tablets once daily for 8 weeks in patients without cirrhosis and for 12 weeks in those with compensated (Class A) cirrhosis. Longer courses of therapy (12 or 16 weeks) are recommended for patients previously treated with HCV NS3/4 protease inhibitors or NS5A inhibitors. Mavyret has not been approved for use in patients with decompensated cirrhosis. Side effects are uncommon but are generally mild and can include fatigue, headache and nausea.
Hepatotoxicity
In large randomized controlled trials, serum aminotransferase levels decreased rapidly during Mavyret therapy and there were only rare instances of late, de novo elevations in ALT or AST that were usually mild-to-moderate in degree and rising to more than 5 times ULN in less than 1% of treated subjects. In addition, Mavyret has been linked to only rare instances of hepatic decompensation during treatment of patients with preexisting cirrhosis (<1%) and possibly with instances of reactivation of hepatitis B, two rare but potentially serious complications that have been linked to other oral regimens to treat chronic hepatitis C. Episodes of decompensation typically arise after 2 to 6 weeks of treatment and are marked by symptoms of fatigue, pruritus and jaundice with marked elevations in serum bilirubin, but minimal increase in serum aminotransferase and alkaline phosphatase levels. Mavyret is not approved for use in patients with advanced cirrhosis (Child Class B or C). The product label for Mavyret has a boxed warning for reactivation of hepatitis B, and screening for HBsAg and anti-HBc is recommended before starting therapy, with careful monitoring during treatment if these markers are present.
Likelihood score: C (rare cause of clinically apparent liver injury due to transient hepatic decompensation or reactivation of hepatitis B).
Mechanism of Injury
The mechanism by which glecaprevir and pibrentasvir might cause liver injury is not known. Both are metabolized in the liver largely via the cytochrome P450 system, predominantly CYP 1A2, and liver injury may be due to production of a toxic or immunogenic metabolite. Mavyret is also susceptible to drug-drug interactions with strong inducers or inhibitors of CYP 3A4.
Outcome and Management
While 8 to 16 weeks of therapy with Mavyret can be associated with transient mild-to-moderate serum aminotransferase elevations, it has not been convincingly linked to cases of clinically apparent idiosyncratic liver injury. Nevertheless, Mavyret is labelled as having the potential of causing decompensation of preexisting cirrhosis and reactivation of hepatitis B in susceptible patients. Assessment of patients for cirrhosis and screening for HBsAg and anti-HBc is recommended before treatment with Mavyret. Patients with markers of ongoing (HBsAg) or previous (anti-HBc) hepatitis B virus infection should be monitored for HBV DNA levels before and at 4 week intervals during therapy. Initiation of oral antiviral therapy for hepatitis B is warranted if levels of HBV DNA rise significantly. In addition, Mavyret should be permanently discontinued if jaundice or symptoms of liver injury arise, or if serum ALT or AST levels are persistently above 5 times the ULN.
Drug Class: Antiviral Agents, Hepatitis C Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Glecaprevir, Pibrentasvir – Mavyret®
DRUG CLASS
Hepatitis C Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Glecaprevir | 1365970-03-1 | C38-H46-F4-N6-O9-S |
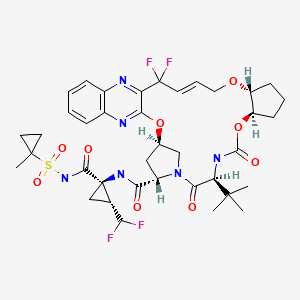
|
| Pibrentasvir | 1353900-92-1 | C57-H65-F5-N10-O8 | SID:252163521 |
ANNOTATED BIBLIOGRAPHY
References updated: 07 February 2022
Abbreviations: HCV, hepatitis C virus; DAA, direct acting antiviral agent; SVR, sustained virological response; ULN, upper limit of the normal range.
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Multi-authored textbook of hepatotoxicity published in 2013; does not discuss oral, direct acting antiviral agents used to treat hepatitis C).
- European Association for Study of Liver. EASL Recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63:199–236. [PubMed: 25911336](Guidelines for the antiviral therapy of chronic hepatitis C from the European liver disease research and academic society).
- AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–54. [PubMed: 26111063](Guidelines for the antiviral therapy of chronic hepatitis C from the US liver and infectious diseases research and academic societies).
- Gane E, Poordad F, Wang S, Asatryan A, Kwo PY, Lalezari J, Wyles DL, et al. High Efficacy of ABT-493 and ABT-530 treatment in patients with HCV genotype 1 or 3 infection and compensated cirrhosis. Gastroenterology. 2016;151:651–659.e1. [PubMed: 27456384](Among 82 cirrhotic patients with chronic HCV infection genotypes 1 or 3 who were treated with glecaprevir and pibrentasvir for 12 or 16 weeks with or without ribavirin, the overall SVR rate was 98% and no patient developed hepatic decompensation).
- Kwo PY, Poordad F, Asatryan A, Wang S, Wyles DL, Hassanein T, Felizarta F, et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J Hepatol. 2017;67:263–71. [PubMed: 28412293](Among 449 noncirrhotic patients with chronic HCV infection of all genotypes treated with glecaprevir and pibrentasvir with or without ribavirin for 8 or 12 weeks in 2 open label trials, SVR rates were excellent [97-100%], except in patients with genotype 3 [83-94%], and there were no ALT elevations after the initial decreases from baseline).
- Poordad F, Felizarta F, Asatryan A, Sulkowski MS, Reindollar RW, Landis CS, Gordon SC, et al. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment. Hepatology. 2017;66:389–97. [PMC free article: PMC5573922] [PubMed: 28128852](Among 91 noncirrhotic patients with chronic HCV infection, genotype 1, previously treated with NS3/4 or NS5A inhibitors, who received glecaprevir and pibrentasvir, SVR rates were 86-100% and there were no liver related serious adverse events or ALT elevations above 3 times ULN).
- AASLD. http://www
.hcvguidelines.org/ (Guidelines for therapy of hepatitis C maintained and regularly updated by the American Association for the Study of Liver Diseases [AASLD] and the Infectious Diseases Society of America [IDSA]). - Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Bräu N, Brown A, Pol S, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med. 2017;377:1448–55. [PubMed: 29020583](Among 104 patients with chronic hepatitis C [any genotype] and severe renal dysfunction [82% on dialysis] treated with glecaprevir and pibrentasvir for 12 weeks, the SVR rate was 98% and no patient developed hepatic decompensation or ALT elevations above 3 times ULN).
- Forns X, Lee SS, Valdes J, Lens S, Ghalib R, Aguilar H, Felizarta F, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 2017;17:1062–8. [PubMed: 28818546](Among 146 patients with compensated cirrhosis due to chronic hepatitis C [genotypes 1, 2, 4, 5 and 6] treated with glecaprevir and pibrentasvir for 12 weeks, 99% had an SVR, while 11 patients [8%] had a serious adverse event, but none had late ALT elevations above 5 times ULN or hepatic decompensation).
- Mavyret and Vosevi--two new combinations for chronic HCV infection. Med Lett Drugs Ther. 2017;59(1531):166–70. [PubMed: 28977807](Concise review of mechanism of action, clinical efficacy, safety and costs of Mavyret; mentions that common adverse effects are headache, fatigue and nausea and that the product label has a boxed warning of HBV reactivation).
- Asselah T, Kowdley KV, Zadeikis N, Wang S, Hassanein T, Horsmans Y, Colombo M, et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 Weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol. 2018;16:417–26. [PubMed: 28951228](Among 526 patients with chronic hepatitis C, genotypes 2, 4, 5 and 6, treated with Mavyret, SVR rates were 93-98% with 8 weeks and 99% with 12 weeks, ALT elevations above 5 times ULN occurred in 3 subjects, one becoming jaundiced at week 12, but resolving completely within 2 weeks of discontinuation and accompanied by an SVR).
- Chayama K, Suzuki F, Karino Y, Kawakami Y, Sato K, Atarashi T, Naganuma A, et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 1 hepatitis C virus infection with and without cirrhosis. J Gastroenterol. 2018;53:557–65. [PMC free article: PMC5866824] [PubMed: 28948366](Among 129 Japanese noncirrhotic patients with chronic hepatitis C, genotype 1, treated with glecaprevir/pibrentasivir for 8 weeks, the SVR rate was 99%, while among 38 cirrhotic patients treated for 12 weeks the SVR rate was 100%).
- Zeuzem S, Foster GR, Wang S, Asatryan A, Gane E, Feld JJ, Asselah T, et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med. 2018;378:354–69. [PubMed: 29365309](Among 1208 patients with chronic hepatitis C without cirrhosis who were treated with glecaprevir and pibrentasvir for 8 or 12 weeks, SVR rates were 99% with 8 weeks of therapy in genotype 1 and 95% with 8 or 12 weeks of therapy in genotype 3 infected patients and there were no ALT elevations above 5 times ULN, no instances of decompensated liver disease or early termination of therapy for hepatic adverse events).
- Poordad F, Pol S, Asatryan A, Buti M, Shaw D, Hézode C, Felizarta F, et al. Glecaprevir/pibrentasvir in patients with HCV genotype 1 or 4 and prior direct-acting antiviral treatment failure. Hepatology. 2018;67:1253–60. [PMC free article: PMC5901397] [PubMed: 29152781](Among 91 patients with chronic hepatitis C, genotypes 1 and 4, who had failed previous DAA therapy and were treated with glecaprevir and pibrentasvir, SV rates were 89% with 12 weeks and 91% for 16 weeks, relapse occurring only in those with previous exposure to NS5A inhibitors; there were no ALT elevations above 5 times ULN during therapy, but 3 patients were found to have liver cancer within 12 weeks of completing treatment).
- Wyles D, Poordad F, Wang S, Alric L, Felizarta F, Kwo PY, Maliakkal B, et al. Glecaprevir/ pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience: A partially randomized phase 3 clinical trial. Hepatology. 2018;67:514–23. [PMC free article: PMC5817409] [PubMed: 28926120](Among 131 patients with chronic hepatitis C, genotype 3, with cirrhosis or previous failure of antiviral therapy who received glecaprevir and pibrentasvir, the SVR rate was 91% with 12 weeks and 96% with 16 weeks of treatment, the differences being largely among those with previous therapy; no patient had a late increase in ALT levels or episode of hepatic decompensation).
- Rockstroh JK, Lacombe K, Viani RM, Orkin C, Wyles D, Luetkemeyer AF, Soto-Malave R, et al. Efficacy and safety of glecaprevir/pibrentasvir in patients co-infected with hepatitis C virus and human immunodeficiency virus-1: the EXPEDITION-2 Study. Clin Infect Dis. 2018;67:1010–7. [PMC free article: PMC6137115] [PubMed: 29566246](Among 153 patients with chronic hepatitis C [genotype 1 to 6] who were coinfected with HIV and were treated with glecaprevir and pibrentasvir for 8 or 12 weeks, SVR rates were 98% with either duration of treatment, and there were no ALT or AST elevations above 3 times ULN or instances of decompensated liver disease).
- Garrison KL, German P, Mogalian E, Mathias A. The drug-drug interaction potential of antiviral agents for the treatment of chronic hepatitis C infection. Drug Metab Dispos. 2018;46:1212–1225. [PubMed: 29695614](Extensive summary of drug-drug interactions of the drugs used to treat chronic hepatitis C; Glecaprevir and pibrentasvir are substrates of P-glycoprotein [Pgp] and weak inhibitors of Pgp, CYP3A, CYP1A2 and UGT1A1).
- Caroleo B, Caroleo MC, Cimellaro A, Colangelo L, Perticone M, Di Mizio G, De Sarro G, et al. Glecaprevir/ pibrentasvir induced cholestatic jaundice in a HCV patient with renal failure. a case presentation. Curr Drug Saf. 2019;14:67–71. [PubMed: 30444202](86 year old man with chronic hepatitis C and possible cirrhosis developed jaundice within 4 weeks of starting Mavyret [bilirubin 5.8 mg/dL, ALT 43 U/L, GGT 34 U/L, INR and albumin normal], which resolved within 1 month of stopping and patient was HCV RNA negative in follow up).
- Hammami MB, Aboushaar R, Alsabbagh E. Glecaprevir/pibrentasvir-associated acute liver injury in non-cirrhotic, chronic HCV infection without HBV co-infection. BMJ Case Rep. 2019;12:e226622. [PMC free article: PMC6536190] [PubMed: 31129632](A 53 year old woman with chronic hepatitis C without cirrhosis developed jaundice 2-3 weeks after starting Mavyret [bilirubin 6.8 mg/dL, ALT 974 U/L, Alk P 177 U/L, INR 1.2], symptoms resolving within a week of stopping and with normal liver tests and no detectable HCV RNA in serum in follow up).
- Yoon JH, Kim SM, Kang G, Kim HJ, Jun CH, Choi SK. A case report of glecaprevir/pibrentasvir-induced severe hyperbilirubinemia in a patient with compensated liver cirrhosis. Medicine (Baltimore). 2019;98:e17343. [PMC free article: PMC6775421] [PubMed: 31574875](77 year old man with chronic hepatitis C, cirrhosis, a history of hepatocellular cancer and tuberculosis developed jaundice 3 weeks after starting Mavyret [bilirubin 21.6 mg/dL, ALT normal], which resolved within a few weeks of stopping therapy; in follow up he was HCV RNA negative and bilirubin fell to 1.2 mg/dL).
- Persico M, Aglitti A, Milella M, Coppola C, Messina V, Claar E, Gentile I, et al. Real-life glecaprevir/pibrentasvir in a large cohort of patients with hepatitis C virus infection: The MISTRAL study. Liver Int. 2019;39:1852–1859. [PubMed: 31175707](Among 1177 patients with chronic hepatitis C enrolled in an observational study of therapy with Mavyret, the overall SVR was 99% and adverse event rate was 5.2% with 6 serious adverse events, two of which were jaundice).
- Gane E, Poordad F, Zadeikis N, Valdes J, Lin CW, Liu W, Asatryan A, et al. Safety and pharmacokinetics of glecaprevir/pibrentasvir in adults with chronic genotype 1-6 hepatitis C virus infections and compensated liver disease. Clin Infect Dis. 2019;69:1657–1664. [PMC free article: PMC6821220] [PubMed: 30923816](Among 2369 patients with chronic hepatitis C treated with Mavyret for 8-16 weeks in 9 clinical trials, the 308 with cirrhosis compared to those without had similar rates of SVR [96% vs 97.5%], adverse events [74% vs 67%], serious adverse events [6% vs 2%], ALT elevations above 5 times ULN [0% vs <1%]; and one patient with cirrhosis and esophageal varices had a variceal bleed without evidence of hepatic worsening on day 22 and continued therapy and achieved an SVR).
- Brown RS Jr, Buti M, Rodrigues L, Chulanov V, Chuang WL, Aguilar H, Horváth G, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial. J Hepatol. 2020;72:441–449. [PubMed: 31682879](Among 343 patients with chronic hepatitis C and compensated cirrhosis treated with Mavyret for 8 weeks, the overall SVR rate was 98%, and 46% had adverse events, 2% serious, no patient developed hepatic decompensation, and only one had an isolated single elevation of ALT above 5 times ULN).
- Liu YC, Jeng WJ, Cheng YT, Hsieh YC, Teng W, Chen YC, Lin CY, et al. Incidence and predictors for abnormal liver function during direct-acting antiviral agents in chronic hepatitis C patients. Medicine (Baltimore). 2020;99:e21898. [PMC free article: PMC7489670] [PubMed: 32925725](Among 1563 patients with chronic hepatitis C treated with direct acting antiviral agents between 2015 -2019 at a single referral center in Taiwan, the SVR rate was 98% and on treatment ALT elevations arose in 11%, with higher rates in those with cirrhosis, with elevated levels pre-treatment, and those receiving asunaprevir with daclatasvir [40%], compared to Zepatier [12.3%], sofosbuvir-based regimens [11.6%] and Mavyret [5.4%]; on treatment ALT elevations were not associated with a lower SVR rate).
- Aghemo A, Alberti A, Andreone P, Angelico M, Brunetto MR, Chessa L, Ciancio A, et al. MARS Study Group. Effectiveness and safety of glecaprevir/pibrentasvir in chronic hepatitis C patients: Results of the Italian cohort of a post-marketing observational study. Dig Liver Dis. 2021;53:612–619. [PubMed: 32917546](Among 321 patients with chronic hepatitis C treated with Mavyret followed in a postmarketing observational study, the SVR rate in those with follow up was 99% and adverse event rate 9%, which were serious in 1.2%, one patient developing ascites).
- Wedemeyer H, Erren P, Naumann U, Rieke A, Stoehr A, Zimmermann T, Lohmann K, et al. Glecaprevir/pibrentasvir is safe and effective in hepatitis C patients with cirrhosis: Real-world data from the German Hepatitis C-Registry. Liver Int. 2021;41:949–955. [PubMed: 33592123](Among 182 patients with chronic hepatitis C and cirrhosis who were treated with Mavyret and followed in a prospective observation study, the SVR rate was 100% in patients with follow up and serious adverse events arose in 4% including 1 patient with liver transplant, 1 with hepatic decompensation, and 4 with ALT elevations above 3 times ULN).
- Forns X, Feld JJ, Dylla DE, Pol S, Chayama K, Hou J, Heo J, et al. Safety of patients with hepatitis C virus treated with glecaprevir/pibrentasvir from clinical trials and real-world cohorts. Adv Ther. 2021;38:3409–3426. [PMC free article: PMC8189955] [PubMed: 34021887](Among 5106 patients with chronic hepatitis C treated with Mavyret in clinical trials or postmarketing observational studies, the overall SVR rate was 97.5% and adverse event rate in clinical trials was 61%, scored as serious in 1.7%; ALT elevations above 5 times ULN arose in 0.2% and 2 patients developed jaundice with ALT elevations and 1 patient [with preexisting cirrhosis) had hepatic decompensation).
- Klinker H, Naumann U, Rössle M, Berg T, Bondin M, Lohmann K, Koenig B, et al. Glecaprevir/pibrentasvir for 8 weeks in patients with compensated cirrhosis: safety and effectiveness data from the German Hepatitis C-Registry. Liver Int. 2021;41:1518–1522. [PubMed: 33966349](Among 148 patients with chronic hepatitis C and cirrhosis who were treated with Mavyret and enrolled in a German Registry, the SVR rate was 98% among those with follow up and no patient had a drug-related severe adverse event, although one developed jaundice which persisted after stopping and despite an SVR).
- Hara T, Ohara T, Taniguchi M, Sakai H, Oka K, Iwai N, Tsuji T, et al. Severe liver injury associated with glecaprevir plus pibrentasvir therapy in a patient with treatment-naïve hepatitis C virus infection. Intern Med. 2021;60:2437–2443. [PMC free article: PMC8381168] [PubMed: 33612683](49 year old Japanese man with chronic hepatitis C, HBsAg carrier state and history of alcoholism developed jaundice 6 weeks after starting Mavyret [pre-treatment bilirubin 1.4 rising to 20.6 mg/dL, ALT 50 to 65 U/L, AST 100 to 205 U/L, Alk P 436 to 442 U/L, INR 1.2, HBV DNA remaining negative] and evidence of hepatic encephalopathy, but ultimately recovered and was HCV RNA negative).
- Torgersen J, Newcomb CW, Carbonari DM, Rentsch CT, Park LS, Mezochow A, Mehta RL, et al. Protease inhibitor-based direct-acting antivirals are associated with increased risk of aminotransferase elevations but not hepatic dysfunction or decompensation. J Hepatol. 2021;75:1312–1322. [PMC free article: PMC8604762] [PubMed: 34333102](Among propensity matched subgroups of 71,391 adults with chronic hepatitis C treated in the Veterans Administration Medical system with oral, direct acting antiviral agents, patients with higher baseline fibrosis scores compared to those with lower scores were more likely to have serum ALT elevations [>200 U/L] [0.5% vs 0.3%] and hepatic decompensation [0.41% vs 0.03%], and ALT elevations were more frequent with Viekira Pak and Technive [1.1-1.2%] compared to Mavyret, Zepatier, Epclusa, and Harvoni [0.11-0.39%]).
- Huang CF, Kuo HT, Chang TS, Lo CC, Hung CH, Huang CW, Chong LW, et al. Nationwide registry of glecaprevir plus pibrentasvir in the treatment of HCV in Taiwan. Sci Rep. 2021;11:23473. [PMC free article: PMC8648748] [PubMed: 34873250](Among 3144 patients with chronic hepatitis C treated with Mavyret and enrolled in a nationwide Taiwanese HCV registry, the SVR rate was 99% and adverse events included ALT elevations in 2%, but most were mild and transient, only 0.6% were above 5 times ULN, and no patient developed clinically apparent liver injury or hepatic decompensation).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Zepatier.[LiverTox: Clinical and Researc...]Review Zepatier.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Safety and Pharmacokinetics of Glecaprevir/Pibrentasvir in Adults With Chronic Genotype 1-6 Hepatitis C Virus Infections and Compensated Liver Disease.[Clin Infect Dis. 2019]Safety and Pharmacokinetics of Glecaprevir/Pibrentasvir in Adults With Chronic Genotype 1-6 Hepatitis C Virus Infections and Compensated Liver Disease.Gane E, Poordad F, Zadeikis N, Valdes J, Lin CW, Liu W, Asatryan A, Wang S, Stedman C, Greenbloom S, et al. Clin Infect Dis. 2019 Oct 30; 69(10):1657-1664.
- Drug-induced liver injury by glecaprevir/pibrentasvir treatment for chronic hepatitis C infection: a systematic review and meta-analysis.[Ann Med. 2022]Drug-induced liver injury by glecaprevir/pibrentasvir treatment for chronic hepatitis C infection: a systematic review and meta-analysis.Hung HY, Hung WL, Shih CL, Chen CY. Ann Med. 2022 Dec; 54(1):108-120.
- Review Sofosbuvir.[LiverTox: Clinical and Researc...]Review Sofosbuvir.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Efficacy of Glecaprevir/Pibrentasvir for 8 or 12 Weeks in Patients With Hepatitis C Virus Genotype 2, 4, 5, or 6 Infection Without Cirrhosis.[Clin Gastroenterol Hepatol. 2018]Efficacy of Glecaprevir/Pibrentasvir for 8 or 12 Weeks in Patients With Hepatitis C Virus Genotype 2, 4, 5, or 6 Infection Without Cirrhosis.Asselah T, Kowdley KV, Zadeikis N, Wang S, Hassanein T, Horsmans Y, Colombo M, Calinas F, Aguilar H, de Ledinghen V, et al. Clin Gastroenterol Hepatol. 2018 Mar; 16(3):417-426. Epub 2017 Sep 22.
- Mavyret - LiverToxMavyret - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...